Abstract
Data has emerged, largely from non-thromboembolic animal models of stroke, that suggests that statins, which have efficacy in preventing strokes when given pre-ischemically, may have a positive effect on stroke even when given post-ischemically, possibly through pleitropic cerebrovascular effects. The goal of this study was to characterize the effects of IV tPA in a clinically-relevant model of stroke utilizing a vascular occlusion with a freshly-formed clot, and evaluate the effects of post-ischemic administration of simvastatin on stroke outcome in this model. Neurological deficit, clot burden, and lesion volume were assessed after treatment with tPA in one experiment, and after treatment with simvastatin in another. In the tPA experiment, treatment with 10mg/kg of tPA IV (with 20% given as an initial bolus, and 80% given as an infusion over the remaining 30 minutes), starting within an hour after stroke, resulted in significant reductions, compared with control animals, in neurological deficit (mean ± SD neuroscores of 21.5 ± 21.1 and 30 ± 29.3, respectively, p = 0.005), clot burden (p = 0.010) and lesion volume (p=0.049) at 24 hours. In the simvastatin experiment on the other hand, treatment with a 20mg/kg of simvastatin as a single intraperitoneal dose within an hour after stroke resulted in no salutary effects on neurological deficit, clot burden or lesion volume compared with controls at 24 hours. These results suggest that more research needs to be done to fully ascertain the therapeutic potential and optimal dosing paradigm of a post-ischemic treatment with a statin.
Keywords: Simvastatin; Hydroxymethylglutaryl-CoA Reductase Inhibitors; Tissue Plasminogen Activator; Stroke; Disease Models, Animal; Thromboembolism
1. Introduction
In the SPARCL trial, 80 mg of atorvastatin per day reduced the overall incidence of strokes and cardiovascular events (Amarenco 2006). The efficacy of statins in the primary and secondary prevention of acute ischemic stroke is well established (Sacco 2006, Fuentes 2009, Milionis 2009). In recent years, it has become apparent that their effects may be more pleitropic than simply lowering low-density lipoprotein (LDL) cholesterol (Cimino 2005, Endres 2005).
In rodent studies, pre-treatment with statins prior to experimental stroke has been shown improve lesion volume and neurological deficit (Shabanzadeh 2005, Yrjänheikki 2005), and in this context up-regulates endothelial nitric oxide synthase (eNOS) (Amin-Hanjani 2001, Sironi 2003, Asahi 2005), increases tissue-plasminogen activator (tPA) activity (Essig1998, Asahi 2005) and increases cerebral blood flow (Endres 1998, Amin-Hanjani 2001). The administration of statins as an acute post-ischemic treatment of experimental ischemic stroke may also have an effect. In a rat study with permanent middle cerebral artery occlusion (MCAO) achieved via direct bipolar coagulation, treatment with simvastatin after ischemic injury resulted in decreased infarct progression (Sironi 2003). An intravenous dose of rosuvastatin given post-ischemically in a model of stroke utilizing a filamentous occlusion in mice resulted in improvements in lesion size and neurological deficit (Prinz 2008). Diabetic rats treated with atorvastatin shortly after a transient middle cerebral artery occlusion with a nylon filament had lower infarct volumes than those in untreated animals at 24 hours (Elewa 2009).
