Abstract
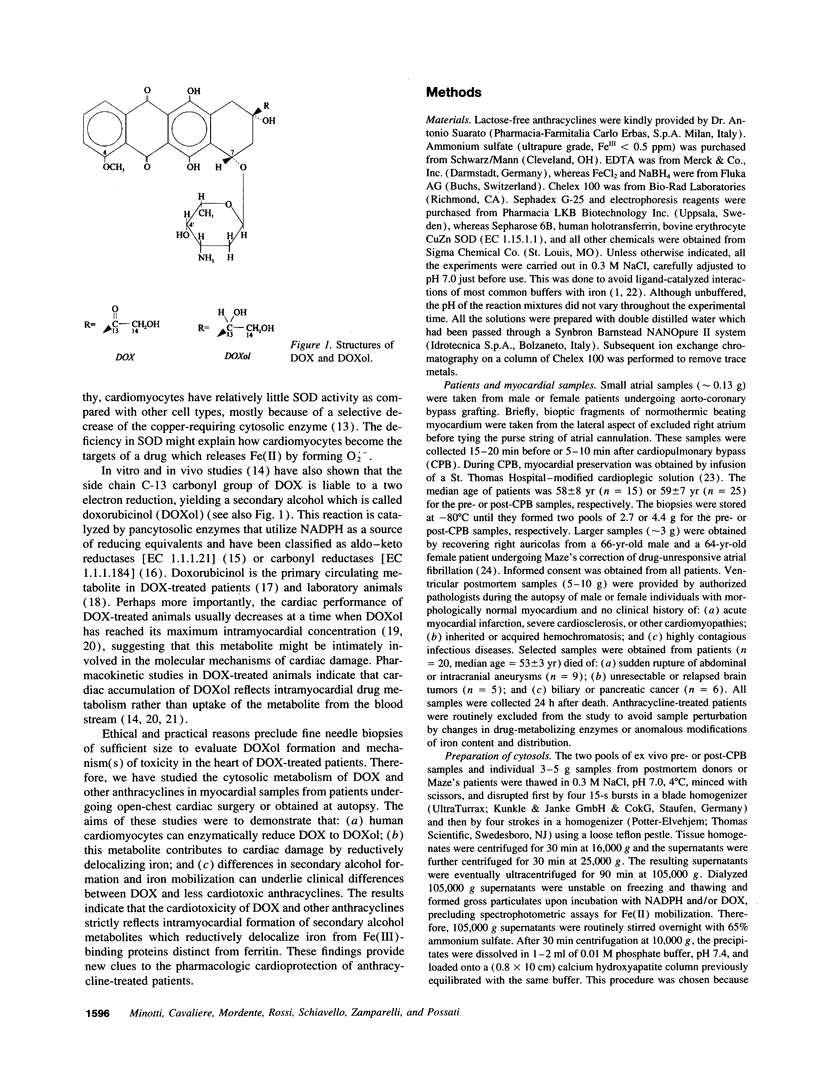
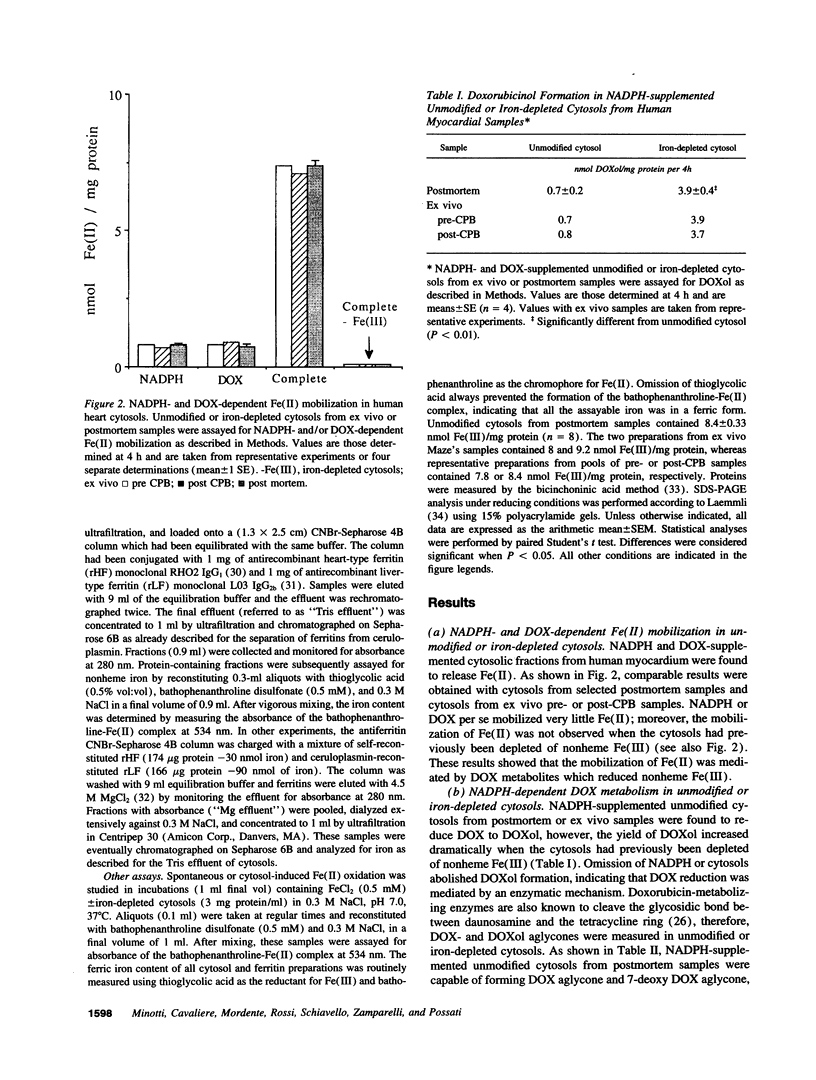
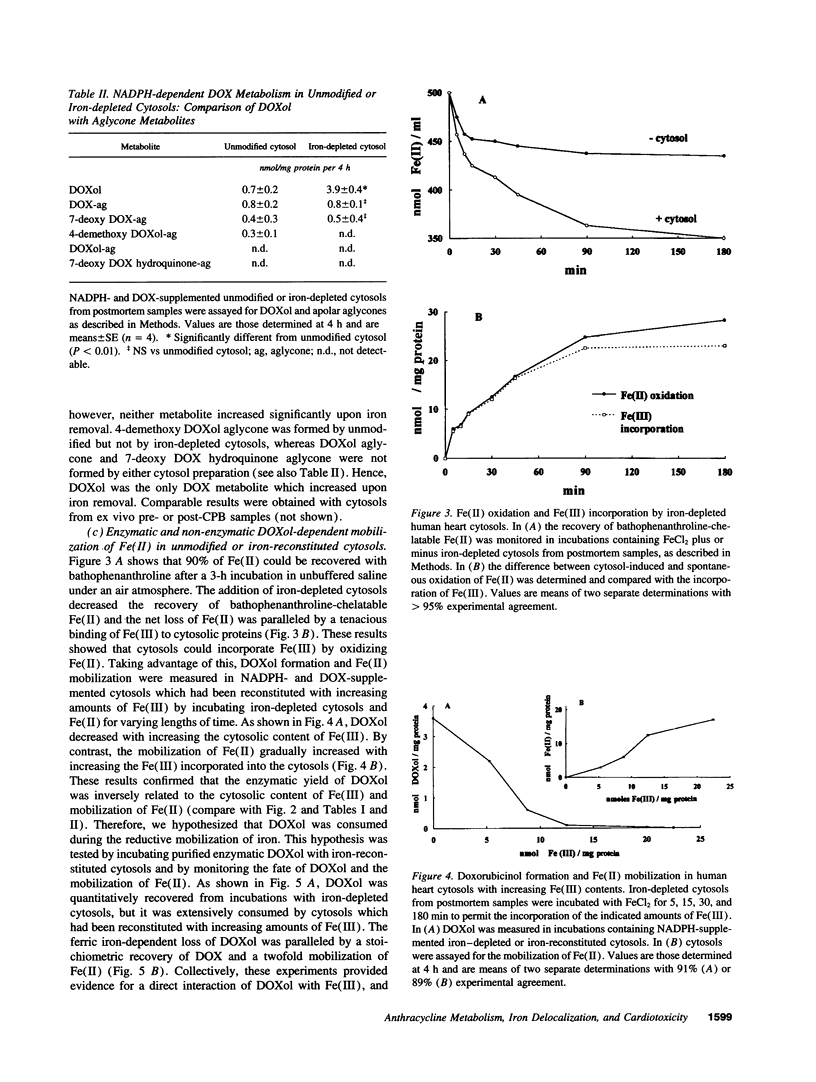
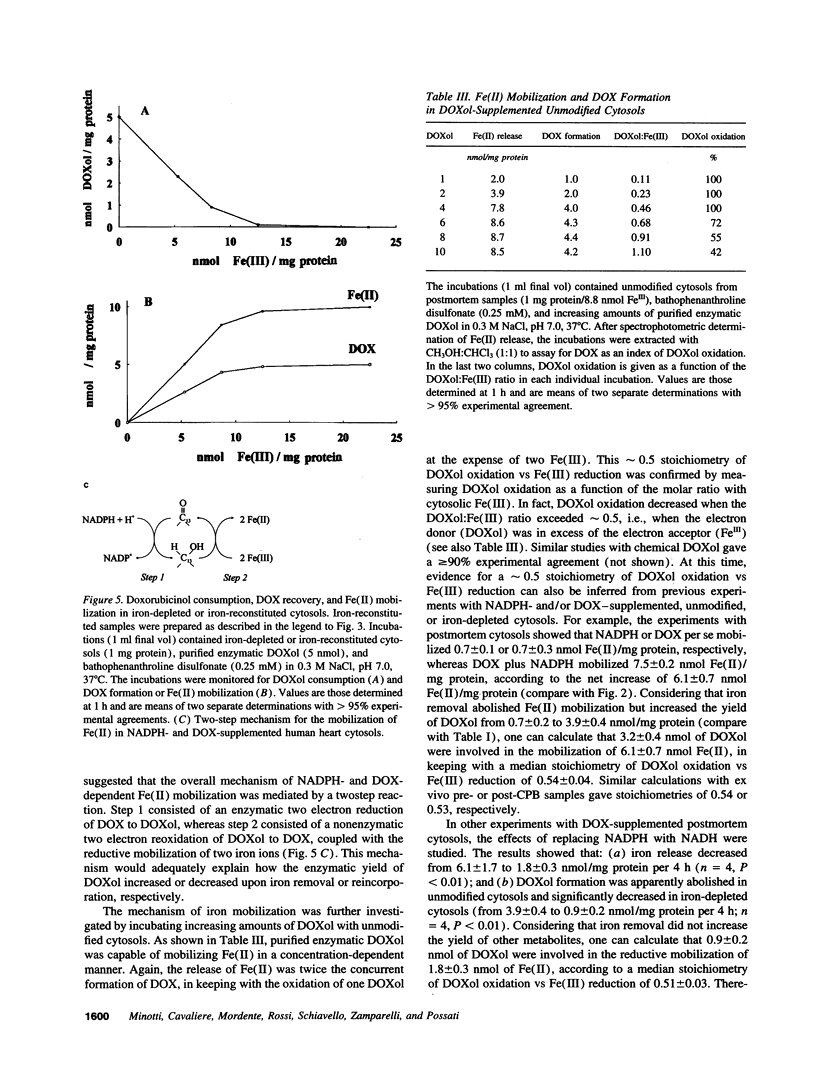
The cardiotoxicity of doxorubicin (DOX) and other quinone-containing antitumor anthracyclines has been tentatively attributed to the formation of drug semiquinones which generate superoxide anion and reduce ferritin-bound Fe(III), favoring the release of Fe(II) and its subsequent involvement in free radical reactions. In the present study NADPH- and DOX-supplemented cytosolic fractions from human myocardial biopsies are shown to support a two-step reaction favoring an alternative mechanism of Fe(II) mobilization. The first step is an enzymatic two-electron reduction of the C-13 carbonyl group in the side chain of DOX, yielding a secondary alcohol metabolite which is called doxorubicinol (3.9 +/- 0.4 nmoles/mg protein per 4 h, mean +/- SEM). The second step is a nonenzymatic and superoxide anion-independent redox coupling of a large fraction of doxorubicinol (3.2 +/- 0.4 nmol/mg protein per 4 h) with Fe(III)-binding proteins distinct from ferritin, regenerating stoichiometric amounts of DOX, and mobilizing a twofold excess of Fe(II) ions (6.1 +/- 0.7 nmol/mg protein per 4 h). The formation of secondary alcohol metabolites decreases significantly (Pi < 0.01) when DOX is replaced by less cardiotoxic anthracyclines such as daunorubicin, 4'-epi DOX, and 4-demethoxy daunorubicin (2.1 +/- 0.1, 1.2 +/- 0.2, and 0.6 +/- 0.2 nmol/mg protein per 4 h, respectively). Therefore, daunorubicin, 4'-epi DOX, and 4-demethoxy daunorubicin are significantly (P < 0.01) less effective than DOX in mobilizing Fe(II) (3.5 +/- 0.1, 1.8 +/- 0.2, and 0.9 +/- 0.3 nmol/mg protein per 4 h, respectively). These results highlight the formation of secondary alcohol metabolites and the availability of nonferritin sources of Fe(III) as novel and critical determinants of Fe(II) delocalization and cardiac damage by structurally distinct anthracyclines, thus providing alternative routes to the design of cardioprotectants for anthracycline-treated patients.