Abstract
Background
Necrotizing enterocolitis (NEC) is a devastating disease that affects primarily the intestine of premature infants. Despite recent advances in neonatology, NEC remains a major cause of morbidity and mortality in neonates. Neonatal mucosal defenses and adherence of bacterial pathogens may play an important role in the pathogenesis of NEC.
Methods
Review and synthesis of pertinent literature.
Results
Putative factors that have been implicated in the pathogenesis of NEC include abnormal patterns of gut colonization by bacteria, immaturity of the host immune system and mucosal defense mechanisms, intestinal ischemia, formula feeding, and loss of intestinal epithelial barrier integrity.
Conclusion
Host defenses and intestinal microbial ecology are believed to play important roles in the pathogenesis of NEC. Commensal bacteria and probiotic therapy may be of therapeutic utility in the maintenance of the gut epithelial barrier.
Necrotizing enterocolitis (NEC) is the most frequent and lethal disease that affects the intestine of premature infants. The incidence of NEC is reported to be approximately 2–5% [1,2]. However, it is likely to continue to rise because of the increased survival of infants born at 24 weeks gestation, which results in a greater number of neonates at risk of developing NEC. Indeed, data from the U.S. Centers for Disease Control and Prevention indicate that the incidence of premature births has almost doubled since 1995 [3]. According to epidemiologic studies, the principal risk factors associated with NEC include low gestational age, low birth weight, low Apgar scores, hyaline membrane disease, formula feeding, umbilical vessel catheterization, and intestinal ischemia [4]. The mortality rate due to NEC ranges between 10% and 50%; however, in infants with the most severe form of the disease, mortality approaches 100% [5]. Survivors of NEC often experience severe long-term complications. These include intestinal strictures, short bowel syndrome, recurrent sepsis, poor growth, and cerebral palsy [5]. These adverse short-term and long-term sequelae underscore the importance of defining the pathophysiology of this devastating disease.
Risk Factors for the Development of NEC
Multiple risk factors including prematurity [6], age at initiation, composition and rate of enteral feeding [7,8], bacterial infection [9], and intestinal ischemia [10,11] have been implicated in the pathogenesis of NEC. Nonetheless, the complex interacting etiologies of the disease remain undefined. The only consistent epidemiologic precursors of NEC are prematurity and enteral alimentation. There is continuing debate, however, regarding the importance of enteral alimentation in the pathogenesis of NEC, because a prospective randomized study failed to show any increase in the incidence of NEC despite an aggressive feeding strategy [12]. Furthermore, up to 10% of infants with NEC have never received any form of enteral nutrition [13,14].
The indigenous intestinal microbial flora has been postulated to play a central role in the pathogenesis of NEC. In fact, bacterial colonization may be a prerequisite for the development of NEC [15] based on data demonstrating that oral prophylaxis with vancomycin or gentamicin reduced the incidence of NEC [16]. Common bacterial isolates from blood, peritoneal fluid, and stool from infants with advanced NEC include Escherichia coli, Enterobacter, Klebsiella species, and occasionally, coagulase-negative Staphylococcus species [17]. As we shall see later, these infections may be the result of translocation of indigenous bacteria through a previously injured intestinal epithelium. Our goal in this review is to define the mechanisms that lead to bacterial invasion and necrosis of the intestinal wall [18,19].
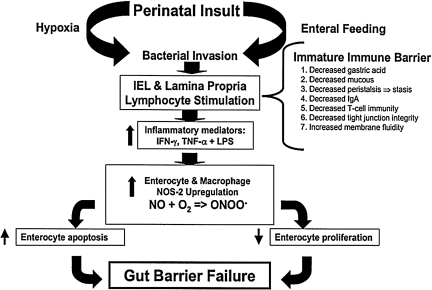
The histologic hallmark of NEC is a “bland infarct” characterized by full-thickness coagulation (ischemic) necrosis, a paucity of acute inflammatory cells (neutrophils), and a predominantly lymphocytic infiltrate [20,21]. Therefore, investigators have postulated that intestinal ischemia resulting from respiratory distress syndrome or cyanotic congenital heart disease, for instance, may be an important inciting event in the pathogenesis of NEC by causing mucosal injury. According to this theory, splanchnic hypoperfusion resulting from various perinatal insults may induce intestinal mucosal injury. Indigenous microorganisms may then breach the immature gut barrier, which has been compromised further by the ischemic insult, and incite a local inflammatory response. As a result, an inflammatory cascade develops, highlighted by the release of various humoral mediators that may be responsible, in part, for the systemic manifestations of NEC. Figure 1 illustrates our current hypothesis regarding the pathogenesis of NEC. In this review, we will examine the principal factors responsible for maintenance of gut barrier integrity, and how disruption of these processes in the premature infant may contribute to the development of NEC.
FIG. 1.
Pathogenesis of necrotizing enterocolitis.
The Gut Epithelial Barrier
The intestinal epithelium harbors the most rapidly proliferating cells in the body. It consists of a single layer of polarized epithelial cells that serve as a selective barrier to the entry of pathogenic bacteria and antigens into the systemic circulation [22]. The selective permeability of the intestinal epithelium is the result of a dynamic equilibrium between extrinsic and intrinsic barriers, which normally protect the host from various microbes. Gastric acidity, intestinal peristalsis, the mucus coat [23], and secretory immunoglobulin A (sIgA), for instance, comprise some of the extrinsic barriers that limit intestinal colonization and restrict bacterial attachment to the epithelium [24,25]. Intrinsic barriers, on the other hand, include the semipermeable epithelial cell plasma membrane and the tight junctions (TJs) that work by sealing the intercellular spaces, preventing paracellular passage of bacteria and restricting the diffusion of most macromolecules, as well as the cellular defense mechanisms [26].
Extrinsic barrier
In the following sections, we focus mainly on antibacterial factors such as defensins and sIgA, the mucus layer, and the intestinal microflora.
Defensins and sIgA
At the epithelial level, various innate mechanisms are available to preserve gut barrier integrity. These include secretion of mucin from the intestinal goblet cells, as well as defensins (in particular, alpha defensins) from Paneth cells [27]. Alpha defensins are cysteine-rich cationic peptides with antibiotic activity against a variety of bacteria. Local secretion of defensins by Paneth cells at the bottom of the intestinal crypts may serve to protect the intestinal stem cell population [28].
Intestinal humoral immunity is provided mainly by sIgA within the gut lumen. sIgA is quantitatively one of the most abundant immunoglobulins within the body; nearly 40 mg/kg are produced daily in mucosal tissues. Following its secretion from local plasma cells, IgA binds to an epithelial cell surface receptor on the basolateral surface of the enterocyte [25]. This complex is then internalized, transported through the epithelial cell, and ultimately secreted into the intestinal lumen as sIgA. Unlike other immunoglobulins, sIgA does not participate in the inflammatory response and does not bind complement; instead, it serves as an immunologic barrier that inhibits uptake of antigens. This antibody forms complexes with antigens and bacteria in the gut lumen, thereby preventing their binding to the mucosal surface and subsequent absorption [29]. As a result, the presence of sIgA within the gut lumen is important in maintaining gut barrier integrity.
