Abstract
Background
Acinetobacter baumannii is gaining importance as a cause of nosocomial infections, but its role in skin and soft tissue infection (SSTI) is not well defined. As a result of the outbreak of A. baumannii occurring in military personnel in Iraq and Afghanistan, reports of severe wound infections and SSTI caused by this pathogen are increasing in frequency.
Methods
We describe four cases of monomicrobial and polymicrobial A. baumannii–associated necrotizing SSTI accompanied by A. baumannii bacteremia and offer a review of similar experiences published in the literature.
Results
Our comparative analysis reveals four unique features associated with necrotizing SSTI associated with A. baumannii: i) Occurs in hosts with underlying comorbidities (e.g., trauma, cirrhosis); ii) is often accompanied by bacteremia; iii) multiple drug resistance and the presence of co-pathogens frequently complicated treatment (64% of cases); iv) the cases reported here and in our review required surgical debridement (84% of cases) and led to substantial mortality (∼30%).
Conclusions
As the prevalence of A. baumannii continues to increase in our health care system, SSTIs caused by this organism may become more common. Clinicians must be aware that the spectrum of disease caused by A. baumannii could include severe necrotizing SSTI and that vigilance for potential complications is necessary.
Introduction
Acinetobacter baumannii, often with a multi-drug-resistant (MDR) phenotype, is responsible for an increasing number of cases of blood stream infection, urinary tract infection, and healthcare- and ventilator-associated pneumonia. Multi-drug-resistant A. baumannii usually affects individuals with serious underlying illnesses [1]. Additionally, it is reported as a cause of outbreaks worldwide, especially in personnel involved in military operations in Iraq and Afghanistan [2, 3]. A. baumannii is also a cause of community-acquired respiratory tract infections in Northern Australia and Asia [4]. The increasing prevalence and capacity of A. baumannii to express resistance to multiple classes of antibiotics designate this organism as a pathogen of global importance.
Surveillance efforts indicate that A. baumannii is infrequently involved in skin and soft tissue infection (SSTI) [5], although numerous studies do not assign A. baumannii a clear role in this condition, based on the difficulty of discerning infection from skin and wound colonization [6–8]. In the healthcare setting, many different organisms, A. baumannii included, may colonize the skin; therefore, assessing the pathogen responsible for SSTI in the absence of bacteremia or tissue cultures is problematic [9].
Herein, we describe four cases of severe A. baumannii–associated SSTI with bacteremia between 2006 and 2008: one case related to a blast injury in Iraq and three additional cases in civilians that occurred at our institutions. In addition, we offer a comprehensive, in-depth analysis of previously published cases of severe SSTI caused by A. baumannii. Taken together, these reports help to characterize a novel and emerging clinical entity that is unappreciated in the spectrum of disease caused by A. baumannii and that may become more relevant in clinical practice as the prevalence of this organism continues to increase in our health care system.
Case 1
A 23-year-old soldier serving in Iraq presented for medical attention after suffering a blast injury. He sustained open right femoral shaft and neck fractures with laceration of the superficial femoral artery and injury of the soft tissues of the groin, penis, testicles, and right wrist. In addition, he incurred a right ulnar fracture and right pneumothorax. He was stabilized with the administration of crystalloids and blood products and placement of a chest tube. Surgical repair of vascular, abdominal, and orthopedic injuries was performed rapidly. He was transferred to a tertiary medical care center while on mechanical ventilation.
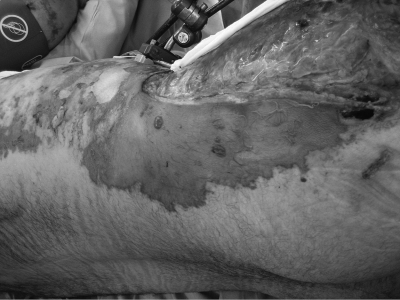
On the second day after the blast injury, he developed fever with septic shock. Laboratory evaluation revealed lactic acidemia, thrombocytopenia, and evidence of disseminated intravascular coagulation. On examination, there was extensive purpura and sloughing of the skin in the lower extremities (Fig. 1). He was empirically treated with vancomycin, imipenem/cilastatin, and amikacin. On the third day, he required additional vasopressor support with norepinephrine and underwent extensive debridement of soft tissue wounds. Central vein catheters were removed, and an abdominal re-exploration was unremarkable. On the fourth day, he improved and underwent further flank and lower extremity skin debridement.
FIG. 1.
Ecchymosis and sloughing of skin in flank and lower extremity. Extensive debridement performed.