These results were obtained, however, in models of experimental stroke utilizing non-biological occlusions. Acute ischemic stroke in humans is somewhat heterogeneous in character, in that what starts out as an acute thromboembolic occlusion of a major branch cerebral artery can result in a spectrum of events at 24 hours, ranging from a transient ischemic attack with no neurological sequelae in one instance to a malignant cerebral infarction in another (Koudstaal 1991, NINDS 1995, Kimura 1999). The acute thromboembolic arterial occlusions that underlie these strokes tend to spontaneously and variably re-canalize (or “dissolve”, re-establishing flow) over time (Toni 1998, Baracchini 2000, Alexandrov 2002), and the extent and immediacy of this recanalization (which presumably depends on a delicate balance of prevailing hematological, hemodynamic, and vascular factors) has a concordant effect on outcome (Rha 2007). Given vascular effects of statins noted above, and the fact that statins produce an anti-thrombotic hemostatic profile in cardiovascular disease (Dangas 1999, Laufs 2000), their potential as a post-ischemic treatment for acute stroke may be most relevantly studied in a thromboembolic, vascular model of acute ischemic stroke adapted to closely approximate the natural physiology of this event in humans, utilizing an occlusion with fresh, spontaneously formed blood clot. The goal of this study was two-fold: 1) to evaluate the effects of intravenous tissue-plasminogen activator (tPA) on post-treatment behavior, clot burden, and lesion volume at 24 hours in such a model, and 2) use the model to assess the effect of a post-ischemic administration of a statin, simvastatin, on those same endpoints.
2. Results
In the tPA experiment, 14 animals received a weight-based dose of tPA intravenously, and ten animals received a weight-based dose of control solution. At 24 hours, four and two of the tPA-treated and control animals, respectively, had died (no significant difference in mortality, p = 0.63 per Chi-square analysis), leaving ten animals in the tPA group and 8 animals in the control group for analysis.
In the simvastatin experiment, 15 animals received simvastatin, 20mg/kg intraperitoneally, and 12 animals received a comparable weight-based volume of vehicle intraperitoneally after embolization. At 24 hours, five and three of the simvastatin-treated and vehicle treated (control) animals, respectively, had died (no significant difference in mortality, p = 0.64 per Chi-square analysis), leaving ten animals in the simvastatin group and nine animals in the vehicle group for analysis.
2.1. Effects of tPA and simvastatin on neurological deficit
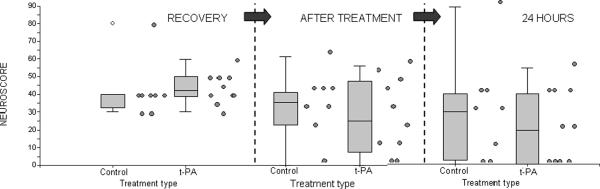
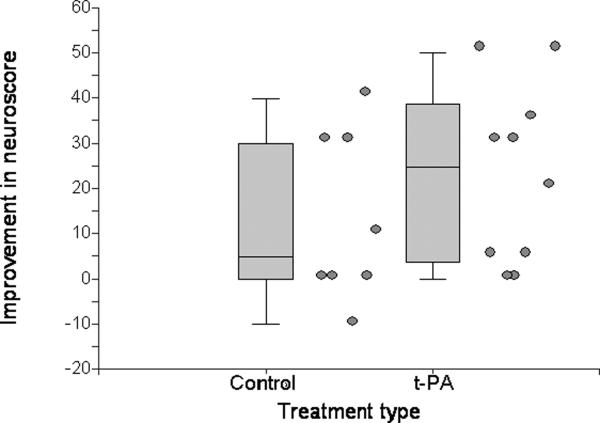
Overall, neurological deficit improved over time in both tPA-treated and contemporaneous control animals in the tPA experiment (Table), as it did in simvastatin and contemporaneous vehicle-treated animals in the simvastatin experiment. In the tPA experiment, there was a trend towards a greater number of improvements to no deficit at all (neuroscore = 0) in the tPA treated animals, compared to the control animals (Figure 1, difference NS), and the group of tPA-treated animals appeared to have a more notable improvement in deficit overall by 24 hours (Figure 2). Given the variability in neuroscores seen, an exploratory paired t-test was performed in each group in the tPA experiment to assess the significance of the change between each pre-treatment neuroscore and its associated post-treatment neuroscore, and revealed that the improvement in neuroscores in tPA treated animals was statistically significant at both the post-treatment timepoint (p = 0.018) and at 24 hours (p = 0.005), but was not in the contemporaneous control animals at either timepoint (p = 0.12 and 0.095, respectively).
Table.