Protective mucus coat and its components
Water, mucin, and lipids comprise the mucus coat that lines the apical surfaces of the gastrointestinal (GI) tract [30]. Mucin is produced by goblet cells in the GI tract and is regulated by specific genes that are part of the MUC family [31]. Various forms of mucins exist, which include secretory, membrane bound, and soluble mucins [32]. Mucins have many important functions that benefit the epithelial cells lining the GI tract. This viscoelastic mucus gel layer acts as a protective barrier against the harsh luminal environment by serving as a natural lubricant, providing mechanical protection and preventing epithelial damage by acidic secretions from the duodenum and stomach. One of the most important functions of mucin is the binding of bacteria, which prevents them from attaching to the intestinal epithelium. The presence of a large number of sulfate and sialic acids on the carbohydrate chains of mature mucins contribute to its higher viscosity and acidity [33,34]. Thus, the acidic mucins that are present in the mucus protect the underlying epithelium from attack by bacterial enzymes [35]. Lack of MUC3A gene, as well as decreased synthesis of MUC2 gene, which produces MUC2 mucin, the most important mucin in the GI tract, has been associated with the development of inflammatory bowel disease [33]. Therefore, mucin plays an important role in the gut barrier by exerting a protective effect against pathogenic bacteria.
Normal intestinal flora
Colonization of the GI tract with bacteria begins immediately after birth. Neonates have a markedly different intestinal flora compared with adults, which may influence the development of NEC. In the neonate, indigenous bacteria colonize the mucosal surfaces and the intestinal lumen in a typical pattern called succession [36,37]. There are four phases of succession. Phase 1 is also referred to as the initial acquisition phase and lasts from birth to 2 weeks. During phase I, streptococci and coliforms predominate in the intestine. Gram-positive, non-spore-forming anaerobes appear and include predominantly bifidobacteria in breast-fed infants and lactobacilli in formula-fed infants. Clostridia and Bacteroides can also be found but in lower numbers than later in development. Phase II is the remaining period of breast-feeding and occurs from the end of phase I to the beginning of consumption of solid food. During phase II, Bacteroides increase progressively in number. Phase III is the remaining time between beginning of supplementation and complete cessation of breast feeding. This phase continues until the flora resembles that of adults (phase IV)—an event known as climax [38].
The indigenous intestinal microflora, especially the anaerobes, normally restricts the number of coliforms to relatively low concentrations [39]. Because there is a relative paucity of anaerobes during phase I of succession, pathogenic bacteria may be more likely to colonize the neonatal gut during that period and thus contribute to the pathogenesis of NEC, which typically develops during the first two weeks of life. Consistent with this theory, commensal bacteria acquired during the early postnatal period are required for the development of intestinal tolerance to luminal antigens [40]. In fact, studies have shown that commensal bacteria can regulate the expression of certain protective barrier genes as well as genes involved in digestion and angiogenesis [41]. Commensal bacteria protect the host by reducing the ability of the pathogenic bacteria to adhere to the epithelial surface. They accomplish this task through different methods such as by producing toxic substances against aerobic bacteria, competing for binding sites, as well as reducing the intraluminal pH [42,43]. Hence, the normal flora plays an important role in overall maintenance of the intestinal barrier.
Intrinsic barrier
Intrinsic barriers include the semipermeable epithelial cell plasma membrane and the TJs that seal the intercellular spaces, preventing paracellular passage of macromolecules and bacteria, as well as the cellular defense mechanisms.
TJs and intestinal permeability
The GI tract represents the largest body surface area that is exposed to environmental pathogens. Intestinal epithelial barrier function depends to a large extent on the presence of TJs, which connect adjacent enterocytes at their apical surface, leading to the formation of a barrier impenetrable to bacteria and large macromolecules [44]. Tight junctions are formed by the tenth week of gestation [45]. The interaction of these TJs with cytoskeletal proteins such as actin helps maintain polarity and the structure of the TJ assembly. Multiple cell culture studies have demonstrated the relationship between the density of TJs and the resistance of the epithelial barrier to breakage [46]. They consist of integral proteins (occludin and claudins) that seal the paracellular spaces and associated cytosolic proteins (zonula occludens-1 [ZO-1] and junction-associated molecule-A) that connect TJ to intracellular actinomyosin complexes.
In vitro studies have demonstrated that, following exposure to inflammatory cytokines, disruption of TJs occurs in model epithelial monolayers. Other studies have helped to define the critical role of myosin light chain kinase (MLCK), a serine–threonine protein kinase activated by Ca2+/calmodulin that phosphorylates the light (regulatory) chain of myosin, in maintaining TJ integrity [47]. For example, in histamine-treated corneal epithelial cell monolayers, increase in barrier permeability was observed when MLCK was activated along with increased MLC phosphorylation [48]. A similar effect was observed in tumor necrosis factor alpha (TNF-α)-treated endothelial monolayers [49] as well as various other proinflammatory cytokine-treated enterocyte monolayers [50,51]. Recent evidence suggests that TJ are not static structures and can be altered during various pathologic processes, as well as in the presence of pathogenic bacteria [52]. Enteric pathogens can disrupt TJs utilizing a variety of mechanisms. For instance, enteropathogenic E. coli alters occludin and leads to disruption of TJ [53], whereas Clostridium difficile toxins A and B cause disorganization of apical and basal F actin and dissociation of occludin, ZO-1, and ZO-2 from the lateral TJ membrane [54].
Cellular immunity
One of the major components of the intrinsic mucosal barrier is a network of lymphoid cells in the epithelium (intraepithelial lympocytes or IELs), lamina propria as well as Peyer patches of the intestine, which is collectively referred to as gut-associated lymphoid tissue (GALT). The various components of the GALT, however, are not activated until postnatal antigenic exposure occurs. The IELs represent a unique population of T cells of mostly CD8+ phenotype that reside superficial to the basement membrane of the epithelium in both small and large intestines. The lamina propria consists of a loose connective tissue matrix between the muscularis mucosa and the epithelium. It contains T cells with mostly CD4+ phenotype. These cells, along with antigen-presenting cells such as dendritic cells and B cells in the lamina propria, are responsible for sampling of luminal antigens and bacterial factors [55]. This process sensitizes the GALT against potential pathogenic bacteria or antigens and serves to restrict their transfer across the epithelium.
In the next section, we will examine how prematurity and enteral feeding, two of the principal risk factors associated with the development of NEC, may affect gut barrier function and predispose the vulnerable host to this devastating disease.
Prematurity and Host Defense Mechanisms
There is an inverse relationship between gestational age and the incidence of NEC. Prematurity, defined as delivery prior to 37 weeks of gestation, is often associated with low birth weight and intrauterine growth retardation. In most cases of NEC, the infants weigh less than 2,500 g at birth. In fact, the incidence of and morbidity and mortality from NEC are significantly greater among very low birth weight (VLBW) infants, defined as birth weight below 1,500 g, and extremely low birth weight infants, defined as birth weight less than 1,000 g [56]. There are approximately 60,000 VLBW infants born in the United States each year [57]. Recent reports from the National Institute of Child Health and Human Development show that 7% of these infants develop NEC Bell stage II or higher [58]. Other population-based studies have similarly reported the incidence of NEC to be 6–7% in this population [59,60].
Evidence suggests that in premature infants, the immune system is relatively immature. This immaturity is characterized by deficiency in local antibacterial products such as sIgA, defensins, and intestinal trefoil factor, altered immune cell production, as well as delayed intestinal colonization by protective commensal bacteria. In addition, functional maturation of the GI tract is delayed in premature infants, and this may play a key role in the development of NEC. For instance, compared with the term infant, the preterm neonate produces smaller amounts of gastric acid and mucus and exhibits increased mucosal permeability and diminished peristaltic activity. The latter is particularly important because intestinal peristalsis propels luminal contents along the GI tract. This function not only aids in digestion, but it also prevents prolonged bacterial stasis in the intestine, thereby reducing the time available for bacteria to penetrate the mucus layer and attach to the intestinal epithelium, which is an important and necessary step for bacterial invasion or bacterial translocation across the gut barrier.