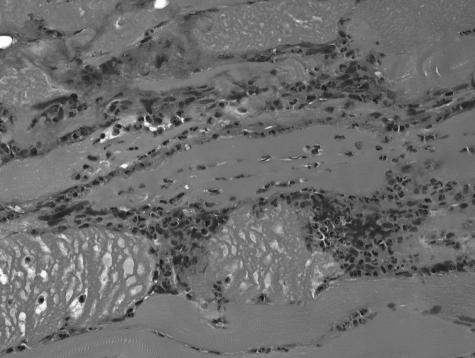
Histopathological analysis of surgical specimens from the lower extremities revealed changes consistent with necrotizing fasciitis (Fig. 2). Cultures from sputum, blood, central venous catheter tip, and wound and skin tissue grew A. baumannii. A wound culture also grew Klebsiella pneumoniae (Table 1). The patient survived after a prolonged hospitalization and rehabilitation.
FIG. 2.
Soft tissue biopsy revealed the presence of bacteria, thrombosis of capillaries, and necrosis of subcutaneous fascia and intrafascicular bundles.
Table 1.
Source, Identification, and Antibacterial Susceptibility of Bacterial Isolates
| Case | Organism | Source | Ampicillin-Sulbactam | Cefepime | Ceftazidime | Ciprofloxacin | Gentamicin | Tobramycin | Imipenem | Meropenem | Colistin | Ampicillin | Vancomycin | Linezolid |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1+ | Acinetobacter baumannii | Blood*, central line, skin biopsy | R | R | R | R | R | R | I | I | S | |||
| Klebsiella pneumoniae | wound | S | S | R | R | R | S | S | S | S | ||||
| 2+ | A. baumannii | Blood**, skin biopsy | R | R | R | R | R | R | R | R | S | |||
| Enterococcus faecium | skin biopsy | R | R | S | ||||||||||
| 3++ | A. baumannii | Blood***, wound | S | S | S | S | S | S | S | S | n/a | |||
| Citrobacter freundii | wound | S | S | S | S | S | S | S | S | n/a | ||||
| 4+ | A. baumannii | Blood****, ascitic fluid, skin biopsy | R | R | R | R | R | R | S | S | S |
Breakpoints interpreted according to Clinical and Laboratory Standards Institute criteria.
Identification and susceptibility of isolates performed using MicroScan Walkaway (Siemens Healthcare Diagnostics, IL).
Identification and susceptibility of isolates performed usingVitek 2 (bioMerieux, NC).
Two sets of blood cultures obtained peripherally and from central line.
Two sets of blood cultures obtained peripherally on different days.
One set of blood cultures obtained from an arterial line.
Two sets of blood cultures obtained peripherally.
S = susceptible; R = resistant; n/a = not available.
Case 2
A 75-year-old nursing home resident with Parkinson dementia, chronic kidney disease, traumatic brain injury, and recent healthcare-associated pneumonia with A. baumannii and Pseudomonas aeruginosa was admitted to the hospital for severe sepsis and SSTI associated with a percutaneous endoscopic gastrostomy (PEG) tube. He presented with extensive erythema and swelling surrounding the PEG site that rapidly progressed. Computed tomography (CT) of the abdomen revealed subcutaneous emphysema. He was initially treated with vancomycin, imipenem-cilastatin, tobramycin, and micafungin. Infusions with crystalloids and norepinephrine were necessary. On the day of admission, he underwent debridement of the abdominal wall, including skin, fat, fascia, and muscle. Histopathology was consistent with necrotizing fasciitis. Intraoperative cultures from skin and soft tissue grew A. baumannii and vancomycin-resistant Enterococcus faecium, and blood cultures grew A. baumannii (Table 1). Antibiotics were changed to colistin and linezolid. He required two additional debridements and placement of a vacuum-assisted closure device to facilitate healing before his discharge to a long-term acute care facility.
Case 3
A 50-year-old morbidly obese male (body mass index 60 kg/m2) presented with progressive right lower extremity edema. He had a chronic ulcer in that leg resulting from minor trauma sustained six months before this admission. He worked as a licensed practical nurse in a nursing home and was a smoker and moderate alcohol drinker.
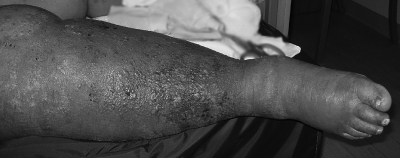
On presentation, he was afebrile and tachycardic, and his blood pressure was 80/50 mmHg. Evaluation of the lower extremities showed stasis changes on both legs and an ulcer above the right lateral malleolus, with erythema extending above the knee and marked edema with “peau d'orange” appearance. The presence of vesicles and early bullae formation were noted (Figure 3). CT of the leg showed diffuse soft tissue edema without focal fluid collections or air. Blood and wound cultures grew A. baumannii; the wound culture also grew Citrobacter freundii (Table 1). He improved on intravenous ampicillin-sulbactam 3 g every 4 h, and although surgical intervention was considered, it was not required.