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderton P. M., Gross J., Green M. D. Comparative study of doxorubicin, mitoxantrone, and epirubicin in combination with ICRF-187 (ADR-529) in a chronic cardiotoxicity animal model. Cancer Res. 1992 Jan 1;52(1):194–201. [PubMed] [Google Scholar]
- Arosio P., Cozzi A., Ingrassia R., Levi S., Luzzago A., Ruggeri G., Iacobello C., Santambrogio P., Albertini A. A mutational analysis of the epitopes of recombinant human H-ferritin. Biochim Biophys Acta. 1990 Jun 19;1039(2):197–203. doi: 10.1016/0167-4838(90)90186-j. [DOI] [PubMed] [Google Scholar]
- Borrello S., De Leo M. E., Wohlrab H., Galeotti T. Manganese deficiency and transcriptional regulation of mitochondrial superoxide dismutase in hepatomas. FEBS Lett. 1992 Oct 5;310(3):249–254. doi: 10.1016/0014-5793(92)81342-j. [DOI] [PubMed] [Google Scholar]
- Boucek R. J., Jr, Olson R. D., Brenner D. E., Ogunbunmi E. M., Inui M., Fleischer S. The major metabolite of doxorubicin is a potent inhibitor of membrane-associated ion pumps. A correlative study of cardiac muscle with isolated membrane fractions. J Biol Chem. 1987 Nov 25;262(33):15851–15856. [PubMed] [Google Scholar]
- Brieland J. K., Clarke S. J., Karmiol S., Phan S. H., Fantone J. C. Transferrin: a potential source of iron for oxygen free radical-mediated endothelial cell injury. Arch Biochem Biophys. 1992 Apr;294(1):265–270. doi: 10.1016/0003-9861(92)90167-u. [DOI] [PubMed] [Google Scholar]
- Cox J. L., Schuessler R. B., Cain M. E., Corr P. B., Stone C. M., D'Agostino H. J., Jr, Harada A., Chang B. C., Smith P. K., Boineau J. P. Surgery for atrial fibrillation. Semin Thorac Cardiovasc Surg. 1989 Jul;1(1):67–73. [PubMed] [Google Scholar]
