Abstract
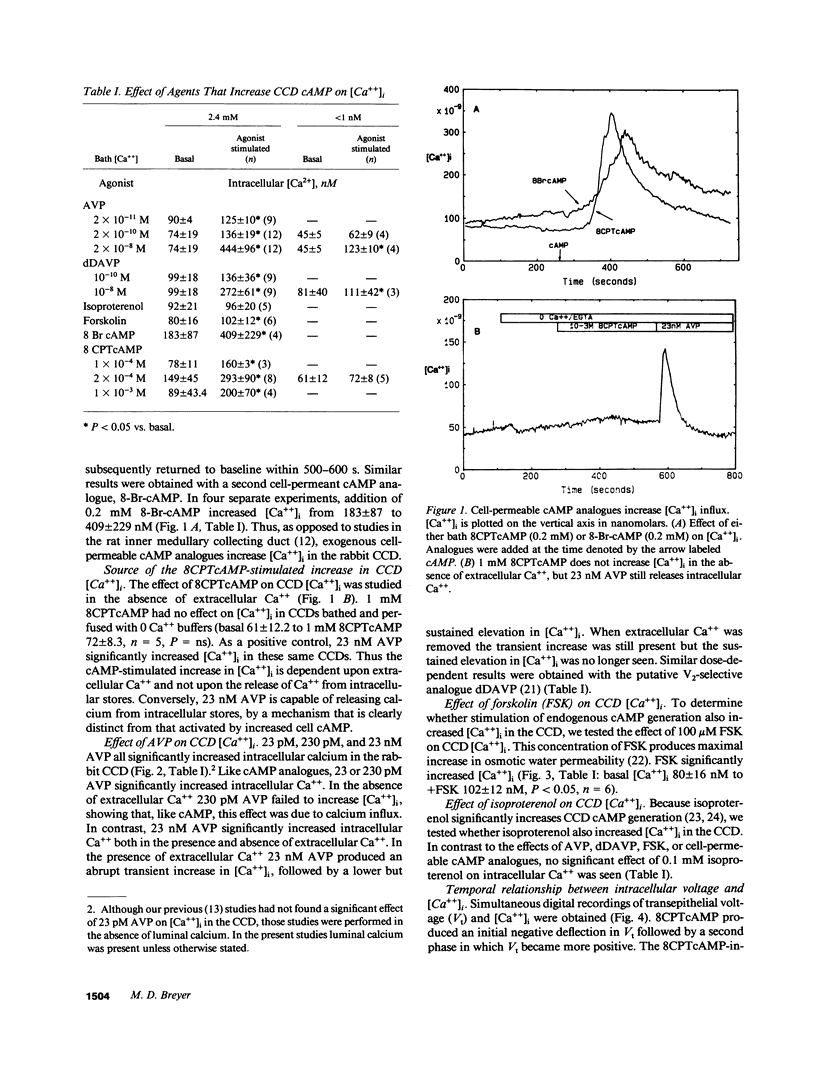
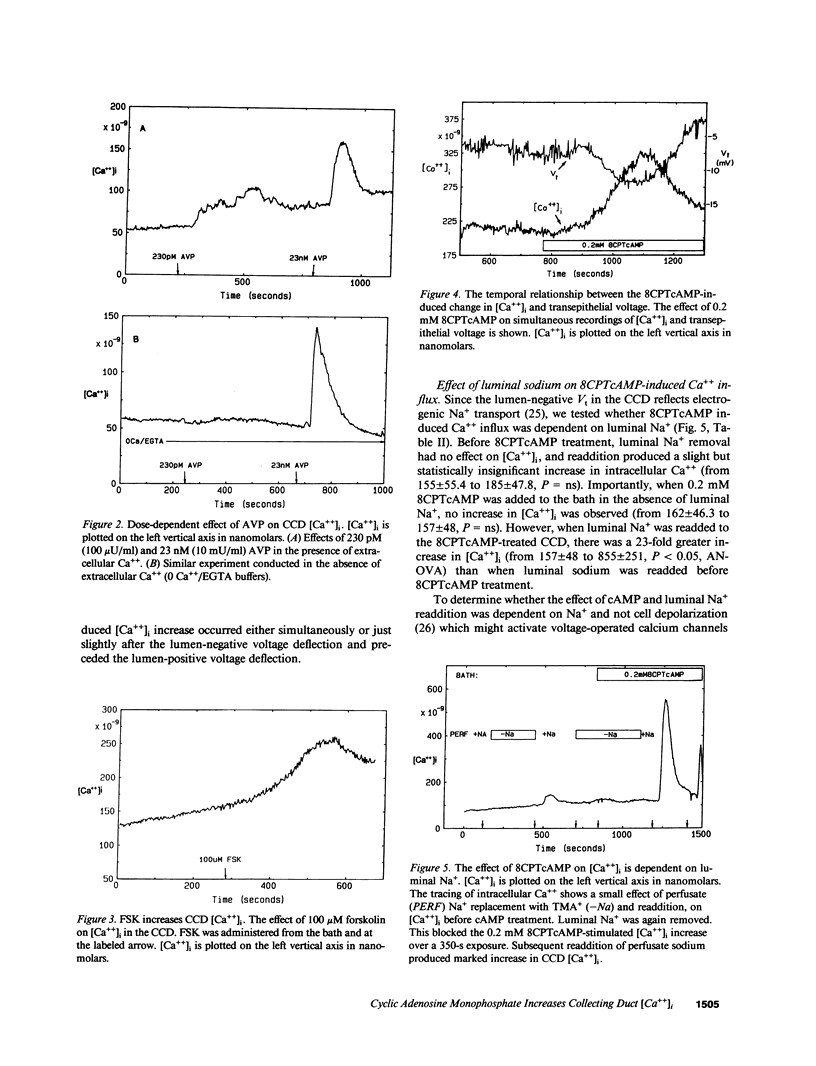
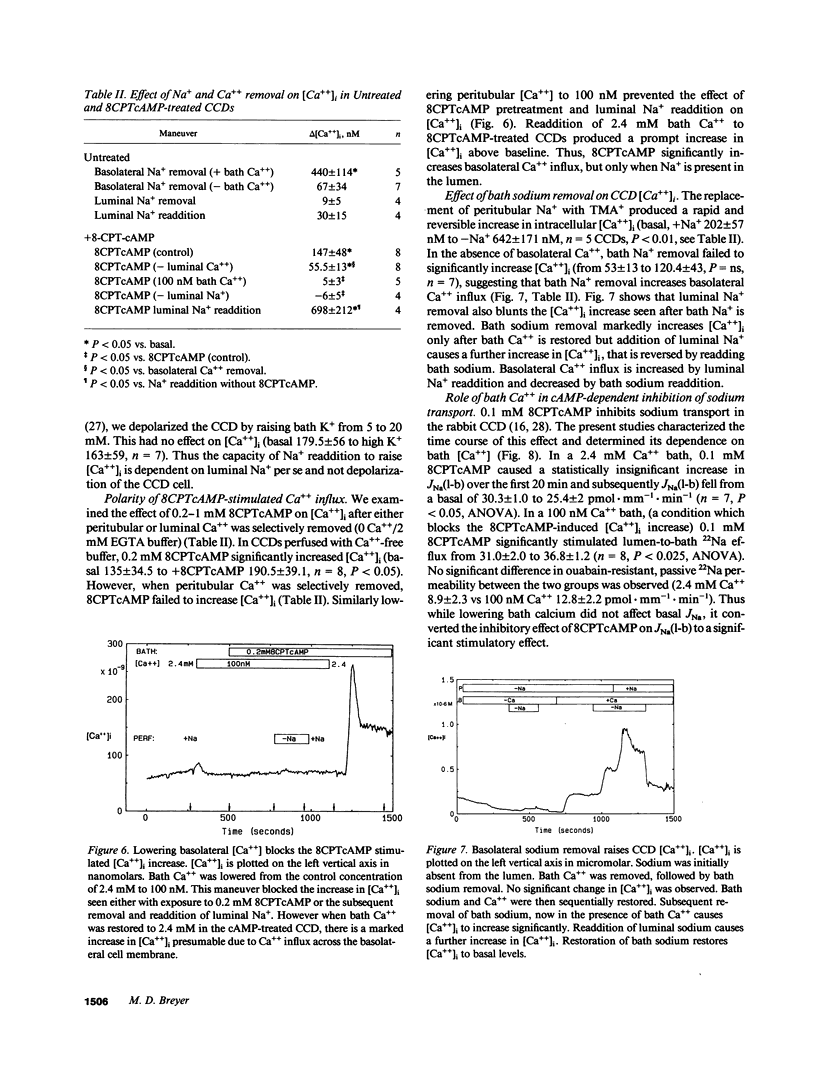
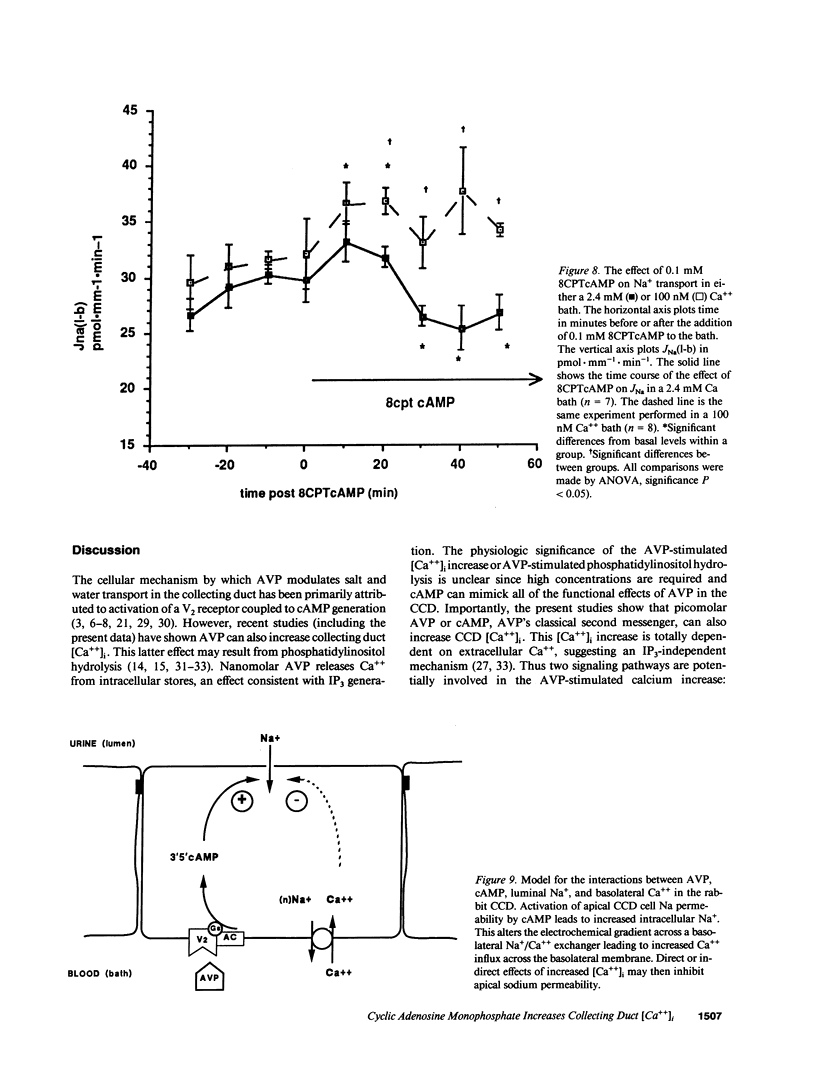
Arginine vasopressin (AVP) transiently stimulates Na+ transport in the rabbit cortical collecting duct (CCD). However, the sustained effect of both AVP and its putative second messenger, cyclic adenosine monophosphate (cAMP), on Na+ transport in the rabbit CCD is inhibitory. Because maneuvers that increase [Ca++]i inhibit Na+ transport, the effects of AVP and cell-permeable cAMP analogues, on [Ca++]i were investigated in fura-2-loaded in vitro microperfused rabbit CCDs. Low-dose AVP (23-230 pM) selectively stimulated Ca++ influx, whereas 23 nM AVP additionally released calcium from intracellular stores. 8-chlorophenylthio-cAMP (8CPTcAMP) and 8-bromo-cAMP (8-Br-cAMP) also increased CCD [Ca++]i. The 8CPTcAMP-stimulated [Ca++]i increase was totally dependent on basolateral [Ca++]. In the absence of cAMP, peritubular Na+ removal produced a marked increase in [Ca++]i, which was also dependent on bath [Ca++], suggesting the existence of basolateral Na+/Ca++ exchange. Luminal Na+ removal in the absence of cAMP did not alter CCD [Ca++]i, but it completely blocked the cAMP-stimulated [Ca++]i increase. Thus the cAMP-dependent Ca++ increase is totally dependent on both luminal Na+ and basolateral Ca++, suggesting the [Ca++]i increase is secondary to cAMP effects on luminal Na+ entry and its coupling to basolateral Na+/Ca++ exchange. 8CPTcAMP inhibits lumen-to-bath 22Na flux [JNa(l-b)] in CCDs bathed in a normal Ca++ bath (2.4 mM). However, when bath Ca++ was lowered to 100 nM, a maneuver that also blocks the 8CPTcAMP [Ca++]i increase, 8CPTcAMP stimulated, rather than inhibited JNa(l-b). These results suggest that cAMP formation initially stimulates CCD Na+ transport, and that increased apical Na+ entry secondarily activates basolateral Ca++ entry. The cAMP-dependent [Ca++]i increase leads to inhibition Na+ transport in the rabbit CCD.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramow M., Beauwens R., Cogan E. Cellular events in vasopressin action. Kidney Int Suppl. 1987 Aug;21:S56–S66. [PubMed] [Google Scholar]
