Abstract
Purpose
We describe a comparative study between an enhanced air-cushion tactile sensor and a wheeled indentation probe. These laparoscopic tools are designed to rapidly locate soft-tissue abnormalities during minimally invasive surgery (MIS).
Materials and Methods
The air-cushion tactile sensor consists of an optically based sensor with a 7.8 mm sphere “floating” on a cushion of air at the tip of a shaft. The wheeled indentation probe is a 10 mm wide and 5 mm in diameter wheel mounted to a force/torque sensor. A continuous rolling indentation technique is used to pass the sensors over the soft-tissue surfaces. The variations in stiffness of the viscoelastic materials that are detected during the rolling indentations are illustrated by stiffness maps that can be used for tissue diagnosis. The probes were tested by having to detect four embedded nodules in a silicone phantom. Each probe was attached to a robotic manipulator and rolled over the silicone phantom in parallel paths. The readings of each probe collected during the process of rolling indentation were used to achieve the final results.
Results
The results show that both sensors reliably detected the areas of variable stiffness by accurately identifying the location of each nodule. These are illustrated in the form of two three-dimensional spatiomechanical maps.
Conclusions
These probes have the potential to be used in MIS because they could provide surgeons with information on the mechanical properties of soft tissue, consequently enhancing the reduction in haptic feedback.
Introduction
Minimally invasive surgery (MIS), which includes but is not limited to laparoscopic surgery or robot-assisted surgery, can be defined simply as major or minor surgery that is performed through very small incisions, varying from 3 mm to 12 mm in diameter.1 An endoscopic camera is inserted through one of the incisions to transmit an enhanced view of the anatomy and pathology to the surgeon, and endoscopic instruments are inserted through the other incisions. The main accepted advantages of MIS are reduced tissue damage, reduced postoperative analgesic requirements, a decreased period of hospitalization, a reduction in blood loss and transfusion, and a better cosmetic result. On the other hand. potential disadvantages2 include the surgeon's inability to directly palpate the tissues in question. There is also friction between trocar ports and the laparoscopic instruments, and thus the actual contact forces with the tissue are skewed, making sensing of any tissue reaction forces through the instrument's shaft extremely difficult.
Finally, the surgical tools' movements are constrained at the insertion point, meaning that the tools' degrees of freedom (DOF) are limited to only four (insertion, roll, yaw, and pitch); this leads to loss of hand-eye coordination3 because of the fulcrum effect, which reverses the directions of the tools at their tips. In short, reduced visual, haptic, and tactile feedback, along with issues of lack of dexterity, can lead to accidental tissue damage.4
Haptics describes what the human hand can feel, and incorporates both force (kinesthetic) sensing and tactile (cutaneous) sensing.5 The benefits of haptic feedback during minimally invasive robot-assisted surgery (MIRS) are extensively detailed in and article by Puangmali and associates.6 This feedback may enable surgeons to reduce positive surgical margins, particularly when significant palpable differences in mechanical tissue properties exist.
The novel air-cushion sensor and the wheeled probe were developed to provide the surgeon with improved haptic feedback and an increased opportunity to locate abnormalities in soft tissues, such as tumor nodules, during MIS. Mechanical images can then be generated quickly to illustrate the stiffness distribution in the soft tissue. We carried out a comparative study between two different sensors that are being developed at our institution to establish their ability to accurately detect tissue viscoelastic differences.
Materials and Methods
Design
The MRI compatible air-cushion tactile sensor is made of an acrylonitrile butadiene styrene (ABS) plastic structure and sphere and is equipped with an optical fiber. The dimensions of the sensor are within the acceptable MIS standards with a shaft of outer diameter 12 mm enabling it to pass down a standard laparoscopic trocar. The structure and the working principle of the sensor are illustrated in Figure 1.
FIG. 1.
Structure and principle of the air cushion sensor.