- Crichton R. R., Ward R. J. Iron metabolism--new perspectives in view. Biochemistry. 1992 Nov 24;31(46):11255–11264. doi: 10.1021/bi00161a001. [DOI] [PubMed] [Google Scholar]
- Cusack B. J., Young S. P., Driskell J., Olson R. D. Doxorubicin and doxorubicinol pharmacokinetics and tissue concentrations following bolus injection and continuous infusion of doxorubicin in the rabbit. Cancer Chemother Pharmacol. 1993;32(1):53–58. doi: 10.1007/BF00685876. [DOI] [PubMed] [Google Scholar]
- Del Tacca M., Danesi R., Ducci M., Bernardini C., Romanini A. Might adriamycinol contribute to adriamycin-induced cardiotoxicity? Pharmacol Res Commun. 1985 Nov;17(11):1073–1084. doi: 10.1016/0031-6989(85)90113-4. [DOI] [PubMed] [Google Scholar]
- Doroshow J. H. Doxorubicin-induced cardiac toxicity. N Engl J Med. 1991 Mar 21;324(12):843–845. doi: 10.1056/NEJM199103213241210. [DOI] [PubMed] [Google Scholar]
- Dorr R. T., Shipp N. G., Lee K. M. Comparison of cytotoxicity in heart cells and tumor cells exposed to DNA intercalating agents in vitro. Anticancer Drugs. 1991 Feb;2(1):27–33. doi: 10.1097/00001813-199102000-00003. [DOI] [PubMed] [Google Scholar]
- Felsted R. L., Gee M., Bachur N. R. Rat liver daunorubicin reductase. An aldo-keto reductase. J Biol Chem. 1974 Jun 25;249(12):3672–3679. [PubMed] [Google Scholar]
- Fischer J. G., Tackett R. L., Howerth E. W., Johnson M. A. Copper and selenium deficiencies do not enhance the cardiotoxicity in rats due to chronic doxorubicin treatment. J Nutr. 1992 Nov;122(11):2128–2137. doi: 10.1093/jn/122.11.2128. [DOI] [PubMed] [Google Scholar]
- Forrest G. L., Akman S., Doroshow J., Rivera H., Kaplan W. D. Genomic sequence and expression of a cloned human carbonyl reductase gene with daunorubicin reductase activity. Mol Pharmacol. 1991 Oct;40(4):502–507. [PubMed] [Google Scholar]
- Furukawa T., Taketani S., Kohno H., Tokunaga R. A newly identified iron-binding protein in rat liver: purification and characterization. Biochem Biophys Res Commun. 1991 Nov 27;181(1):409–415. doi: 10.1016/s0006-291x(05)81434-2. [DOI] [PubMed] [Google Scholar]