- Aiyar N., Nambi P., Stassen F. L., Crooke S. T. Vascular vasopressin receptors mediate phosphatidylinositol turnover and calcium efflux in an established smooth muscle cell line. Life Sci. 1986 Jul 7;39(1):37–45. doi: 10.1016/0024-3205(86)90435-2. [DOI] [PubMed] [Google Scholar]
- Ando Y., Breyer M. D., Jacobson H. R. Dose-dependent heterogenous actions of vasopressin in rabbit cortical collecting ducts. Am J Physiol. 1989 Apr;256(4 Pt 2):F556–F562. doi: 10.1152/ajprenal.1989.256.4.F556. [DOI] [PubMed] [Google Scholar]
- Ando Y., Jacobson H. R., Breyer M. D. Phorbol ester and A23187 have additive but mechanistically separate effects on vasopressin action in rabbit collecting tubule. J Clin Invest. 1988 May;81(5):1578–1584. doi: 10.1172/JCI113491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartter F. C., Schwartz W. B. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967 May;42(5):790–806. doi: 10.1016/0002-9343(67)90096-4. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bourdeau J. E., Lau K. Basolateral cell membrane Ca-Na exchange in single rabbit connecting tubules. Am J Physiol. 1990 Jun;258(6 Pt 2):F1497–F1503. doi: 10.1152/ajprenal.1990.258.6.F1497. [DOI] [PubMed] [Google Scholar]
- Burnatowska-Hledin M. A., Spielman W. S. Vasopressin V1 receptors on the principal cells of the rabbit cortical collecting tubule. Stimulation of cytosolic free calcium and inositol phosphate production via coupling to a pertussis toxin substrate. J Clin Invest. 1989 Jan;83(1):84–89. doi: 10.1172/JCI113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlen D., Guillon G., Rajerison R. M., Jard S., Sawyer W. H., Manning M. Structural requirements for activation of vasopressin-sensitive adenylate cyclase, hormone binding, and antidiuretic actions: effects of highly potent analogues and competitive inhibitors. Mol Pharmacol. 1978 Nov;14(6):1006–1017. [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Seagar M. J., Takahashi M. Molecular properties of dihydropyridine-sensitive calcium channels in skeletal muscle. J Biol Chem. 1988 Mar 15;263(8):3535–3538. [PubMed] [Google Scholar]