FIG. 3.
Cellulitis caused by Acinetobacter baumannii. There is characteristic edematous “peau d'orange” erythema with associated vesicles that may coalesce to form non-hemorrhagic bullae.
Case 4
A 50-year-old male presented with scrotal edema and pain. Ultrasound showed bilateral hydroceles and soft tissue edema. Initially, he was prescribed oral ciprofloxacin. He returned five days later with worsening discomfort and edema, as well as abdominal pain and distension and lower extremity swelling. A history of hepatitis C virus infection and cirrhosis was documented.
On examination, the patient was lethargic and somnolent and had a temperature of 39°C, pulse of 128 beats/min, respiration rate of 30 breaths/min, and blood pressure of 80/40 mm Hg. The abdomen was distended and diffusely tender; the scrotum was edematous, and its inferior aspect revealed dark discoloration and crepitus. Laboratory evaluation revealed neutropenia (absolute neutrophil count of 210 cells/mm3), thrombocytopenia, prolonged prothrombin and partial thromboplastin times, and acute renal failure. The patient was hypoxic and acidemic and required crystalloids, vasopressor support, and mechanical ventilation. Treatment with ertapenem and vancomycin was initiated. He underwent emergency debridement of extensive necrotic tissue at the base of the scrotum. The patient remained in septic shock with multiple organ dysfunction syndrome and ongoing hypoxia and acidemia, despite maximal pressor and crystalloid support. There was persistent bleeding at the surgical site due to coagulopathy. He died the following day. Cultures of blood, ascitic fluid, and scrotal biopsies grew A. baumannii susceptible only to imipenem-cilastatin and meropenem (Table 1).
Discussion
A. baumannii is a ubiquitous pathogen commonly found in water, soil, and the healthcare environment [6]. The ability of A. baumannii to acquire genetic determinants of resistance is responsible for the emergence of MDR strains. Beta-lactamases, alterations in porin channels, efflux pumps (responsible for resistance to β-lactam antibiotics), mutations in deoxyribonucleic acid topoisomerase (mediating quinolone resistance), and genes encoding aminoglycoside-modifying enzymes are among the mechanisms of resistance. Metallo-β-lactamases and oxacillinases (e.g., blaOXA24/40, blaOXA23, and blaOXA58) contribute to carbapenem resistance. Elements (such as the insertion sequence ISABA1) located upstream in the bacterial chromosome often regulate the expression of these carbapenemase and cephalosporinase genes [7]. In addition to the plethora of resistance determinants, new niches for infection and colonization are being reported (e.g., community-acquired pneumonia in Australia, blood stream infections in the United States). The determinants of virulence and their expression in strains from diverse origins (nosocomial versus community) are unknown [8].
These four cases illustrate contemporary clinical presentations of severe SSTI in which A. baumannii is the primary pathogen. In one of the cases (Case 4), A. baumannii appeared as the sole pathogen associated with necrotizing SSTI, whereas other organisms (E. faecium and C. freundii) were isolated as co-pathogens in two other instances (Cases 2 and 3). In the first case, K. pneumoniae was isolated from a superficial wound culture but not from cultures of skin and deep soft tissue obtained during surgical exploration. In all four cases, patients were bacteremic with A. baumannii, and the presence of other aerobic and anaerobic bacteria was excluded using conventional modern methods of clinical microbiology. As such, these cases suggest that A. baumannii is emerging as a cause of SSTI. Sadly, Case 4 illustrates the inherent difficulties physicians face when choosing empiric therapy because Acinetobacter spp. are uniformly resistant to ertapenem.
Complicated and uncomplicated SSTIs are common in healthy and compromised hosts and in various clinical settings (determined by travel history, animal exposure, trauma or surgery) [10,11]. Beta-hemolytic streptococci (Group A predominantly, but also B, C, and G) are often the cause of severe cellulitis and necrotizing SSTI, representing what is termed Type 1 (or monomicrobial) necrotizing SSTI. Streptococcal toxic shock syndrome often accompanies this clinical entity. Community-associated methicillin-resistant Staphylococcus aureus is recognized, potentially with greater virulence than its nosocomial counterpart, as another cause of necrotizing SSTI [12]. A mixture of anaerobic bacteria (Bacteroides spp, Clostridium spp, microaerophilic streptococci) and enterobacteria (e.g., Escherichia coli, Klebsiella spp.), typically resulting from a gastrointestinal source, commonly cause Type 2 (or polymicrobial) necrotizing SSTI. This latter syndrome localizes to the perineum, giving rise to Fournier's gangrene [13]. Although the above-described types encompass the majority of presentations of necrotizing SSTI, different organisms may also be recovered. For example, gram-negative bacteria such as Vibrio vulnificus and Aeromonas hydrophila cause monobacterial necrotizing SSTI accompanied by sepsis and a high mortality rate in patients with underlying illnesses such as cirrhosis and diabetes [14]. Similarly, K. pneumoniae (with the virulent hypermucous K1 phenotype) is associated with a similar pattern of disease in patients with cirrhosis, diabetes mellitus, or cancer [15,16].