- Goebel M. Oral idarubicin--an anthracycline derivative with unique properties. Ann Hematol. 1993 Jan;66(1):33–43. doi: 10.1007/BF01737687. [DOI] [PubMed] [Google Scholar]
- Homesley H. D., Harry D. S., O'Toole R. V., Hoogstraten B., Franklin E. W., Cavanagh D., Nahhas W. A., Smith J. J., Lovelace J. V. Randomized comparison of cisplatin plus epirubicin or doxorubicin for advanced epithelial ovarian carcinoma. A multicenter trial. Am J Clin Oncol. 1992 Apr;15(2):129–134. doi: 10.1097/00000421-199204000-00007. [DOI] [PubMed] [Google Scholar]
- Kennedy C. H., Mason R. P. A reexamination of the cytochrome P-450-catalyzed free radical production from a dihydropyridine. Evidence of trace transition metal catalysis. J Biol Chem. 1990 Jul 15;265(20):11425–11428. [PubMed] [Google Scholar]
- Kuffel M. J., Reid J. M., Ames M. M. Anthracyclines and their C-13 alcohol metabolites: growth inhibition and DNA damage following incubation with human tumor cells in culture. Cancer Chemother Pharmacol. 1992;30(1):51–57. doi: 10.1007/BF00686485. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levi S., Yewdall S. J., Harrison P. M., Santambrogio P., Cozzi A., Rovida E., Albertini A., Arosio P. Evidence of H- and L-chains have co-operative roles in the iron-uptake mechanism of human ferritin. Biochem J. 1992 Dec 1;288(Pt 2):591–596. doi: 10.1042/bj2880591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe G., Comelli M., Mazzilis D., Sala F. D., Mavelli I. The inactivation of mitochondrial F1 ATPase by H2O2 is mediated by iron ions not tightly bound in the protein. Biochem Biophys Res Commun. 1991 Dec 16;181(2):764–770. doi: 10.1016/0006-291x(91)91256-c. [DOI] [PubMed] [Google Scholar]
- Luzzago A., Arosio P., Iacobello C., Ruggeri G., Capucci L., Brocchi E., De Simone F., Gamba D., Gabri E., Levi S. Immunochemical characterization of human liver and heart ferritins with monoclonal antibodies. Biochim Biophys Acta. 1986 Jul 25;872(1-2):61–71. doi: 10.1016/0167-4838(86)90147-0. [DOI] [PubMed] [Google Scholar]