Human and animal studies have demonstrated that the motility of the GI tract is tightly regulated throughout development [61]. In brief, the fetal intestine does not mature until the third trimester despite the fact that intestinal motility can be detected as early as the second trimester. Migrating motor complexes, which serve as the “housekeepers” of the GI tract, do not appear in the intestine until the thirty-fourth week of gestation [62]. Migratory motor complexes regulate distal propulsion of intestinal contents.
Hypoxic conditions are known to affect GI motility and decrease the propulsion of intestinal contents [63]. Thus, in the premature infant, who already has relatively impaired GI motility, any superimposed hypoxic or ischemic insult can further exacerbate the defect in intestinal motility and result in bacterial overgrowth and stasis. This phenomenon may facilitate bacterial invasion, which in turn will elicit the inflammatory cascade leading to NEC.
Enteral Feeding
The premature infant's intestine is sterile at birth and does not become colonized with bacteria until passage through the vaginal canal or the initiation of enteral feedings or instrumentation. Breast feeding is known to promote intestinal maturation and increase local protective mechanisms. In addition, breast milk is a major source of immunoglobulins and lysozymes, which serve to enhance the baby's immature immune system [64]. Further, breast milk contains trophic factors such as epidermal growth factor, as well as antiinflammatory cytokines such as interleukin-10 (IL-10). Indeed, IL-10 has been hypothesized to play a protective role in the intestinal epithelium in experimental NEC [65,66]. Breast-fed infants also have increased levels of beneficial probiotic organisms such as bifidobacteria and decreased levels of pathogenic gram-negative bacteria such as Enterobacter species [67].
Unfortunately, most premature infants are fed formula rather than breast milk and are exposed to the nosocomial flora of the neonatal intensive care unit (NICU). Thus, formula feeding results in colonization of the immature intestine with pathogenic bacteria, partly as a result of contamination. Contaminated formula, although rare, has been associated with outbreaks of NEC. A review of 125 cases of NEC showed that Enterobacter spp. were the most common bacterial isolates recovered from 29% of patients [68]. In particular, Enterobacter sakazakii (ES) has been shown to be one of the most virulent causative agents of NEC. Other bacteria, fungi, and some viruses have also been implicated in outbreaks of NEC in NICUs. In contrast to formula feeding, increased use of breast milk has been shown to decrease the incidence of NEC [69,70]. A recent study suggests a dose-dependent protective effect of breast milk in reducing the incidence of NEC in VLBW infants [71].
Antigen transport in the neonatal period is less restricted than in adults, a phenomenon that is important for mucosal antigen sampling and gut maturation. Udall et al. [72] measured plasma radioactivity in rabbits fed radiolabeled bovine serum albumin (BSA) at birth, one, two, six weeks, and one year of age [73]. They demonstrated a marked increase in transport of radiolabeled BSA in the younger age groups. Breast-fed animals exhibited lower intestinal passage of radiolabeled BSA [74]. Similar findings have been demonstrated in human neonates [75,76]. Changes in the enterocytes that occur during development may also promote increased mucosal passage of macromolecules. The cell membrane lipid composition changes with development [77]. Thus, differences in the biological and physical properties of the intestinal epithelium may contribute to the increased mucosal transport of bacteria and macromolecules in neonates.
However, there is abundant evidence that antigen sampling may be impaired or dysregulated in premature neonates, thus placing them at risk for bacterial invasion and the development of NEC. The intestinal microclimate, local mucosal mucin production, and composition changes with age [78]. This alteration in mucin composition may allow antigens and pathogenic bacteria to more easily penetrate the mucus layer. Further, synthesis of sIgA does not occur for several weeks postnatal; as a result, there is decreased binding of luminal bacteria. Taken together, altered mucus composition and decreased sIgA production may facilitate bacterial attachment to the epithelium and subsequent translocation to incite the inflammatory cascade leading to NEC.
Inflammatory Mediators and NEC
Activation of the cellular component of the intrinsic barrier results in the release of proinflammatory mediators and cytokines. Multiple inflammatory factors have been implicated in the pathogenesis of NEC. These include platelet-activating factor, interferon gamma (IFN-γ), TNF-α, IL-1, IL-6, IL-12, and nitric oxide (NO•). Our group has shown increased production of IFN-γ, IL-1β, and TNF-α in the intestinal epithelium, and sustained overproduction of NO• in the intestinal epithelium during NEC.
A recent study suggests that IFN-γ-deficient mice may be partially protected from NEC [79], which supports the role of IFN-γ in disease pathogenesis. In addition, Nadler et al. have demonstrated in an animal model of NEC that both IL-6 and IL-12 are elevated in the intestinal epithelium [80]. In fact, previous studies by Hsueh et al. have shown that coinjection of TNF-α and lipopolysaccharides or LPS leads to significant bowel necrosis in newborn rats, consistent with the morphological changes seen in NEC [81]. These findings corroborate earlier studies that demonstrated that oral or intravenous administration of LPS in newborn rats and piglets combined with hypoxia resulted in intestinal findings that resembled human NEC [82,83]. These data support the importance of bacteria and their products in the activation of the inflammatory cascade leading to NEC.
NO• and the pathogenesis of NEC
NO• plays a paradoxical role in the pathogenesis of NEC. At low levels, NO• acts as a local vasodilator that improves mucosal blood flow. However, sustained upregulation of NO• and its oxidative by-products has been shown to cause direct epithelial injury and to promote epithelial cell apoptosis [84]. We have previously demonstrated that the inducible isoform of NO• synthase or iNOS, and IFN-γ messenger ribonucleic acid are upregulated in the intestine of infants undergoing intestinal resection for acute NEC, and that NO• mediates intestinal epithelial injury (enterocyte apoptosis and necrosis) via the formation of peroxynitrite [85,86]. Upregulation of iNOS and peroxynitrite in the intestinal epithelium colocalized with enterocyte apoptosis suggests that NO• mediates epithelial injury via the formation of peroxynitrite. Under normal circumstances, following intestinal mucosal injury, epithelial integrity is restored in relatively short time via a process known as epithelial restitution, which involves migration of adjacent enterocytes to fill the gap created by the missing cells, followed by crypt cell proliferation to replace the missing enterocytes. Accelerated rates of villus destruction can result in an imbalance in this process and leave the intestinal epithelium with bare areas that are exposed to pathogens [87]. Data from our lab show that NO• not only causes epithelial injury but also disrupts the repair mechanisms, namely epithelial restitution and enterocyte proliferation [88]. This phenomenon creates persistent epithelial defects or bare areas in the intestinal epithelium through which bacteria can translocate and incite the inflammatory cascade. The translocation of bacteria can lead to further epithelial injury, intestinal perforation, and systemic sepsis which are characteristics of NEC [89].
In summary, evidence suggests that the pathogenesis of NEC is multifactorial in etiology. The inciting event may be an initial injury to the immature mucosal epithelium caused, in part, by environmental factors such as hypoxia, intestinal ischemia resulting from splanchnic hypoperfusion, or enteral alimentation. Immaturity of the neonatal mucosal immune system and intestinal epithelial barrier may predispose the premature infant to bacterial invasion (Fig. 1). Indigenous microorganisms may then breach the immature gut barrier, which has been further compromised by the ischemic insult. Moreover, bacterial–epithelial interactions and local intestinal invasion lead to the release of various proinflammatory cytokines and NO•. Initiation of this inflammatory cascade leads to propagation of a vicious cycle of injury and inflammation, leading to widespread microbial invasion of the intestinal wall, intestinal necrosis or perforation, and systemic sepsis (Fig. 1).