The inner diameter ranges between 7.4 mm at the tip of the shaft to 10 mm at the distal end. The total length of the shaft is 60 mm. The sphere, which is located at the tip of the shaft, has a diameter of 7.8 mm. The optical fiber, which has an outer jacket of 2.2 mm and a core of 1 mm, is kept in place just above the sphere by two brackets. The fibre optic, which is connected to an optical sensing scheme, is used to detect the motion of the sphere along the longitudinal axis of the shaft. The optical fiber that both emits light onto the sphere and collects the reflected light back from the sphere is able in this way to continuously monitor the distance between the tip and the sphere. As a result, a change in this distance during the process of rolling indentation would indicate a change in the mechanical properties of the tissue under inspection (Fig. 1).
The distal end of the sensor is connected to a compressor that generates a steady flow of air at a pressure of 7 kPa into the shaft, applying pressure onto the sphere. Consequently, during rolling indentation, the sphere rolls on a cushion of air, resulting in a virtually frictionless roll. The design and materials used for this novel sensor make it entirely MRI compatible.
The wheeled probe sensor7 (Fig. 2) is a force sensor made of an aluminium wheel and force/torque sensor. The wheel of the sensor is also within the MIS standards with a diameter of 5 mm and a width of 10 mm. The wheel, which is made of aluminium, acts as an end effector for the sensor. The wheel benefits from a smooth surface to reduce the effects of friction during the process of rolling indentation. The cylindrical wheel is mounted to a force/torque sensor (ATI NANO17 SI-12-0.12 with NI PCI 6034E 16-bit DAQ). This sensor allows for the measurements of the three force components (x, y, and z direction) acting on the wheel during the process of rolling indentation.
FIG. 2.
Wheeled probe sensor.
Experimental procedure
An experimental procedure using a silicone phantom with embedded hard and soft nodules made of rubber and silicone, respectively, was used for the direct comparative study between the MRI compatible air cushion tactile sensor and a wheel indentation probe. During the trial, four differing types of nodule were used: Nodule A, a semispherical hard nodule with a diameter of 9 mm and height of 8 mm; nodule C, a semispherical soft silicone nodule with a diameter of 10 mm and height of 7 mm; nodule D, a semispherical soft silicone nodule with a diameter of 10 mm and height of 5 mm; and nodule B, a cylindrical soft silicone nodule with a diameter and height of 9 mm and 8 mm, respectively.
The four nodules were placed on a metal board facing down and were then covered with a silicone phantom that measured 100 × 100 × 10 mm (Fig. 3). The dimensions of the soft nodules are altered once covered with the silicone phantom. The silicone phantom represented the soft tissue under inspection, and the nodules acted as differing tissue abnormalities.
FIG. 3.
Silicone phantom with nodules.
To achieve rolling indentation, each sensor was attached consecutively to the distal tip of the Mitsubishi RV-6SL 6-DOF robotic arm to achieve a controlled motion for each pass in terms of direction and velocity. Each sensor was rolled over the silicone phantom in 10 parallel and adjacent paths of 10 mm width and 100 mm length. An indentation depth of 4 mm into the silicone phantom was kept during the rolling indentation for both sensors. The speed of travel of 20 mm/s was kept constant throughout each roll. During the rolling indentation for the MRI compatible air-cushion sensor, the compressor was set to generate a pressure of 6.9 kPa, which represents a gentle pressure down a laparoscopic instrument.
Results
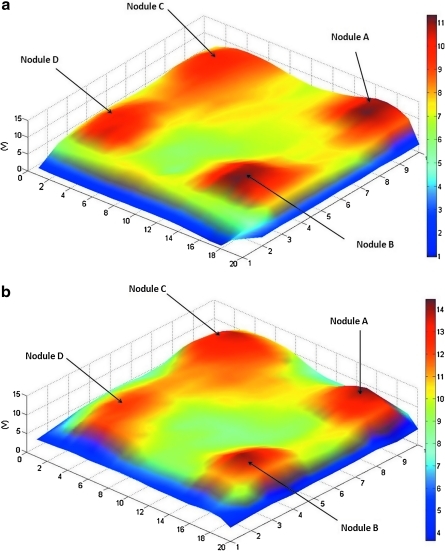
The MRI compatible air-cushion sensor and the wheeled probe both accurately located the position of all four nodules in the silicone block despite their varying shapes and elasticities. Spatiomechanical images were generated for each sensor's readings to illustrate the stiffness distribution in the silicone phantom (Figs. 4a and 4b). The spatiomechanical stiffness images are color-coded to illustrate the variation in stiffnesses with blue at the bottom end of the spectrum representing an area of low stiffness and red at the other end of the spectrum representing an area of high stiffness.