- Mazzanti L., Breschi M. C., Scalori V., Giovannini L., Mian M. Comparative histochemical study of adriamycin and adriamycinol in rat liver and heart. In Vivo. 1988 May-Aug;2(3-4):189–193. [PubMed] [Google Scholar]
- Miller D. M., Buettner G. R., Aust S. D. Transition metals as catalysts of "autoxidation" reactions. Free Radic Biol Med. 1990;8(1):95–108. doi: 10.1016/0891-5849(90)90148-c. [DOI] [PubMed] [Google Scholar]
- Mimnaugh E. G., Trush M. A., Ginsburg E., Gram T. E. Differential effects of anthracycline drugs on rat heart and liver microsomal reduced nicotinamide adenine dinucleotide phosphate-dependent lipid peroxidation. Cancer Res. 1982 Sep;42(9):3574–3582. [PubMed] [Google Scholar]
- Minotti G. Adriamycin-dependent release of iron from microsomal membranes. Arch Biochem Biophys. 1989 Jan;268(1):398–403. doi: 10.1016/0003-9861(89)90601-2. [DOI] [PubMed] [Google Scholar]
- Minotti G., Aust S. D. Redox cycling of iron and lipid peroxidation. Lipids. 1992 Mar;27(3):219–226. doi: 10.1007/BF02536182. [DOI] [PubMed] [Google Scholar]
- Minotti G., Ikeda-Saito M. Fe(II) oxidation and Fe(III) incorporation by the M(r) 66,000 microsomal iron protein that stimulates NADPH oxidation. J Biol Chem. 1992 Apr 15;267(11):7611–7614. [PubMed] [Google Scholar]
- Minotti G. Sources and role of iron in lipid peroxidation. Chem Res Toxicol. 1993 Mar-Apr;6(2):134–146. doi: 10.1021/tx00032a001. [DOI] [PubMed] [Google Scholar]
- Minotti G. The role of an endogenous nonheme iron in microsomal redox reactions. Arch Biochem Biophys. 1992 Sep;297(2):189–198. doi: 10.1016/0003-9861(92)90661-f. [DOI] [PubMed] [Google Scholar]
- Monteiro H. P., Vile G. F., Winterbourn C. C. Release of iron from ferritin by semiquinone, anthracycline, bipyridyl, and nitroaromatic radicals. Free Radic Biol Med. 1989;6(6):587–591. doi: 10.1016/0891-5849(89)90065-8. [DOI] [PubMed] [Google Scholar]