- Chabardès D., Imbert-Teboul M., Montégut M., Clique A., Morel F. Catecholamine sensitive adenylate cyclase activity in different segments of the rabbit nephron. Pflugers Arch. 1975 Dec 19;361(1):9–15. doi: 10.1007/BF00587334. [DOI] [PubMed] [Google Scholar]
- Chase H. S., Jr Does calcium couple the apical and basolateral membrane permeabilities in epithelia? Am J Physiol. 1984 Dec;247(6 Pt 2):F869–F876. doi: 10.1152/ajprenal.1984.247.6.F869. [DOI] [PubMed] [Google Scholar]
- Fejes-Tóth G., Náray-Fejes-Tóth A. Isolated principal and intercalated cells: hormone responsiveness and Na+-K+-ATPase activity. Am J Physiol. 1989 Apr;256(4 Pt 2):F742–F750. doi: 10.1152/ajprenal.1989.256.4.F742. [DOI] [PubMed] [Google Scholar]
- Frindt G., Burg M. B. Effect of vasopressin on sodium transport in renal cortical collecting tubules. Kidney Int. 1972 Apr;1(4):224–231. doi: 10.1038/ki.1972.32. [DOI] [PubMed] [Google Scholar]
- Frindt G., Windhager E. E. Ca2(+)-dependent inhibition of sodium transport in rabbit cortical collecting tubules. Am J Physiol. 1990 Mar;258(3 Pt 2):F568–F582. doi: 10.1152/ajprenal.1990.258.3.F568. [DOI] [PubMed] [Google Scholar]
- Garty H., Benos D. J. Characteristics and regulatory mechanisms of the amiloride-blockable Na+ channel. Physiol Rev. 1988 Apr;68(2):309–373. doi: 10.1152/physrev.1988.68.2.309. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Burg M. B. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966 Jul;211(1):255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guillon G., Butlen D., Cantau B., Barth T., Jard S. Kinetic and pharmacological characterization of vasopressin membrane receptors from human kidney medulla: relation to adenylate cyclase activation. Eur J Pharmacol. 1982 Dec 3;85(3-4):291–304. doi: 10.1016/0014-2999(82)90216-3. [DOI] [PubMed] [Google Scholar]