Role of bacteria in the pathogenesis of NEC
As discussed in previous sections, the indigenous intestinal microbial flora has been postulated to play a central role in the pathogenesis of NEC. In fact, bacterial colonization may be a prerequisite for the development of NEC. Common bacterial isolates from blood and peritoneal and stool cultures from infants with advanced NEC include E. coli, Enterobacter [90], Klebsiella species, and occasionally, coagulase-negative Staphylococcus species [91,92]. Other organisms that have been isolated include Streptococcus and Lactobacillus. In formula-fed infants, obligate anaerobes such as enterococci, coliforms, and clostridia have also been reported [93]. Anaerobic organisms tend to appear somewhat later during the postnatal period.
Outbreaks of NEC within NICUs further support the role of pathogenic bacteria in the pathogenesis of NEC. Table 1 represents a list of all bacterial pathogens that have been implicated in the development of NEC [94]. Our laboratory, in particular, has studied the role of ES in the pathogenesis of NEC.
Table 1.
List of Pathogenic Bacteria Implicated in Cases of Necrotizing Enterocolitis
| Gram-negative bacteria | Gram-positive bacteria |
|---|---|
| Clostridia | Staphylococci |
| Clostridium butyricum | Staphylococcus aureus (MRSA) |
| Clostridium difficile | Staphylococcus epidermis |
| Clostridium perfringes | |
| Escherichia coli | |
| Enterobactericeae | |
| Enterobacter cloacae | |
| Enterobacter sakazakii | |
| Klebsiella pneumoniae | |
| Pseudomonas aeroginosa | |
| Salmonella species |
MRSA, methicillin-resistant staphylococcus aureus.
Molecular Mechanism of Bacterial Interaction with the Gut Barrier
There are ubiquitous bacterial factors that interact with both the innate as well as the adaptive intestinal immune system. Therefore, many studies have focused on the nature of these interactions. An example of these conserved bacterial factors is a group of molecules called PAMPs, or bacterial pattern-associated molecular pattern molecules. These molecules include compounds such as LPS, glycolipids, and nucleic acids, which are present on the surface of bacteria. There are certain receptors at the epithelial level that recognize these conserved bacterial patterns. These receptors are called pattern recognition receptors (PRRs), which play a key role in the maintenance of intestinal integrity as a result of their interaction with bacterial PAMPs. Toll-like receptors (TLRs) are a great example of PRRs. Currently, there are 10 different TLRs named by their numbers. Among them, TLR-4 has been the most studied because of its binding of LPS [95]. This relationship was established when it was demonstrated that mice with a single mutation in the TLR-4 gene are resistant to the systemic effects of LPS [96].
Both in vivo and in vitro studies of NEC have shown that LPS binding and signaling through TLR-4 promotes increased apoptosis and results in the development of the symptoms of NEC. The important role of TLR-4 signaling in NEC was further shown by Leaphart et al., who demonstrated that TLR-4 knockout mice are protected from the development of NEC [97]. Caplan et al. showed a temporal decrease in the expression of TLR-4 receptor in the intestinal epithelium of breast-fed animals, which are less susceptible to developing experimental NEC [98]. Consistent with these observations, circulating concentrations of LPS are also shown to be elevated in the plasma of infants with NEC. In experimental rat models of NEC, LPS concentrations are increased not only in the plasma of the animals but also in their stool samples [99]. Therefore, LPS signaling through TLR-4 is an important molecular mechanism involved in the pathogenesis of NEC.
Role of Enterobacter sakazakii in NEC
Enterobacter sakazakii is a rare microorganism classified previously as yellow-pigmented Enterobacter cloacae, but reclassified subsequently as a separate species in 1980 [100]. Enterobacter sakazakii is resistant to osmotic pressure, heat, and dry stresses. These properties may explain, in part, its ability to survive in desiccated infant powder and formulas. Enterobacter sakazakii is highly virulent and this may explain how a very low inoculum, ranging from 0.36 to 66 colony-forming units/100 g, is sufficient to cause disease [101].
Enterobacter sakazakii has been implicated in several cases of infant sepsis and meningitis as well as several outbreaks of NEC. The first report of an outbreak in the literature was from the Netherlands; it described eight cases of sepsis and meningitis due to ES [102]. In addition, Van Acker et al. reported on 12 infants with NEC in a NICU setting in which ES was implicated as the pathogenic cause of the outbreak. Two of the 12 infants died as a result of this infection. These cases were directly associated with the use of contaminated infant formula. The authors recommended the use of sterilized liquid milk formula in NICU setting to prevent future similar outbreaks [103]. Another outbreak in the United States occurred in Memphis, TN, and involved four neonates. Three of the four patients had sepsis and bloody diarrhea associated with NEC. The ES cultured in all four cases had the same plasmid profile and was traced back to the same can of powdered milk [104].
These reports direct our attention to the fact that bacteria play a central role in the pathogenesis of NEC. As a result, we have set out to investigate further the mechanisms by which ES elicits epithelial injury in our animal models of NEC. It has been established that bacterial adhesion to the intestinal epithelium is the first step in the process of intestinal invasion by ES. Hunter et al. showed that ES binds to the intestinal epithelial lining in neonatal rats [105]. Most pathogens exert their effects on the host through the binding of their virulence factors to specific receptors, such as TLRs, on the surface of target cells. As a gram-negative bacterium, ES has LPS as well as another surface receptor, Omp A, which is a protein that has been shown to play a role in the invasion of brain endothelium in meningitis cases of ES. This surface protein is also found in E. coli species and interacts with different immunocytes such as dendritic cells and macrophages [106]. Hunter et al. have shown that ES binds to IEC-6 cells and induces apoptosis in this rat epithelial cell line. Essentially, our experiments have shown that although ES binds to IEC-6 cells, it does not invade these cells.
Our studies have demonstrated that ES can induce clinical and morphological changes characteristic of NEC in an experimental rat model of NEC [105]. We demonstrated that binding of ES results in the upregulation of inflammatory cytokines such as IL-6. This phenomenon was replicated both in vitro using rat intestinal epithelial cells and in vivo through analysis of the serum of rats exposed to ES. Other proinflammatory cytokines such as IFN-γ and TNF-α were assayed but did not reach the threshold required for detection. Thus, studying ES-induced NEC provides important insight into the role of pathogenic bacteria in the pathogenesis of NEC. Although we do not suggest that a single bacterium fits Koch's postulates for NEC, we found that abnormal patterns of bacterial colonization alter intestinal inflammatory signaling, epithelial restitution, and enterocyte proliferation, ultimately causing clinical NEC.
Role of Probiotics in the Treatment and Prevention of NEC
According to the World Health Organization, probiotics are “live microorganisms, which when administered in adequate amounts, confer a health benefit on the host” [107]. The use of probiotics has focused on normalization of the infant's gut flora via delivery of some of these commensal bacteria. Examples of such protective bacterial species include Bifidobacterium and Lactobacillus families. The most commonly used probiotics include multiple species of Bifidobacterium such as B. breve and B. lactis, Lactobacillus such as L. acidophilus and L. casei, and Streptococcus species such as S. salivaris [108]. Table 2 provides a list of some of the most commonly studied probiotics [109,110,111].
Table 2.