- Mulligan M., Althaus B., Linder M. C. Non-ferritin, non-heme iron pools in rat tissues. Int J Biochem. 1986;18(9):791–798. doi: 10.1016/0020-711x(86)90055-8. [DOI] [PubMed] [Google Scholar]
- Olson R. D., Mushlin P. S., Brenner D. E., Fleischer S., Cusack B. J., Chang B. K., Boucek R. J., Jr Doxorubicin cardiotoxicity may be caused by its metabolite, doxorubicinol. Proc Natl Acad Sci U S A. 1988 May;85(10):3585–3589. doi: 10.1073/pnas.85.10.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson R. D., Mushlin P. S. Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. FASEB J. 1990 Oct;4(13):3076–3086. [PubMed] [Google Scholar]
- Peters J. H., Gordon G. R., Kashiwase D., Acton E. M. Tissue distribution of doxorubicin and doxorubicinol in rats receiving multiple doses of doxorubicin. Cancer Chemother Pharmacol. 1981;7(1):65–69. doi: 10.1007/BF00258216. [DOI] [PubMed] [Google Scholar]
- Polosa P. L., Attardi G. Distinctive pattern and translational control of mitochondrial protein synthesis in rat brain synaptic endings. J Biol Chem. 1991 May 25;266(15):10011–10017. [PubMed] [Google Scholar]
- Powis G. Free radical formation by antitumor quinones. Free Radic Biol Med. 1989;6(1):63–101. doi: 10.1016/0891-5849(89)90162-7. [DOI] [PubMed] [Google Scholar]
- Reif D. W. Ferritin as a source of iron for oxidative damage. Free Radic Biol Med. 1992;12(5):417–427. doi: 10.1016/0891-5849(92)90091-t. [DOI] [PubMed] [Google Scholar]
- Robert J. Epirubicin. Clinical pharmacology and dose-effect relationship. Drugs. 1993;45 (Suppl 2):20–30. doi: 10.2165/00003495-199300452-00005. [DOI] [PubMed] [Google Scholar]
- Rothman R. J., Serroni A., Farber J. L. Cellular pool of transient ferric iron, chelatable by deferoxamine and distinct from ferritin, that is involved in oxidative cell injury. Mol Pharmacol. 1992 Oct;42(4):703–710. [PubMed] [Google Scholar]