- Holt W. F., Lechene C. ADH-PGE2 interactions in cortical collecting tubule. I. Depression of sodium transport. Am J Physiol. 1981 Oct;241(4):F452–F460. doi: 10.1152/ajprenal.1981.241.4.F452. [DOI] [PubMed] [Google Scholar]
- Hébert R. L., Jacobson H. R., Breyer M. D. Prostaglandin E2 inhibits sodium transport in rabbit cortical collecting duct by increasing intracellular calcium. J Clin Invest. 1991 Jun;87(6):1992–1998. doi: 10.1172/JCI115227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M. Effects of parathyroid hormone and N6,O2'-dibutyryl cyclic AMP on Ca2+ transport across the rabbit distal nephron segments perfused in vitro. Pflugers Arch. 1981 May;390(2):145–151. doi: 10.1007/BF00590197. [DOI] [PubMed] [Google Scholar]
- Jones S. M., Frindt G., Windhager E. E. Effect of peritubular [Ca] or ionomycin on hydrosmotic response of CCTs to ADH or cAMP. Am J Physiol. 1988 Feb;254(2 Pt 2):F240–F253. doi: 10.1152/ajprenal.1988.254.2.F240. [DOI] [PubMed] [Google Scholar]
- Kimmel P. L., Goldfarb S. Effects of isoproterenol on potassium secretion by the cortical collecting tubule. Am J Physiol. 1984 Jun;246(6 Pt 2):F804–F810. doi: 10.1152/ajprenal.1984.246.6.F804. [DOI] [PubMed] [Google Scholar]
- Kirk K. L., Buku A., Eggena P. Cell specificity of vasopressin binding in renal collecting duct: computer-enhanced imaging of a fluorescent hormone analog. Proc Natl Acad Sci U S A. 1987 Aug;84(16):6000–6004. doi: 10.1073/pnas.84.16.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen B. M., Biagi B. A., Giebisch G. H. Intracellular microelectrode characterization of the rabbit cortical collecting duct. Am J Physiol. 1983 Jan;244(1):F35–F47. doi: 10.1152/ajprenal.1983.244.1.F35. [DOI] [PubMed] [Google Scholar]
- Lau K., Bourdeau J. E. Evidence for cAMP-dependent protein kinase in mediating the parathyroid hormone-stimulated rise in cytosolic free calcium in rabbit connecting tubules. J Biol Chem. 1989 Mar 5;264(7):4028–4032. [PubMed] [Google Scholar]