List of Some of the Commonly Studied Probiotic Species and Their Positive Effects on the Host
| Types of probiotics | Positive role |
|---|---|
| Lactobacillus acidophilus | It has been shown to prevent infectious diseases and favorably alter the intestinal microflora balance, thus inhibiting the growth of harmful bacteria, promoting good digestion, boosting immune function, and increasing resistance to infection. |
| Lactobacillus casei | It has been shown to increase the levels of circulating immunoglobulin A in infants infected with rotavirus, shortening the duration of diarrhea. |
| Lactobacillus rhamnosus(Lactobacillus GGa) | It has been shown to be effective in the management of acute pediatric diarrheal disease (Saavedra, 2001b) and reduces Candida colonization in neonates (Manzoni, 2007c). Lactobacillus GG has also been associated with reduced atopic dermatitis in infants when administered to pregnant women prenatally and during the first 6 months of the infant's life (Michail et al., 2006d) |
| Bifidobacterium lactis | It has been shown to reduce the incidence of antibiotic-associated diarrhea in infants. |
| Bifidobacterium bifidum | It has been shown to strengthen gastrointestinal immunity, especially in children. |
Bifidobacteria are the most common probiotic organisms, among which strains such as B. longum and B. bifidum are found normally in the intestine of the term infants [112,113]. They have been cited as the most common organism detected in the intestine of breast-fed infants [114]. Multiple species of bifidobacteria are known to have beneficial effects, such as stimulation of GALT and induction of resistance to colonization by pathogenic bacteria. Some species of bifidobacteria such as B. animalis and B. lactis are applied in probiotic dairy products, food supplements, and pharmaceutical preparations [115].
The specific mechanisms by which probiotics confer their protective effect to the intestinal epithelium have yet to be elucidated fully [116]. Several studies have suggested that probiotics can be used to protect the premature infant's intestine similar to the use of surfactant in cases of lung immaturity. A Cochrane review of nine separate eligible probiotic trials in NEC, including nearly 1,500 infants, concluded that enteral supplementation of probiotics reduced the risk of severe NEC and mortality in preterm infants. The authors went as far as suggesting a change in practice in premature infants <1,000 g at birth based on their review results [117].
Thus far, there have been four recent randomized studies testing the effect of prophylactic use of probiotics in NICU patients. One such study by Hoyos et al. in 1999 demonstrated a decrease in the incidence of NEC and NEC-associated mortality using L. acidophilus and Bifidobacterium infantis without any adverse side effects [118]. Most of these prophylactic cocktails include different species of lactobacilli, including L. acidophilus as well as L. casei. Lin et al. similarly showed a decrease in the incidence of NEC using the same probiotics. Their study focused on VLBW infants who survived the first week of life [119]. Finally, Bin-Nun et al. studied the effects of administration of Bifidobacterium infantis, Bifidobacterium bifidis, and Salmonella thermophilus to 145 VLBW infants. The incidence of NEC and NEC-related deaths were lower in their study group. However, no difference was seen in the incidence of sepsis or in total days of antibiotic utilization between the study group and control [120].
There are numerous animal studies underway to better elucidate the mechanism by which probiotics exert their protective effect in NEC. Previously it has been shown that newborn rats exposed to hypoxia and formula feeding appear to be protected from intestinal inflammation by oral administration of B. infantis. In our recent studies we have shown that pretreatment with Lactobacillus bulgaricus suppresses the upregulation of inflammatory cytokines and reduces ES-induced apoptosis in our rat model. Further, when provided in a prophylactic manner, L. bulgaricus protects rat pups fed formula contaminated with ES against the development of NEC. Thus, probiotics may represent an important therapeutic modality to reduce the incidence of NEC in vulnerable neonates [121].
Conclusion
The foregoing paragraphs suggest that intestinal damage in NEC may be the result of an imbalance between tissue injury and tissue repair mechanisms. Immaturity of the intestinal epithelial barrier and the mucosal immune system predisposes the premature infant to bacterial infections and/or dysregulated inflammatory responses. Mucosal injury resulting from host or environmental factors leads to bacterial–epithelial interactions and intestinal wall invasion with local release of inflammatory mediators which, in conjunction with LPS, stimulate iNOS upregulation and release of NO• and peroxynitrite by enterocytes and macrophages. Nitric oxide and its cytopathic adduct, peroxynitrite, promote further epithelial injury (apoptosis) with concurrent inhibition of tissue repair mechanisms (epithelial restitution and enterocyte proliferation). This phenomenon results in a vicious cycle of injury and uncontrolled inflammatory response with release of other more potent inflammatory factors. The net effect is further tissue destruction, intestinal perforation, and systemic sepsis, as seen in advanced NEC. In the future, more effective therapeutic modalities or prevention strategies should be directed at the underlying mechanisms responsible for the accelerated enterocyte apoptosis or necrosis and the decreased proliferation that characterize NEC.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Weidmeier SE. Henry V. Baer VL, et al. Center differences in NEC within one health-care system may depend on feeding protocol. Amer J Perinat. 2008;25:5–11. doi: 10.1055/s-2007-995220. [DOI] [PubMed] [Google Scholar]
- 2.Peter C. Feuerhahn B. Bohnhorst M, et al. Necrotising enterocolitis: Is there a relationship to specific pathogens. J Pediatr Surg. 1999;158:67–70. doi: 10.1007/s004310051012. [DOI] [PubMed] [Google Scholar]
- 3.www.cdc.gov/datastatistics/2007/births www.cdc.gov/datastatistics/2007/births
- 4.Boccia D. Stofli I. Lana S. Moro ML. Nosocomial necrotizing enterocolitis outbreaks: Epidemiology and control measures. Eur J Pediatr. 2001;160:385–391. doi: 10.1007/s004310100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blakely ML. Lally KP. McDonald S, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: A prospective cohort study by the NICHD Neonatal Research Network. Ann Surg. 2005;241:984–994. doi: 10.1097/01.sla.0000164181.67862.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford HR. Mechanism of nitric oxide-mediated intestinal barrier failure: Insight into the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 2006;41:294–299. doi: 10.1016/j.jpedsurg.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Grishin A. Ford H. Wang J, et al. Attenuation of apoptosis in enterocytes by blockade of potassium channels. Am J Physiol Gastrointest Liver Physiol. 2005;289:G815–G821. doi: 10.1152/ajpgi.00001.2005. [DOI] [PubMed] [Google Scholar]
- 8.Grishin A. Wang J. Hackam D, et al. p38 MAP kinase mediates endotoxin-induced expression of cyclooxygenase-2 in enterocytes. Surgery. 2004;136:329–335. doi: 10.1016/j.surg.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Grishin AV. Wang J. Potoka DA, et al. Lipopolysaccharide induces cyclooxygenase-2 in intestinal epithelium via a noncanonical p38 MAPK pathway. J Immunol. 2006;176:580–588. doi: 10.4049/jimmunol.176.1.580. [DOI] [PubMed] [Google Scholar]