- Ryan T. P., Aust S. D. The role of iron in oxygen-mediated toxicities. Crit Rev Toxicol. 1992;22(2):119–141. doi: 10.3109/10408449209146308. [DOI] [PubMed] [Google Scholar]
- Ryan T. P., Grover T. A., Aust S. D. Rat ceruloplasmin: resistance to proteolysis and kinetic comparison with human ceruloplasmin. Arch Biochem Biophys. 1992 Feb 14;293(1):1–8. doi: 10.1016/0003-9861(92)90357-3. [DOI] [PubMed] [Google Scholar]
- Ryan T. P., Samokyszyn V. M., Dellis S., Aust S. D. Effects of (+)-1,2-bis(3,5-dioxopiperazin-1-yl)propane (ADR-529) on iron-catalyzed lipid peroxidation. Chem Res Toxicol. 1990 Jul-Aug;3(4):384–390. doi: 10.1021/tx00016a018. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., Schwab M., Thoenen H. Specificity of retrograde transport of nerve growth factor (NGF) in sensory neurons: a biochemical and morphological study. Brain Res. 1975 May 16;89(1):1–14. doi: 10.1016/0006-8993(75)90129-8. [DOI] [PubMed] [Google Scholar]
- Storrie B., Madden E. A. Isolation of subcellular organelles. Methods Enzymol. 1990;182:203–225. doi: 10.1016/0076-6879(90)82018-w. [DOI] [PubMed] [Google Scholar]
- Takanashi S., Bachur N. R. Adriamycin metabolism in man. Evidence from urinary metabolites. Drug Metab Dispos. 1976 Jan-Feb;4(1):79–87. [PubMed] [Google Scholar]
- Thomas C. E., Aust S. D. Release of iron from ferritin by cardiotoxic anthracycline antibiotics. Arch Biochem Biophys. 1986 Aug 1;248(2):684–689. doi: 10.1016/0003-9861(86)90523-0. [DOI] [PubMed] [Google Scholar]
- Thomas C., Vile G. F., Winterbourn C. C. The hydrolysis product of ICRF-187 promotes iron-catalysed hydroxyl radical production via the Fenton reaction. Biochem Pharmacol. 1993 May 25;45(10):1967–1972. doi: 10.1016/0006-2952(93)90005-h. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Rozencweig M., Layard M., Slavik M., Muggia F. M. Daunomycin-induced cardiotoxicity in children and adults. A review of 110 cases. Am J Med. 1977 Feb;62(2):200–208. doi: 10.1016/0002-9343(77)90315-1. [DOI] [PubMed] [Google Scholar]