- Ling B. N., Eaton D. C. Effects of luminal Na+ on single Na+ channels in A6 cells, a regulatory role for protein kinase C. Am J Physiol. 1989 Jun;256(6 Pt 2):F1094–F1103. doi: 10.1152/ajprenal.1989.256.6.F1094. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Billah M. M. Hormonal stimulation of phosphatidylinositol breakdown with particular reference to the hepatic effects of vasopressin. Biochem Soc Trans. 1979 Oct;7(5):861–865. doi: 10.1042/bst0070861. [DOI] [PubMed] [Google Scholar]
- Nadler S. P., Hebert S. C., Brenner B. M. PGE2, forskolin, and cholera toxin interactions in rabbit cortical collecting tubule. Am J Physiol. 1986 Jan;250(1 Pt 2):F127–F135. doi: 10.1152/ajprenal.1986.250.1.F127. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Hayhurst R. A. Functional differentiation of cell types of cortical collecting duct. Am J Physiol. 1985 Mar;248(3 Pt 2):F449–F453. doi: 10.1152/ajprenal.1985.248.3.F449. [DOI] [PubMed] [Google Scholar]
- ORLOFF J., HANDLER J. S. The similarity of effects of vasopressin, adenosine-3',5'-phosphate (cyclic AMP) and theophylline on the toad bladder. J Clin Invest. 1962 Apr;41:702–709. doi: 10.1172/JCI104528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G., Frindt G. Effects of cell Ca and pH on Na channels from rat cortical collecting tubule. Am J Physiol. 1987 Aug;253(2 Pt 2):F333–F339. doi: 10.1152/ajprenal.1987.253.2.F333. [DOI] [PubMed] [Google Scholar]
- Reif M. C., Troutman S. L., Schafer J. A. Sodium transport by rat cortical collecting tubule. Effects of vasopressin and desoxycorticosterone. J Clin Invest. 1986 Apr;77(4):1291–1298. doi: 10.1172/JCI112433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisine T., Guild S. Activators of protein kinase C and cyclic AMP-dependent protein kinase regulate intracellular calcium levels through distinct mechanisms in mouse anterior pituitary tumor cells. Mol Pharmacol. 1987 Oct;32(4):488–496. [PubMed] [Google Scholar]
- Schafer J. A., Troutman S. L. cAMP mediates the increase in apical membrane Na+ conductance produced in rat CCD by vasopressin. Am J Physiol. 1990 Nov;259(5 Pt 2):F823–F831. doi: 10.1152/ajprenal.1990.259.5.F823. [DOI] [PubMed] [Google Scholar]