Acinetobacter baumannii is recovered frequently in association with SSTI and orthopedic surgical site infections in personnel returning from operations in Iraq and Afghanistan [17]. Despite their MDR phenotype, the clinical outcomes of war-related SSTI caused by A. baumannii are usually satisfactory [18,19], although instances of severe A. baumannii–associated SSTI with adverse outcomes, often in conjunction with other organisms as in our report are also described as part of the U.S. military outbreak. Physicians from the U.S. Navy hospital ship USNS Comfort described eight cases of A. baumannii–associated SSTI in wounded personnel. Those cases presented as cellulitis with a “peau d'orange” appearance with overlying vesicles, a presentation reminiscent of Case 3 [20]. There also are reports implicating A. baumannii as the etiologic agent of severe SSTI outside of the military outbreak [21–30]. These include two cases of fatal monomicrobial necrotizing fasciitis in immunosuppressed patients who had undergone abdominal surgery [31], as well as cases of polymicrobial necrotizing SSTI.
Table 2 summarizes 25 cases in the literature describing previous experiences with severe SSTI due to A. baumannii, including the four cases presented in this report. The patients' ages ranged from 13 to 75, and most were male. The preponderance of infections occurring in wounded extremities reflects the nature of military injuries sustained in combat. Perineal involvement is also frequently noted. Patients often had co-morbidities with underlying immune compromise (e.g., cirrhosis, severe trauma, diabetes, cancer) and were previously exposed to the healthcare system. The isolation of a co-pathogen was noted in 64% of cases, whereas blood stream infection with A. baumannii was documented in 44% (11/25) of patients. Surgical intervention was necessary in 84% of the cases, and crude mortality was 28%.
Table 2.
Review of Characteristics of 25 Patients with Acinetobacter Baumannii–Associated Skin and Soft Tissue Infections
| Case | Ref. | Age | Sex | Underlying Conditions | Location | Co-pathogen(s) | Acinetobacter Bacteremia | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | This article | 23 | M | Blast injury with open fractures | Right flank and lower extremity | Klebsiella pneumoniae | Yes | Debridement, line removal, amikacin, imipenem-cilastatin | Survived |
| 2 | This article | 75 | M | Nursing home resident, dementia, percutaneous endoscopic gastrostomy tube | Abdomen and left flank | Enterococcus faecium | Yes | Debridement, colistin, linezolid | Survived |
| 3 | This article | 50 | M | Nursing home worker, obesity | Lower extremity | Citrobacter freundii | Yes | Ampicillin-sulbactam | Survived |
| 4 | This article | 50 | M | Cirrhosis | Scrotum | None | Yes | Debridement, ertapenem, vancomycin | Died |
| 5 | [28] | 21 | M | End-stage renal disease, systemic lupus erythematosus, thrombotic thrombocytopenic purpura, vasculitis, steroid and rituximab use | Abdomen, both flanks, thigh and up to scapula | None | Yes | Debridement, amikacin, vancomycin, clindamycin, imipenem, metronidazole | Died |
| 6 | [28] | 47 | F | Human immunodeficiency virus, end-stage renal disease, laparotomy for ovarian torsion | Right lower extremity | None | Yes | Debridement, vancomycin, piperacillin-tazobactam, clindamycin, colistin | Died |
| 7 | [17] | 16 | M | Pelvic gunshot wound | Neck, chest, abdomen and perineum | Enterobacter cloacae Proteus mirabilis | Yes | Debridement, cefazolin, ceftazidime, clindamycin, vancomycin, imipenem | Died |
| 8 | [17] | 22 | M | Abdominal gunshot wound | Abdomen, right flank | None | Yes | Debridement, cefoxitin, nafcillin, clindamycin, ciprofloxacin, imipenem-cilastatin, vancomycin, fluconazole, doxycycline | Survived |
| 9 | [17] | 30 | M | Shrapnel injury to extremities | Left foot | Proteus vulgaris, E. cloacae | No | Debridement, cefazolin, tobramycin, imipenem-cilastatin | Survived |
| 10 | [17] | 23 | M | Right femur fracture | Right ankle | E. cloacae | No | Debridement, imipenem-cilastatin | Survived |
| 11 | [17] | 13 | M | Bilateral leg gunshot wounds | Left leg | Pseudomonas aeruginosa | No | Debridement, cefazolin, imipenem-cilastatin | Survived |
| 12 | [17] | 35 | M | Shrapnel injury on neck and head | Left face | None | No | Debridement, cefazolin, cephalexin | Survived |