FIG. 4.
(a) Spatiomechanical stiffness images for air-cushion sensor. (b) Spatiomechanical stiffness images for wheel probe.
In Figures 4a and 4b, four peaks can be identified on each map. Nodule A is the most prominent peak because of its properties as a hard nodule. Nodule B is slightly less prominent because of its properties as a soft nodule but is still easily visible because of its large size. Nodule C is slightly more discreet, reflecting its size and soft properties. Nodule D is more discreet, but its peaks can still be identified despite its properties as a soft nodule of smaller size. It must be noted that in both figures, the location of the four peaks accurately represents the location of the actual nodules underneath the silicone phantom, and their stiffness representation is a clear indication of their mechanical properties and size.
Role in Endourology
A long-standing criticism of MIS is the inability of the operating surgeon to palpate target tissues during the operative procedure (haptic feedback). It is thought by many that actually feeling the differences between normal and abnormal structures may lead directly to key decision-making steps in oncologic surgery. The ability to return the loss of tactile feedback experience in MIS to the operating surgeons via intraoperative assessment of tissue properties relayed to real-time imaging is exciting.
Others have investigated the potential of optical coherence tomography (OCT) and intraoperative transrectal ultrasonographic imaging to improve intraoperative decision-making and tissue assessment.8–10 Unlike OCT, these sensors have no coupling problems, because the spherical tip or grooved wheel is moved directly over the tissue. We have previously published an article on the concept of the air-cushion probe in MIS11 and its potential to discriminate not only between hard and soft tissues but also to be calibrated to detect subtle differences in the texture of individual areas of specific tissue surfaces. This trial between two MIS sensors with significant differences in concept and design shows both devices to have good potential in this field.
The air tactile sensor benefits from a simple structure and a straightforward sensing principle. The fact that the sensor is made of ABS plastic and has an optical fiber as a sensing element makes it entirely MRI compatible and easy to sterilize. The sphere at the tip of the sensor allows it to follow straight and curved paths with ease, facilitating the rapid acquisition of data. The influx of air at the top of the sensor, which can be adjusted to change the amount of force applied, can easily be replaced with carbon dioxide to meet MIS standards. Its small size and its “on board” sensor give it the potential to be used around corners, because both the optical fiber and the tube of air are flexible. The design and materials of the sensor are simple and affordable, making it a relatively low-cost sensor. The limitation of this sensor is that its sensing range is constrained to the displacement of the sphere, which can only ever be as much as the radius of the sphere. Hence, the smaller the sphere, the smaller the maximum displacement measured.
The wheel probe sensor benefits from a wheel that can fit through a trocar port and can be used to cover large areas rapidly. Its force/torque sensor is very accurate and is able to detect a wide range of forces, resulting in its potential use for soft-tissue analysis and classification. The force/torque sensor cannot be fitted through a trocar port because of its size, forcing the wheel to be rigidly connected to the sensor, consequently limiting its flexibility. The materials of the wheel and the sensor unfortunately do not make it MRI compatible, and the very high price of the force/torque does not make it a low-cost design. Despite these drawbacks, the sensor nonetheless is still very accurate and efficient.
Future directions
Animal studies using tumor models are not ethically acceptable in the United Kingdom and, because the probe has no significant risk to human tissue, it can thus be tested directly in clinical situations after hospital ethical committee approval. We will shortly be conducting a head-to-head trial of both sensors on their ability to detect palpable and impalpable prostatic nodules during radical prostatectomy. Initially, this will involve ex-vivo assessment of excised prostates before moving to intraoperative trials.
We see these probes, once fully developed and validated, to have substantial potential in the detection of tissue abnormalities in urologic and other MIS.