- Schlatter E., Schafer J. A. Electrophysiological studies in principal cells of rat cortical collecting tubules. ADH increases the apical membrane Na+-conductance. Pflugers Arch. 1987 Jun;409(1-2):81–92. doi: 10.1007/BF00584753. [DOI] [PubMed] [Google Scholar]
- Schuster V. L. Cyclic adenosine monophosphate-stimulated bicarbonate secretion in rabbit cortical collecting tubules. J Clin Invest. 1985 Jun;75(6):2056–2064. doi: 10.1172/JCI111925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster V. L. Mechanism of bradykinin, ADH, and cAMP interaction in rabbit cortical collecting duct. Am J Physiol. 1985 Nov;249(5 Pt 2):F645–F653. doi: 10.1152/ajprenal.1985.249.5.F645. [DOI] [PubMed] [Google Scholar]
- Snowdowne K. W., Borle A. B. Effects of low extracellular sodium on cytosolic ionized calcium. Na+-Ca2+ exchange as a major calcium influx pathway in kidney cells. J Biol Chem. 1985 Dec 5;260(28):14998–14507. [PubMed] [Google Scholar]
- Star R. A., Nonoguchi H., Balaban R., Knepper M. A. Calcium and cyclic adenosine monophosphate as second messengers for vasopressin in the rat inner medullary collecting duct. J Clin Invest. 1988 Jun;81(6):1879–1888. doi: 10.1172/JCI113534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner L. C., Burg M. B., Orloff J. Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol. 1974 Aug;227(2):453–459. doi: 10.1152/ajplegacy.1974.227.2.453. [DOI] [PubMed] [Google Scholar]
- Teitelbaum I. Vasopressin-stimulated phosphoinositide hydrolysis in cultured rat inner medullary collecting duct cells is mediated by the oxytocin receptor. J Clin Invest. 1991 Jun;87(6):2122–2126. doi: 10.1172/JCI115243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. M., Chase H. S., Jr Effect of vasopressin on intracellular [Ca] and Na transport in cultured toad bladder cells. Am J Physiol. 1988 Nov;255(5 Pt 2):F1015–F1024. doi: 10.1152/ajprenal.1988.255.5.F1015. [DOI] [PubMed] [Google Scholar]