| 13 | [17] | 28 | M | Shoulder gunshot wound | Left shoulder | Group B Streptococcus | No | Debridement, cefazolin, gentamicin, imipenem-cilastatin, tobramycin | Survived |
| 14 | [17] | 55 | M | Buttock gunshot wound | Hip, abdomen | None | Yes | Debridement, ampicillin, clindamycin, levofloxacin, ticarcillin, gentamicin, imipenem-cilastatin | Survived |
| 15 | [27] | 69 | M | Hypertension, peripheral vascular disease, esophageal perforation, septic shock | Right lower extremity, buttock and flank | None | No | Unspecified antibiotics | Died |
| 16 | [23] | 45 | F | Obesity, genital pustule | Vulva, perineum | Enterococcus faecalis, Candida albicans | No data | Unspecified antibiotics | Died |
| 17 | [23] | 47 | F | Diabetes, rectal fistula | Vulva | C. albicans | No data | Debridement, unspecified antibiotics | Survived |
| 18 | [23] | 44 | M | None | Penis | E. faecalis | No data | Debridement, unspecified antibiotics | Survived |
| 19 | [23] | 51 | M | None | Penis, scrotum | E. faecalis C. albicans | No data | Debridement, unspecified antibiotics | Survived |
| 20 | [19] | 74 | M | Chronic obstructive pulmonary disease, steroids | Left forearm, hand; Right arm, thorax, thighs | None | Yes | Amputation | Died |
| 21 | [18] | 69 | M | Open reduction, internal fixation tibia, chronic edema | Right leg | E. faecalis, C. albicans | No | Fasciotomy, piperacillin, cefoxitin | Survived |
| 22 | [21] | 60 | M | Remote perianal abscess | Scrotum and perineum | E. coli, Enterococcus spp., B. fragilis, S. epidermidis | No data | Debridement, unspecified antibiotics | Survived |
| 23 | [21] | 49 | M | Obesity, alcoholism | Inguinoperineal | Methicillin-resistant Staphylococcus aureus, P. aeruginosa | No data | Debridement, unspecified antibiotics | Survived |
| 24 | [21] | 53 | M | Diabetes mellitus, diabetic ketoacidosis | Perineum | E. coli, Streptococcus spp. | No data | Debridement, unspecified antibiotics | Survived |
| 25 | [22] | 79 | M | Preexistent scar | Left leg | None | Yes | Meropenem | Survived |
E. = Escherichia.
These descriptions and the cases in this report help to delineate some general characteristics of A. baumannii–associated SSTI, including, in addition to the “peau d'orange” appearance and hemorrhagic bullae, the progression to soft tissue necrosis, the occurrence of bacteremia, and severe sepsis. Overall, these clinical observations suggest that the spectrum of disease caused by A. baumannii includes severe and necrotizing SSTI and that A. baumannii is an important consideration in the contemporary differential diagnosis of severe and necrotizing SSTI.
Several explanations underlying the new importance of A. baumannii as a cause of severe SSTI are likely. The prevalence of infections caused by A. baumannii in hospitals in the United States has increased in the past decade [32]. This suggests that, in a hospital environment where there is endemic colonization and ongoing horizontal transmission of A. baumannii, the clinical presentations of infection with this agent that was previously rare may become more common. Surprisingly, A. baumannii was not reported as an etiologic agent of necrotizing SSTI in clinical series from the United States published as recently as 2002 [33,34]. In contrast, it is frequently reported in case series from Turkey, Argentina, and Singapore, where A. baumannii infection is endemic [26–28,35–37]. As cited above, A. baumannii became an important cause of SSTI in personnel from Iraq in the setting of an outbreak across the U.S. military healthcare system. Some of the patients suffered severe combat wounds as described in this report. As a result, their skin and soft tissue, as well as their immune system [38], were especially vulnerable to invasive infection—in this case by A. baumannii.
Similar conditions exist in critically ill hosts in the modern nosocomial environment, even in the absence of combat wounds. One of the patients described in this report (Case 4) had cirrhosis caused by chronic hepatitis C virus infection. Patients with cirrhosis appear especially susceptible to monomicrobial gram-negative necrotizing SSTI [39–41]. In addition, one of the patients in this report resided (Case 2) and another worked (Case 3) in a nursing home. Although we do not demonstrate that A. baumannii in this case was related to strains circulating in the nursing home, the occupational transmission of A. baumannii to a healthcare worker has previously been documented [42].