Both of the probes under consideration can slide down a standard 12-mm trocar, assess tissue reliably and relatively quickly, and cause no damage to the tissues themselves. The combination of three-dimensional vision and robotic assistance, along with the highly detailed imaging capability of this system, offers the prospect of reducing positive surgical margins in patients with urologic and other malignancies. These probes could be used to characterize the force tissue deflection profile of soft tissues during MIS, providing surgeons with enhanced haptic feedback.
Conclusion
Both the air-cushion sensor and the wheeled probe were able to locate and discriminate between embedded nodules of differing hardness and depth. These devices both consist of designs that allow quick and easy use by minimally invasive surgeons. Once fully validated and tested clinically, these sensors have considerable potential within the field of MIS.
Abbreviations Used
- ABS
acrylonitrile butadiene styrene
- DOF
degrees of freedom
- MIRS
minimally invasive robot-assisted surgery
- MIS
minimally invasive surgery
- MRI
magnetic resonance imaging
- OCT
optical coherence tomography
Acknowledgments
The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St. Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust.
Disclosure Statement
No competing financial interests exist.
References
- 1.Bicchi A. Canepa G. de Rossi D, et al. A sensorised minimally invasive surgery tool for detecting tissutal elastic properties. Proceedings from the IEEE International Conference on Robotics and Automation; Apr;1996 ; Minneapolis, MN. 1996. pp. 884–888. [Google Scholar]
- 2.Treat MR. Computer-Integrated Surgery. Cambridge, MA: MIT Press; 1995. A surgeon's perspective on the difficulties of laparoscopic surgery; pp. 559–560. [Google Scholar]
- 3.Tavakoli M. Aziminejad R. Patel RV. Moallem M. Methods and mechanisms for contact feedback in a robot-assisted minimally invasive environment. Surg Endosc. 2006;20:1570–1579. doi: 10.1007/s00464-005-0582-y. [DOI] [PubMed] [Google Scholar]
- 4.Tendick F. Cavusoglu M. Human machine interfaces for minimally invasive surgery. Proceedings from the 19th International Conference of IEEE = EMBS; Chicago, IL. 1997. pp. 2771–2776. [Google Scholar]
- 5.Okamura A. Methods for haptic feedback in teleoperated robot-assisted surgery. Ind Rob. 2004;31:499–508. doi: 10.1108/01439910410566362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puangmali P. Althoefer K. Seneviratne LD, et al. State-of-the-art in force and tactile sensing for minimally invasive surgery. IEEE Sensors J. 2008;8:371–381. [Google Scholar]
- 7.Liu H. Noonan DP. Challacombe BJ, et al. Rolling mechanical imaging for tissue abnormality localization during minimally invasive surgery. IEEE Trans Biomed Eng. 2010;57:404–414. doi: 10.1109/TBME.2009.2032164. [DOI] [PubMed] [Google Scholar]
- 8.Ukimura O. Magi-Galluzzi C. Gill IS. Real-time transrectal ultrasound guidance during laparoscopic radical prostatectomy: Impact on surgical margins. J Urol. 2006;175:1304–1310. doi: 10.1016/S0022-5347(05)00688-9. [DOI] [PubMed] [Google Scholar]
- 9.Rais-Bahrami S. Levinson AW. Fried NM, et al. Optical coherence tomography of cavernous nerves: A step toward real-time intraoperative imaging during nerve-sparing radical prostatectomy. Urology. 2008;72:198–204. doi: 10.1016/j.urology.2007.11.084. [DOI] [PubMed] [Google Scholar]
- 10.Aron M. Kaouk JH. Hegarty NJ, et al. Second prize: Preliminary experience with the Niris optical coherence tomography system during laparoscopic and robotic prostatectomy. J Endourol. 2007;21:814–818. doi: 10.1089/end.2006.9938. [DOI] [PubMed] [Google Scholar]
- 11.Zbyszewski D. Challacombe B. Liu H, et al. Air-cushion force-sensitive probe for soft tissue investigation during minimally invasive surgery. J Endourol. 2009;23:1421–1424. doi: 10.1089/end.2009.0382. [DOI] [PubMed] [Google Scholar]