- Weenen H., van Maanen J. M., de Planque M. M., McVie J. G., Pinedo H. M. Metabolism of 4'-modified analogs of doxorubicin. unique glucuronidation pathway for 4'-epidoxorubicin. Eur J Cancer Clin Oncol. 1984 Jul;20(7):919–926. doi: 10.1016/0277-5379(84)90165-2. [DOI] [PubMed] [Google Scholar]
- Weiss R. B. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992 Dec;19(6):670–686. [PubMed] [Google Scholar]
- Yeung T. K., Jaenke R. S., Wilding D., Creighton A. M., Hopewell J. W. The protective activity of ICRF-187 against doxorubicin-induced cardiotoxicity in the rat. Cancer Chemother Pharmacol. 1992;30(1):58–64. doi: 10.1007/BF00686486. [DOI] [PubMed] [Google Scholar]
- Youngman R. J., Elstner E. F. On the interaction of adriamycin with DNA: investigation of spectral changes. Arch Biochem Biophys. 1984 Jun;231(2):424–429. doi: 10.1016/0003-9861(84)90406-5. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Gianni L., Muindi J., Myers C. E. Differences in O2 reduction by the iron complexes of adriamycin and daunomycin: the importance of the sidechain hydroxyl group. Biochim Biophys Acta. 1986;884(2):326–336. doi: 10.1016/0304-4165(86)90181-9. [DOI] [PubMed] [Google Scholar]
- de Jong J., Geijssen G. J., Munniksma C. N., Vermorken J. B., van der Vijgh W. J. Plasma pharmacokinetics and pharmacodynamics of a new prodrug N-l-leucyldoxorubicin and its metabolites in a phase I clinical trial. J Clin Oncol. 1992 Dec;10(12):1897–1906. doi: 10.1200/JCO.1992.10.12.1897. [DOI] [PubMed] [Google Scholar]
- de Silva D., Aust S. D. Stoichiometry of Fe(II) oxidation during ceruloplasmin-catalyzed loading of ferritin. Arch Biochem Biophys. 1992 Oct;298(1):259–264. doi: 10.1016/0003-9861(92)90121-c. [DOI] [PubMed] [Google Scholar]
- de Silva D., Guo J. H., Aust S. D. Relationship between iron and phosphate in mammalian ferritins. Arch Biochem Biophys. 1993 Jun;303(2):451–455. doi: 10.1006/abbi.1993.1308. [DOI] [PubMed] [Google Scholar]