A distinguishing and disturbing feature of A. baumannii is its ability to acquire determinants of resistance against multiple classes of antibiotics. Emerging resistance to carbapenems is particularly worrisome [43]. Two of the isolates reported here were resistant to carbapenems, which complicated the choice of empiric and definitive antibiotic therapy. It is unclear what effect infection with carbapenem-resistant and MDR A. baumannii and “inappropriate” antibiotic therapy (use of agents not active against the organism) will have on clinical outcomes [44–48]. The cases in this report and those in the literature represent severe infections with inherently poor outcomes, making it impossible to establish the effect of resistance and of “inappropriate” therapy. Furthermore, it should be emphasized that, for necrotizing SSTI, antibiotic therapy functions as an adjunct to surgical debridement and that the timing of surgery may be as critical as the choice of antimicrobials [49].
Multi-drug-resistant A. baumannii possesses a genomic structure that allows it to acquire resistance markers under antimicrobial pressure [50]. It might be that this same genetic plasticity would allow A. baumannii to acquire factors that result in greater virulence, and this is being actively investigated. Recently, Adams et al. described a series of genes that are presumed to play an important role in the adaptation of A. baumannii to the human host [51]. These are genes that predominantly encode for transport and transcription factors, as well as genes involved in quorum sensing and biofilm formation. Comparative historical and genomic analyses are needed to establish the significance of these genetic elements.
The role of the co-pathogens isolated in the wounds of patients with severe and necrotizing SSTI is also intriguing (Tables 1 and 2). There may have been a synergistic interaction between these co-pathogens and A. baumannii. Synergy has typically been observed in polymicrobial infections with enteric bacilli (E. coli, Klebsiella spp.) and anaerobes (Bacillus spp. and Clostridium spp.), and there is experimental evidence suggesting that exopolysaccharide-producing strains of Acinetobacter spp. enhance the virulence of other gram-negative species in polymicrobial infections [52,53]. One could also speculate that chemical signals elaborated by the co-pathogen may activate biofilm formation by enhancing auto-inducer synthase production in A. baumannii, or vice versa [54].
In conclusion, the four cases of A. baumannii–associated monomicrobial and polymicrobial necrotizing SSTI presented here add to the growing recognition of this syndrome and help to redefine the capacity of A. baumannii to cause disease. The emergence of these infections in an age of increased A. baumannii endemicity and resistance and its occurrence in patients with severe coexisting illnesses underscore the importance of A. baumannii as a successful pathogen adapted to the modern nosocomial environment [55].
Acknowledgments
The National Institutes of Health (RO1 AI072219) and Veterans Affairs Merit Review Program and Geriatric Research, Education and Clinical Care VISN 10 support RAB. FP is supported by the Wyeth Fellowship in Antimicrobial Resistance.
Author Disclosure Statement
The opinions expressed in this report are those of the authors and do not represent the position of the U.S. Air Force. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
References
- 1.Dijkshoorn L. Nemec A. Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 2.Villegas MV. Hartstein AI. Acinetobacter outbreaks, 1977–2000. Infect Control Hosp Epidemiol. 2003;24:284–295. doi: 10.1086/502205. [DOI] [PubMed] [Google Scholar]
- 3.Scott P. Deye G. Srinivasan A, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44:1577–1584. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 4.Leung WS. Chu CM. Tsang KY, et al. Fulminant community-acquired Acinetobacter baumannii pneumonia as a distinct clinical syndrome. Chest. 2006;129:102–109. doi: 10.1378/chest.129.1.102. [DOI] [PubMed] [Google Scholar]
- 5.Moet GJ. Jones RN. Biedenbach DJ, et al. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004) Diagn Microbiol Infect Dis. 2007;57:7–13. doi: 10.1016/j.diagmicrobio.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Maragakis LL. Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46:1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 7.Munoz-Price LS. Weinstein RA. Acinetobacter infection. N Engl J Med. 2008;358:1271–1281. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 8.Peleg AY. Seifert H. Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horan TC. Andrus M. Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Stevens DL. Bisno AL. Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–1406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 11.Cuschieri J. Necrotizing soft tissue infection. Surg Infect (Larchmt) 2008;9:559–562. doi: 10.1089/sur.2008.9952. [DOI] [PubMed] [Google Scholar]