Neuroscores in tPA and control animals
| Group | Recovery | 1-hr post-treatment | 24 hours |
|---|---|---|---|
| Control (n=8) | 42.5 ± 15.8 | 32.5 ± 17.5 | 30 ± 29.3 |
| tPA (n=10) | 44 ± 8.8 | 26 ± 20.8* | 21.5 ± 21.1* |
Values are given as means± standard deviations
p<0.05
t-PA, tissue plasminogen activator
Figure 1.
Neuroscores at recovery (RECOVERY), one-hour after treatment (AFTER-TREATMENT), and at 24 hours (24 HOURS) in control and t-PA (tissue plasminogen activator)-treated animals. Individual values are to the right of the associated box plots. A higher value indicates more severe neurological deficit.
Figure 2.
Improvement in neuroscores between recovery and 24-hours (calculated as Neuroscore-on-recovery minus Neuroscore-at-24-hours) in control and t-PA (tissue plasminogen activator)-treated animals. Individual values are to the right of the associated box plot. A higher value indicates more improvement.
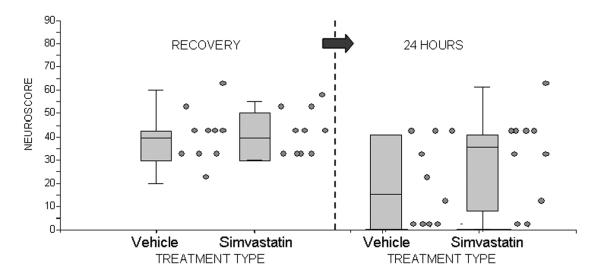
In the simvastatin experiment, the mean (± SD) recovery neuroscores were 38 ± 11.4 in the vehicle-treated group and 39.5 ± 9.6 in simvastatin-treated group, and the mean 24-hour neuroscores were 18 ± 18.1 in the vehicle-treated group and 21.5 ± 21.1 in simvastatin-treated group (Figure 3). A paired t-test performed in each group to assess the significance of the change between each pre-treatment neuroscore and its associated 24-hour neuroscore revealed that the simvastatin-treated animals had a significantly less pronounced improvement in neuroscores at 24 hours than did the vehicle group, to the point that, while the t-test was significant in the vehicle group (p = 0.012) due to spontaneous improvement, it was not in the simvastatin group (p = 0.13).
Figure 3.
Neuroscores at recovery (RECOVERY) and at 24 hours (24 HOURS) in vehicle and simvastatin-treated animals. Individual values are to the right of the associated box plots. A higher value indicates more severe neurological deficit.
2.2. Effects of tPA and simvastatin on clot burden
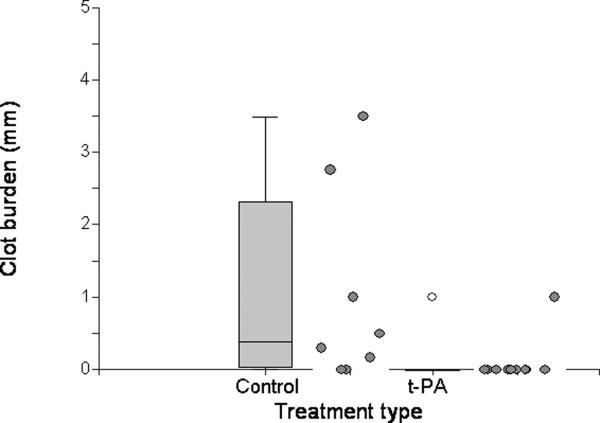
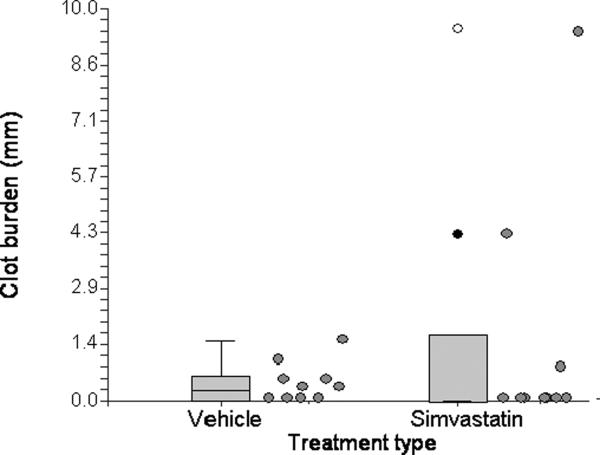