- 10.Hackam DJ. Upperman JS. Grishin A, et al. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:49–57. doi: 10.1053/j.sempedsurg.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Kelly N. Friend K. Boyle P, et al. The role of the glutathione antioxidant system in gut barrier failure in a rodent model of experimental necrotizing enterocolitis. Surgery. 2004;136:557–566. doi: 10.1016/j.surg.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 12.Nadler EP. Boyle P. Murdock AD, et al. Newborn endothelin receptor type B mutant (piebald) mice have a higher resting anal sphincter pressure than newborn C57BL/6 mice. Contemp Top Lab Anim Sci. 2003;42:36–38. [PubMed] [Google Scholar]
- 13.Neal MD. Leaphart C. Levy R, et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176:3070–3079. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 14.Podd BS. Aberg C. Christopher TL, et al. Late postnatal expansion of self-reactive CD8alphaalpha+ intestinal intraepithelial lymphocytes in mice. Autoimmunity. 2004;37:537–547. doi: 10.1080/08916930400027094. [DOI] [PubMed] [Google Scholar]
- 15.Podd BS. Thoits J. Whitley N, et al. T cells in cryptopatch aggregates share TCR gamma variable region junctional sequences with gamma delta T cells in the small intestinal epithelium of mice. J Immunol. 2006;176:6532–6542. doi: 10.4049/jimmunol.176.11.6532. [DOI] [PubMed] [Google Scholar]
- 16.Potoka DA. Nadler EP. Upperman JS, et al. Role of nitric oxide and peroxynitrite in gut barrier failure. World J Surg. 2002;26:806–811. doi: 10.1007/s00268-002-4056-2. [DOI] [PubMed] [Google Scholar]
- 17.Potoka DA. Upperman JS. Nadler EP, et al. NF-kappaB inhibition enhances peroxynitrite-induced enterocyte apoptosis. J Surg Res. 2002;106:7–14. doi: 10.1006/jsre.2002.6423. [DOI] [PubMed] [Google Scholar]
- 18.Sala FG. Curtis JL. Veltmaat JM, et al. Fibroblast growth factor 10 is required for survival and proliferation but not differentiation of intestinal epithelial progenitor cells during murine colon development. Dev Biol. 2006;299:373–385. doi: 10.1016/j.ydbio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Stanford A. Upperman JS. Boyle P, et al. Long-term follow-up of patients with necrotizing enterocolitis. J Pediatr Surg. 2002;37:1048–1050. doi: 10.1053/jpsu.2002.33842. [DOI] [PubMed] [Google Scholar]
- 20.Horbar JD. Onstad L. Wright E. Predicting mortality risk for infants weighing 501 to 1500 grams at birth: A National Institutes of Health Neonatal Research Network report. Crit Care Med. 1993;21:12–8. doi: 10.1097/00003246-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Potoka DA. Upperman JS. Zhang XR, et al. Peroxynitrite inhibits enterocyte proliferation and modulates Src kinase activity in vitro. Am J Physiol Gastrointest Liver Physiol. 2003;285:G861–G869. doi: 10.1152/ajpgi.00412.2002. [DOI] [PubMed] [Google Scholar]
- 22.Upperman JS. Glutathione (GSH) levels in necrotizing enterocolitis (NEC) J Pediatr Surg. 2005;40:1813. doi: 10.1016/j.jpedsurg.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 23.Corfield AP. Myerscough NY. Longman R, et al. Mucin and mucosal protection in the gastrointestinal tract: New prospect for mucins in the pathology of gastrointestinal disease. Gut. 2000;47:589–594. doi: 10.1136/gut.47.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller CA. Autenrieth IB. Peschel A. Innate defenses of the intestinal epithelial barrier. Cell Mol Life Sci. 2005;62:1297–1307. doi: 10.1007/s00018-005-5034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fagarason S. Tasuku H. Intestinal IgA synthesis: Regulation of front-line body defenses. Nat Rev. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 26.Artis D. Epithelial cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 27.Otte JM. Kiehne K. Herzig KH. Antimicrobial peptides in innate immunity of the human intestine. J Gastroenterol. 2003;38:717–726. doi: 10.1007/s00535-003-1136-5. [DOI] [PubMed] [Google Scholar]
- 28.Bevins CH. Events at the host-microbial interface of the gastrointestinal tract V. Paneth cell alpha-defensins in intestinal host defense. Am J Physiol Gastroenterol Liver Physiol. 2005;289:G173–G176. doi: 10.1152/ajpgi.00079.2005. [DOI] [PubMed] [Google Scholar]
- 29.Neu J. Chen M. Beierle E. Intestinal innate immunity: How does it relate to the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:137–144. doi: 10.1053/j.sempedsurg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Rose MC. Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 31.Strous GJ. Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27:57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- 32.Einerhand AW. Renes IB. Makkink MK, et al. Role of mucins in inflammatory bowel disease: Important lessons from experimental models. Eur J Gastroenterol Hepatol. 2002;14:757–765. doi: 10.1097/00042737-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Allen A. Bell A. Mantle M. Pearson JP. The structure and physiology of gastrointestinal mucus. Adv Exp Med Biol. 1982;144:115–133. doi: 10.1007/978-1-4615-9254-9_15. [DOI] [PubMed] [Google Scholar]
- 34.Montagne L. Piel C. Lalles JP. Effect of diet on mucin kinetics and composition: Nutrition and health implications. Nutr Rev. 2004;62:105–114. doi: 10.1111/j.1753-4887.2004.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 35.Rhodes JM. Colonic mucus and mucosal glycoproteins: The key to colitis and cancer? Gut. 1989;30:1660–1666. doi: 10.1136/gut.30.12.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uhlig HH. Powrie F. The role of mucosal T lymphocytes in regulating intestinal inflammation. Springer Semin Immunopathol. 2005;27:167–180. doi: 10.1007/s00281-005-0206-6. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y. Chou K. Fuchs E, et al. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackie R. Sghir A. Gaskins R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann JC. Peters K. Henschke S, et al. Role of T lymphocytes in rat 2,4,6-trinitrobenzene sulphonic acid (TNBS) induced colitis: Increased mortality after gammadelta T cell depletion and no effect of alphabeta T cell depletion. Gut. 2001;48:489–495. doi: 10.1136/gut.48.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hooper LV. Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 41.Hooper LV. Bacterial contributions to mammalian gut development. Trends Microbiol. 2004;12:129–134. doi: 10.1016/j.tim.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Collins MD. Gibson GR. Probiotics, prebiotics, and synbiotics: Approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69:1052S–1057S. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- 43.Bernt MF. Brassart D. Neeser JR, et al. Lactobacillus acidophilus LA 1 binds to culture of human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483–489. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner JR. Molecular basis of epithelial barrier regulation: From basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nusrat A. Turner JR. Madara JL. Molecular physiology and pathophysiology of tight junctions IV. Regulation of tight junctions by extracellular stimuli: Nutrients, cytokines and immune cells. Am J Physiol Gastroenterol Liver Physiol. 2000;279:G851–G857. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 46.Yu QH. Yang Q. Diversity of tight junctions (TJs) between gastrointestinal epithelial cells and their function in maintaining the mucosal barrier. Cell Biol Int. 2009;33:78–82. doi: 10.1016/j.cellbi.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Kamm KE. Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 48.Guo Y. Ramachandran C. Satpathy M. Srinivas SP. Histamine-induced myosin light chain phosphorylation breaks down the barrier integrity of cultured corneal epithelial cells. Pharm Res. 2007;24:1824–1833. doi: 10.1007/s11095-007-9309-1. [DOI] [PubMed] [Google Scholar]