- 12.Young LM. Price CS. Community-acquired methicillin-resistant Staphylococcus aureus emerging as an important cause of necrotizing fasciitis. Surg Infect (Larchmt) 2008;9:469–474. doi: 10.1089/sur.2007.052. [DOI] [PubMed] [Google Scholar]
- 13.Anaya DA. Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44:705–710. doi: 10.1086/511638. [DOI] [PubMed] [Google Scholar]
- 14.Cui H. Hao S. Arous E. A distinct cause of necrotizing fasciitis: Aeromonas veronii biovar sobria. Surg Infect (Larchmt) 2007;8:523–528. doi: 10.1089/sur.2006.046. [DOI] [PubMed] [Google Scholar]
- 15.Chang CM. Lee HC. Lee NY, et al. Community-acquired Klebsiella pneumoniae complicated skin and soft-tissue infections of extremities: emphasis on cirrhotic patients and gas formation. Infection. 2008;36:328–334. doi: 10.1007/s15010-008-7272-3. [DOI] [PubMed] [Google Scholar]
- 16.Kohler JE. Hutchens MP. Sadow PM, et al. Klebsiella pneumoniae necrotizing fasciitis and septic arthritis: an appearance in the Western hemisphere. Surg Infect (Larchmt) 2007;8:227–232. doi: 10.1089/sur.2006.007. [DOI] [PubMed] [Google Scholar]
- 17.Uckay I. Sax H. Harbarth S, et al. Multi-resistant infections in repatriated patients after natural disasters: lessons learned from the 2004 tsunami for hospital infection control. J Hosp Infect. 2008;68:1–8. doi: 10.1016/j.jhin.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Johnson EN. Burns TC. Hayda RA, et al. Infectious complications of open type III tibial fractures among combat casualties. Clin Infect Dis. 2007;45:409–415. doi: 10.1086/520029. [DOI] [PubMed] [Google Scholar]
- 19.Davis KA. Moran KA. McAllister CK. Gray PJ. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg Infect Dis. 2005;11:1218–1224. doi: 10.3201/1108.050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sebeny PJ. Riddle MS. Petersen K. Acinetobacter baumannii skin and soft-tissue infection associated with war trauma. Clin Infect Dis. 2008;47:444–449. doi: 10.1086/590568. [DOI] [PubMed] [Google Scholar]
- 21.Amsel MB. Horrilleno E. Synergistic necrotizing fasciitis: a case of polymicrobic infection with Acinetobacter calcoaceticus. Curr Surg. 1985;42:370–372. [PubMed] [Google Scholar]
- 22.Villalba F. Manana P. Limongi G. [Necrotizing cellulitis caused by Acinetobacter baumannii] Enferm Infecc Microbiol Clin. 2000;18:479–480. [PubMed] [Google Scholar]
- 23.Qazi SA. Mohammed AA. Saber EI. Mirza SM. Necrotizing fasciitis. Role of early surgical intervention. Saudi Med J. 2004;25:890–894. [PubMed] [Google Scholar]
- 24.Comin Novella L. del Val Gil JM. Oset Garcia M. [Fournier gangrene: presentation of 6 cases with no mortality] Cir Esp. 2008;84:28–31. doi: 10.1016/s0009-739x(08)70600-6. [DOI] [PubMed] [Google Scholar]
- 25.Chiang WC. Su CP. Hsu CY, et al. Community-acquired bacteremic cellulitis caused by Acinetobacter baumannii. J Formos Med Assoc. 2003;102:650–652. [PubMed] [Google Scholar]
- 26.Cabrera H. Skoczdopole L. Marini M, et al. Necrotizing gangrene of the genitalia and perineum. Int J Dermatol. 2002;41:847–851. doi: 10.1046/j.1365-4362.2002.01640.x. [DOI] [PubMed] [Google Scholar]
- 27.Taviloglu K. Cabioglu N. Cagatay A, et al. Idiopathic necrotizing fasciitis: risk factors and strategies for management. Am Surg. 2005;71:315–320. [PubMed] [Google Scholar]
- 28.Wong CH. Chang HC. Pasupathy S, et al. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2003;85-A:1454–1460. [PubMed] [Google Scholar]
- 29.Liu YM. Chi CY. Ho MW, et al. Microbiology and factors affecting mortality in necrotizing fasciitis. J Microbiol Immunol Infect. 2005;38:430–435. [PubMed] [Google Scholar]
- 30.Patil NR. Rogers SO. Multi-drug resistant Acinetobacter fasciitis in a patient with perforated esophagus. Chest. 2005;128:480S. [Google Scholar]
- 31.Charnot-Katsikas A. Dorafshar AH. Aycock JK, et al. Two cases of necrotizing fasciitis due to Acinetobacter baumannii. J Clin Microbiol. 2009;47:258–263. doi: 10.1128/JCM.01250-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hidron AI. Edwards JR. Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 33.Childers BJ. Potyondy LD. Nachreiner R, et al. Necrotizing fasciitis: a fourteen-year retrospective study of 163 consecutive patients. Am Surg. 2002;68:109–116. [PubMed] [Google Scholar]