Residual clot burden at 24 hours was variable in both the tPA and simvastatin experiments. In the tPA experiment, the mean clot burdens were 1.0 ± 1.3 mm in the control group and 0.1 ± 0.3 mm in the tPA group. Compared to animals treated with control solution, treatment with tPA resulted in a marked and statistically significant reduction in clot burden (p = 0.010). Only 1/8 t-PA-treated animals, compared with 6/8 control animals, had residual clot (Figure 4). In the simvastatin experiment, on the other hand, there were no discernable differences in clot burden or in the incidence of clot, with mean (± SD) clot burdens of 0.4 ± 0.5 mm and 1.5 ± 3.1 mm in the vehicle and simvastatin groups, respectively (Figure 5).
Figure 4.
Residual clot burden at 24 hours in control and t-PA (tissue plasminogen activator)-treated animals. Individual values are to the right of their associated box plots.
Figure 5.
Residual clot burden at 24 hours in vehicle and simvastatin-treated animals. Individual values are to the right of their associated box plots.
2.3. Effects of tPA and simvastatin on lesion volume
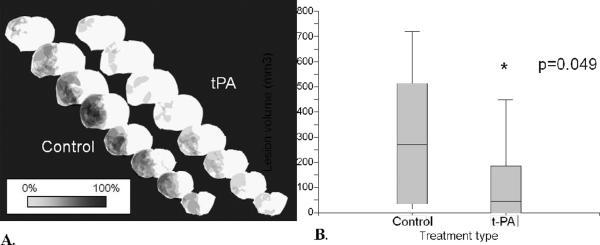
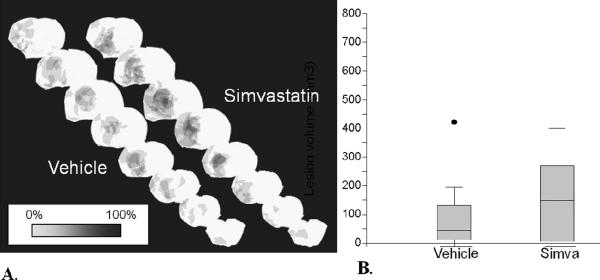
Treatment with tPA resulted in a statistically significant reduction in lesion volume at 24 hours, compared to control (p=0.049, Figure 6). The mean lesion volumes in control animals and tPA-treated animals were 301.8 ± 266.1 mm3 and 112.9 ± 153.2 mm3, respectively. In the simvastatin experiment, on the other hand the mean lesion volumes in the vehicle animals and simvastatin-treated animals were 94.1 ± 129.4 mm3 and 149.3 ± 140.9 mm3, respectively, with what appeared to be a slight trend towards increased lesion volume with simvastatin (Figure 7), although not statistically significant (p = 0.57). Intracranial hemorrhage was rare; there was only one small hemorrhage noted, and it occurred in the tPA experiment, paradoxically in a control animal.
Figure 6.
A. Lesion volume incidence maps for control and t-PA (tissue plasminogen activator)-treated animals, with each lesion incident to each brain slice in each animal overlaid at the location of the corresponding slice in which the lesion was found (areas of darker shade imply areas of increasing lesion overlap). B. Corresponding lesion volume box plots, with median and interquartile ranges for each group (Y-axis is in mm3).
Figure 7.