- 49.Cetin S. Leaphart C. Li J, et al. Nitric oxide inhibits enterocyte migration through activation of RhoA-GTPase in a SHP-2 dependent manner. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1347–G1358. doi: 10.1152/ajpgi.00375.2006. [DOI] [PubMed] [Google Scholar]
- 50.Boivin MA. Ye D. Kennedy JC, et al. Mechanism of glucocorticoid regulation of the intestinal tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2007;292:G590–G598. doi: 10.1152/ajpgi.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clayburgh DR. Rosen S. Witkowski ED, et al. A differentiation-dependent splice variant of myosin light chain kinase, MLCK1, regulates epithelial tight junction permeability. J Biol Chem. 2004;279:55506–55513. doi: 10.1074/jbc.M408822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han X. Fink MP. Delude RL. Proinflammatory cytokines cause NO*-dependent and -independent changes in expression and localization of tight junction proteins in intestinal epithelial cells. Shock. 2003;19:229–237. doi: 10.1097/00024382-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Nusrat A. von Eichel-Streiber C. Turner JR, et al. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001;69:1329–1336. doi: 10.1128/IAI.69.3.1329-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simonovic I. Rosenberg J. Koutsouris A. Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2000;2:305–315. doi: 10.1046/j.1462-5822.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- 55.Forcielli M. Walker A. The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr. 2005;93:S41–S48. doi: 10.1079/bjn20041356. [DOI] [PubMed] [Google Scholar]
- 56.Holman RC. Stoll BJ. Clarke MJ, et al. The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health. 1997;87:2026–2031. doi: 10.2105/ajph.87.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin JA. Hamilton BE. Sutton PD, et al. Births: Final data for 2004. Natl Vital Stat Rep. 2006;55:1–101. [PubMed] [Google Scholar]
- 58.Lin P. Nasr T. Stoll JB. Necrotizing enterocolitis: Recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008;37:70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 59.Horbar JD. Badger GI. Carpenter GH, et al. Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics. 2002;110:143–151. doi: 10.1542/peds.110.1.143. [DOI] [PubMed] [Google Scholar]
- 60.Sankaran K. Puckett B. Lee DS, et al. Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J Pediatr Gastroenterol Nutr. 2004;39:366–372. doi: 10.1097/00005176-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 61.Sase M. Miwa I. Sumie M, et al. Ontogeny of gastric emptying patterns in human fetus. Am J Matern Fetal Med. 2005;17:213–217. doi: 10.1080/14767050500073340. [DOI] [PubMed] [Google Scholar]
- 62.Sanderson IR. The physiochemical environment of the neonatal intestine. Am J Clin Nutr. 1999;69:1028S–1034S. doi: 10.1093/ajcn/69.5.1028s. [DOI] [PubMed] [Google Scholar]
- 63.Berseth CL. McCoy HH. Birth asphyxia alters neonatal intestine motility in term neonates. Pediatrics. 1992;90:669–673. [PubMed] [Google Scholar]
- 64.Goldman AS. Ogra PL. Anti-infectious and infectious agents in human milk. In: Mestecky J, editor; Lamm M, editor; Strober W, et al., editors. Mucosal Immunology (Vol. 1) San Diego CA: Academic Press; 1999. pp. 72–85. [Google Scholar]
- 65.Ozturk H. Dokucu AI. Ogun C, et al. Protective effects of recombinant human interleukin-10 on intestines of hypoxia induced necrotizing enterocolitis in immature rats. J Pediatr Surg. 2002;37:1330–1333. doi: 10.1053/jpsu.2002.35002. [DOI] [PubMed] [Google Scholar]
- 66.Fituch CC. Palkowetz KH. Goldman AS, et al. Concentration of IL-10 in preterm human milk and in milk from mothers of infants with necrotizing enterocolitis. Acta Paediatr. 2004;93:1496–1500. doi: 10.1080/08035250410022314. [DOI] [PubMed] [Google Scholar]
- 67.Favier CF. Vaughan EE. De Vos WM. Akkermans AD. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002;68:219–226. doi: 10.1128/AEM.68.1.219-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan KL. Saing H. Yung RW, et al. A study of pre-antibiotic bacteriology in 125 patients with necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:45–48. doi: 10.1111/j.1651-2227.1994.tb13242.x. [DOI] [PubMed] [Google Scholar]
- 69.McGuire W. Anthonyn MY. Donor milk versus formula for preventing necrotizing enterocolitis in preterm infants: Systemic review. Arch Dis Child Fetal Neonatal Ed. 2003;88:F11–F14. doi: 10.1136/fn.88.1.F11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henderson G. Craig S. Brockehurst P, et al. Enteral feeding regimens and necrotizing enterocolitis in preterm infants: A multicenter case control study. Arch Dis Child Fetal Neonatal Ed. 2009;94:F120–F123. doi: 10.1136/adc.2007.119560. [DOI] [PubMed] [Google Scholar]
- 71.Meinzen-Derr J. Poindexter B. Wrage L, et al. Role of human milk in extremely low birth weight infants' risk of necrotizing enterocolitis or death. J Perinatol. 2009;29:57–62. doi: 10.1038/jp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Udall JN. Pang K. Fritz L. Development of gastrointestinal mucosal barrier: I. effect of age on intestinal permeability to macromolecules. Ped Res. 1981;15:241–244. doi: 10.1203/00006450-198103000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Gordon JI. Hermiston ML. Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr Opin Cell Biol. 1994;6:795–803. doi: 10.1016/0955-0674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 74.Herbst JJ. Sunshine P. Postnatal development of the small intestine of the rat. Pediatr Res. 1969;3:27–33. doi: 10.1203/00006450-196901000-00004. [DOI] [PubMed] [Google Scholar]
- 75.Bandeira A. Itohara S. Bonneville M, et al. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-cell antigen receptor gamma delta. Proc Natl Acad Sci USA. 1991;88:43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Colgan SP. Resnick MB. Parkos CA, et al. IL-4 directly modulates function of a model human intestinal epithelium. J Immunol. 1994;153:2122–2129. [PubMed] [Google Scholar]
- 77.Penttila IA. van Spriel AB. Zhang MF, et al. Transforming growth factor-beta levels in maternal milk and expression in postnatal rat duodenum and ileum. Pediatr Res. 1998;44:524–531. doi: 10.1203/00006450-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 78.Lyscom N. Brueton MJ. The impact of post-weaning protein malnutrition on intraepithelial and Peyer's patch lymphocyte sub-types in rat small bowel. Immunology. 1983;50:511–514. [PMC free article] [PubMed] [Google Scholar]
- 79.Leaphart CL. Qureshi F. Cetin S, et al. Interferon-gamma inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology. 2007;132:2395–2411. doi: 10.1053/j.gastro.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 80.Nadler EP. Dickinson E. Kinsely A, et al. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res. 2000;92:71–77. doi: 10.1006/jsre.2000.5877. [DOI] [PubMed] [Google Scholar]
- 81.Hsueh W. Caplan M. Qu X, et al. Neonatal necrotising enterocolitis: Clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. 2002;6:6–23. doi: 10.1007/s10024-002-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan KL. Ho JC. Chan KW, et al. A study of gut immunity to enteral endotoxin in rats of different ages: A possible cause of necrotizing enterocolitis. J Pediatr Surg. 2002;37:1435–1440. doi: 10.1053/jpsu.2002.35407. [DOI] [PubMed] [Google Scholar]
- 83.Chan KL. Hui WC. Chan KW, et al. Revisiting ischemia and reperfusion injury as possible cause of necrotizing enterocolitis: Role of nitric oxide and super oxide dismutase. J Pediatr Surg. 2002;37:828–834. doi: 10.1053/jpsu.2002.32882. [DOI] [PubMed] [Google Scholar]