- 34.Norton KS. Johnson LW. Perry T, et al. Management of Fournier's gangrene: an eleven year retrospective analysis of early recognition, diagnosis, and treatment. Am Surg. 2002;68:709–713. [PubMed] [Google Scholar]
- 35.Koh TH. Gram-negative resistance in Singapore: a historical perspective. Ann Acad Med Singapore. 2008;37:847–854. [PubMed] [Google Scholar]
- 36.Barbolla RE. Centron D. Di Martino A, et al. Identification of an epidemic carbapenem-resistant Acinetobacter baumannii strain at hospitals in Buenos Aires City. Diagn Microbiol Infect Dis. 2003;45:261–264. doi: 10.1016/s0732-8893(02)00538-2. [DOI] [PubMed] [Google Scholar]
- 37.Korten V. Ulusoy S. Zarakolu P. Mete B. Antibiotic resistance surveillance over a 4-year period (2000–2003) in Turkey: results of the MYSTIC Program. Diagn Microbiol Infect Dis. 2007;59:453–457. doi: 10.1016/j.diagmicrobio.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Tschoeke SK. Ertel W. Immunoparalysis after multiple trauma. Injury. 2007;38:1346–1357. doi: 10.1016/j.injury.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 39.Lee CC. Chi CH. Lee NY, et al. Necrotizing fasciitis in patients with liver cirrhosis: predominance of monomicrobial gram-negative bacillary infections. Diagn Microbiol Infect Dis. 2008;62:219–225. doi: 10.1016/j.diagmicrobio.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Corredoira JM. Ariza J. Pallares R, et al. Gram-negative bacillary cellulitis in patients with hepatic cirrhosis. Eur J Clin Microbiol Infect Dis. 1994;13:19–24. doi: 10.1007/BF02026118. [DOI] [PubMed] [Google Scholar]
- 41.Ruef C. Complicated skin and soft-tissue infections—consider gram-negative pathogens. Infection. 2008;36:295. doi: 10.1007/s15010-008-3408-8. [DOI] [PubMed] [Google Scholar]
- 42.Whitman TJ. Qasba SS. Timpone JG, et al. Occupational transmission of Acinetobacter baumannii from a United States serviceman wounded in Iraq to a health care worker. Clin Infect Dis. 2008;47:439–443. doi: 10.1086/589247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hujer KM. Hujer AM. Hulten EA, et al. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother. 2006;50:4114–4123. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sunenshine RH. Wright MO. Maragakis LL, et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis. 2007;13:97–103. doi: 10.3201/eid1301.060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daniels TL. Deppen S. Arbogast PG, et al. Mortality rates associated with multidrug-resistant Acinetobacter baumannii infection in surgical intensive care units. Infect Control Hosp Epidemiol. 2008;29:1080–1083. doi: 10.1086/591456. [DOI] [PubMed] [Google Scholar]
- 46.Lee NY. Lee HC. Ko NY, et al. Clinical and economic impact of multidrug resistance in nosocomial Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol. 2007;28:713–719. doi: 10.1086/517954. [DOI] [PubMed] [Google Scholar]
- 47.Fournier PE. Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42:692–699. doi: 10.1086/500202. [DOI] [PubMed] [Google Scholar]
- 48.Falagas ME. Kasiakou SK. Rafailidis PI et al. Comparison of mortality of patients with Acinetobacter baumannii bactaeremia receiving appropriate and inappropriate empirical therapy. J Antimicrob Chemother. 2006;57:1251–1254. doi: 10.1093/jac/dkl130. [DOI] [PubMed] [Google Scholar]
- 49.Gunter OL. Guillamondegui OD. May AK. Diaz JJ. Outcome of necrotizing skin and soft tissue infections. Surg Infect (Larchmt) 2008;9:443–450. doi: 10.1089/sur.2007.053. [DOI] [PubMed] [Google Scholar]
- 50.Fournier PE. Vallenet D. Barbe V, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006;2:e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adams MD. Goglin K. Molyneaux N, et al. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J Bacteriol. 2008;190:8053–8064. doi: 10.1128/JB.00834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mastropaolo MD. Evans NP. Byrnes MK, et al. Synergy in polymicrobial infections in a mouse model of type 2 diabetes. Infect Immun. 2005;73:6055–6063. doi: 10.1128/IAI.73.9.6055-6063.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Obana Y. Pathogenic significance of Acinetobacter calcoaceticus: analysis of experimental infection in mice. Microbiol Immun. 1986;30:645–657. doi: 10.1111/j.1348-0421.1986.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 54.Niu C. Clemmer KM. Bonomo RA. Rather PN. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J Bacteriol. 2008;190:3386–3392. doi: 10.1128/JB.01929-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perez F. Endimiani A. Bonomo RA. Why are we afraid of Acinetobacter baumannii? Expert Rev Anti Infect Ther. 2008;6:269–271. doi: 10.1586/14787210.6.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]