- 84.Chokshi N. Guner Y. Hunter CJ, et al. The role of nitric oxide in intestinal epithelial injury and restitution in neonatal necrotizing enterocolitis. Semin Perinatol. 2008;32:92–99. doi: 10.1053/j.semperi.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Potoka DA. Upperman JS. Nadler EP, et al. NF-kappaB inhibition enhances peroxynitrite-induced enterocyte apoptosis. J Surg Res. 2002;106:7–14. doi: 10.1006/jsre.2002.6423. [DOI] [PubMed] [Google Scholar]
- 86.Ford H. Watkins S. Reblock K, et al. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 1997;32:275–282. doi: 10.1016/s0022-3468(97)90194-9. [DOI] [PubMed] [Google Scholar]
- 87.Hacham D. Upperman JS. Grishin A. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Pediatr Crit Care Med. 2005;6:S108–S111. doi: 10.1053/j.sempedsurg.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 88.Potoka DA. Nadler EP. Upperman JS. Ford HR. Role of nitric oxide and peroxynitrite in gut barrier failure. World J Surg. 2002;26:806–811. doi: 10.1007/s00268-002-4056-2. [DOI] [PubMed] [Google Scholar]
- 89.Ford H. Reblock K. Teramana C, et al. The role of nitric oxide in the pathogenesis of necrotizing enterocolitis. Surg Forum. 1995;46:642–644. [Google Scholar]
- 90.Fast C. Rosegger H. Necrotizing enterocolitis prophylaxis: Oral antibiotics and lyophilized enterobacteria vs oral immunoglobulins [see comments] Acta Paediatr Suppl. 1994;396:86–90. doi: 10.1111/j.1651-2227.1994.tb13253.x. [DOI] [PubMed] [Google Scholar]
- 91.Peter CS. Feuerhahn M. Bohnhorst B, et al. Necrotising enterocolitis: Is there a relationship to specific pathogens? Eur J Pediatr. 1999;158:67–70. doi: 10.1007/s004310051012. [DOI] [PubMed] [Google Scholar]
- 92.Chan KL. Saing H. Yung RW, et al. A study of pre-antibiotic bacteriology in 125 patients with necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:45–48. doi: 10.1111/j.1651-2227.1994.tb13242.x. [DOI] [PubMed] [Google Scholar]
- 93.Martin CR. Walker WA. Probiotics: Role in pathophysiology and prevention in necrotizing enterocolitis. Semin Perinatol. 2008;32:127–137. doi: 10.1053/j.semperi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 94.Hunter CJ. Upperman JS. Ford HR. Camerini V. Understanding the susceptibility of premature infant to necrotizing enterocolitis (NEC) Pediatr Res. 2008;63:117–123. doi: 10.1203/PDR.0b013e31815ed64c. [DOI] [PubMed] [Google Scholar]
- 95.Medzhitov R. Preston P. Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 96.Poltorak A. Smirnova M. Liu C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10Sc CR mice: Mutations in the TLR4 genes. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 97.Leaphart CT. Cavallo J. Gribar SC, et al. A critical role for TLR 4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179:4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 98.Caplan MS. Miller R. Kaup S, et al. Bifidobacterium supplementation reduces the incidence of encrotising enterocolitis in neonatal rat model. Gastroenterology. 1999;117:577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 99.Jilling T. Simon D. Lu J, et al. The role of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177:3273–3282. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Farmer JJ. Ashbury F. Hickman DJ, et al. Enterobacter sakazakii: A new species of Enterobacteriaceae isolated from clinical material. Int J Syst Bacteriol. 1980;30:568–584. [Google Scholar]
- 101.Hunter CJ. Petrosyan M. Ford HR. Prasadarao NV. Enterobacter sakazakii: An emerging pathogen in infants and neonates. Surg Infect. 2008;9:533–539. doi: 10.1089/sur.2008.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muytjens HL. Zanen HC. Sonderkamp HJ, et al. Analysis of eight cases of neonatal meningitis and sepsis due to Enterobacter sakazakii. J Clin Microbiol. 1983;18:115–120. doi: 10.1128/jcm.18.1.115-120.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Acker J. de Smet F. Muyldermans G, et al. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J Clin Microbiol. 2001;39:293–297. doi: 10.1128/JCM.39.1.293-297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simmons BP. Gelfand MH. Metts L, et al. Enterobacter sakazakii infections in neonates associated with intrinsic contamination of powdered infant formula. Control Hosp Epidemiol. 1989;10:389–401. doi: 10.1086/646060. [DOI] [PubMed] [Google Scholar]
- 105.Hunter CJ. Singamsetty VK. Chokshi NK, et al. Enterobacter sakazakii enhances epithelial cell injury by inducing apoptosis in a rat model of necrotizing enterocolitis. J Infect Dis. 2008;198:586–593. doi: 10.1086/590186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Selvaraj SK. Prasadarao NV. E. coli K1 inhibits pro inflammatory cytokine induction in monocytes by preventing NF-kB activation. J Leukoc Biol. 2005;78:544–554. doi: 10.1189/jlb.0904516. [DOI] [PubMed] [Google Scholar]
- 107.AO/WHO. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. www.who.int/entity/foodsafety/fs_managementlen/probiotic_guidelines.pdf www.who.int/entity/foodsafety/fs_managementlen/probiotic_guidelines.pdf
- 108.Cabana MD. Shane AL. Cao C, et al. Probiotics in primary care pediatrics. Clin Pediatr (Phila) 2006;45:405–410. doi: 10.1177/0009922806289614. [DOI] [PubMed] [Google Scholar]
- 109.Saavedra JM. Clinical applications of probiotic agents. Am J Clin Nutr. 2001;73:11475–11515. doi: 10.1093/ajcn/73.6.1147S. [DOI] [PubMed] [Google Scholar]
- 110.Manzoni P. Use of lactobacillus casei subspecies rhamnosus gg and gastrointestinal colonization by candida species in preterm neonates. J Ped Gastroent Nutr. 2006;43:550–557. doi: 10.1097/01.mpg.0000302971.06115.15. [DOI] [PubMed] [Google Scholar]
- 111.NASPGHAN Nutrition Report Committee. Michail S. Sylvester F. Fuchs G. Issenman R. Clinical efficacy of probiotics: review of the evidence with focus on children. J Ped Gastroent Nutr. 2006;43:550–557. doi: 10.1097/01.mpg.0000239990.35517.bf. [DOI] [PubMed] [Google Scholar]
- 112.Bin-Nun A. Bromiker R. Wilschanski M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight infants. J Pediatr. 2005;147:192–196. doi: 10.1016/j.jpeds.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 113.Gewolb I. Schwalbe R. Taciak V, et al. Stool microflora in extremely low birth weight infants. Arch Dis Child Fetal Neonatal Ed. 1999;80:F167–F173. doi: 10.1136/fn.80.3.f167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Panders J. Thijis C. Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 115.Masco L. Ventura M. Zink R, et al. Polyphasic taxonomic analysis of B. animalis and B. lactis relatedness at the subspecies level. Int J Syst Evol Microbiol. 2004;54:1137–1143. doi: 10.1099/ijs.0.03011-0. [DOI] [PubMed] [Google Scholar]
- 116.Martin CR. Walker AW. Probiotics: Role in pathophysiology and prevention in necrotizing enterocolitis. Semin Perinatol. 2008;32:127–137. doi: 10.1053/j.semperi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 117.Alfalah KM. Bassler D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2008;(1):CD005496. doi: 10.1002/14651858.CD005496.pub2. [DOI] [PubMed] [Google Scholar]
- 118.Hoyos AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis. 1999;3:197–202. doi: 10.1016/s1201-9712(99)90024-3. [DOI] [PubMed] [Google Scholar]
- 119.Lin HC. Su BH. Chen AC, et al. Oral probiotics reduced the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005;115:1–4. doi: 10.1542/peds.2004-1463. [DOI] [PubMed] [Google Scholar]
- 120.Bin-Nun A. Bromiker R. Wischanski M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005;147:192–196. doi: 10.1016/j.jpeds.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 121.Hunter CJ. Williams M. Petrosyan M, et al. Lactobacillus bulgaris prevents intestinal epithelial cell injury caused by Enterobacter sakazakii-induced nitric oxide both in-vitro and in the newborn rat model of necrotizing enterocolitis. Infect Immun. 2009;77:1031–1043. doi: 10.1128/IAI.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]