A. Lesion volume incidence maps for vehicle and simvastatin-treated animals, with each lesion incident to each brain slice in each animal overlaid at the location of the corresponding slice in which the lesion was found (areas of darker shade imply areas of increasing lesion overlap). B. Corresponding lesion volume box plots, with median and interquartile ranges for each group (Y-axis is in mm3).
3. Discussion
In this study, we found that administration of a standard thrombolytic agent significantly decreased neurological deficit measured at 24 hours after embolization. The behavioral improvement was associated with a significant decrease in clot burden and enhanced cellular survival as determined by TTC staining. We found that, in comparison to the beneficial effects of tPA, the post-ischemic administration of simvastatin, at a dose previously found to have pharmacological effects in models of cerebrovascular disease, did not have a beneficial effect on any of the studied endpoints, and in fact resulted in a significantly less pronounced behavioral recovery at 24 hours than that seen in untreated animals. The lack of an effect from statins has been reported in other studies. Balduini found that the post-ischemic administration of a statin in a model of neonatal hypoxicischemic brain injury did not have the same salutary effect on outcome as it did when given prior to the ischemic insult (Balduini 2003). Montanera found, in a pilot study of humans with acute ischemic stroke, that post-ischemic treatment simvastatin 40 mg/day for the first week followed by a dose of 20 mg/day until day 90 resulted in a trend towards improvement in neurological deficit, but without an increase in functional outcome (mRS), and associated with a non-significant increase in mortality and higher proportion of infections (Montanera 2007). The effect of statins on outcome in clinical trials has not been without contention (Goldstein 2009).
Our model is characterized by variability in the outcome of endpoints studied that, at first glance, would appear to be a liability, but actually represents an asset. First of all, the variability closely approximates the human condition. Some control animals spontaneously improved - on occasion to the point of having no deficit - while others worsened to have mortality, and outcome at 24 hours was variable. The NINDS trial revealed that 40% of human patients with acute ischemic stroke who go untreated will, by 24 hours, spontaneously and noticeably improve (as determined by an National Institutes of Health Stroke Scale (NIHSS) score improvement of 4 points or more) (NINDS 1995). Spontaneous reperfusion (and improvement) or - on the other hand - spontaneous deterioration are typical findings in the first 24 hours of human stroke (Zanette 1995, Bowler 1996, Toni 1998). Secondly, the manner in which the vascular occlusion was induced, which probably underlay some of the variability mentioned above, inherently allows for maximal sensitivity to any treatment effects on ischemic injury that might have been mediated through changes in autolysis or vascular interactions. We used fresh, spontaneously-formed clots in our study (see Experimental Procedure section). Previously described animal models of ischemic stroke using techniques such as direct mechanical occlusion (Tamura et al., 1981, Bederson 1986, Kawamata 1997), applying vascular injury to induce in-situ coagulation (Futrell 1989, Markgraf 1993, Dietrich 1993; Matsuno 1993, Guarini 1996), the embolization or insertion of non-biological material (Longa 1989, Akai 1995, Yang 2002), and the embolization of aged, processed clot (Zhang Z 1997, Kilic 1998, Kilic 1998, Wang 2001) have significant utility in answering very focused biological questions, specifically regarding the fate of neuronal populations down-stream from a vascular occlusion, but they present a limitation in that they are not comprehensively reflective of acute ischemic stroke in humans. They are designed to optimize uniformity of results, and use a relatively hardy occlusion to stabilize the initial ischemic insult over the duration of assessment and minimize any variability. In so doing, however, they lose the capability by which to clearly study effects on vascular thrombomodulation and autolysis that partially underlie the natural variation and outcome in ischemic stroke. The manner in which clot is prepared (and therefore the manner in which an occlusion is sustained) has a significant effect on outcome in experimental ischemic stroke; Neissen found that thrombin-generated clots were significantly more resistant to thrombolysis with t-PA than were fresh, spontaneously formed clots (Niessen 2003). The model we used is optimal for evaluating agents and treatment strategies with potentially pleitropic mechanisms of action.
The evident difference in 24-hour lesion volumes between the tPA control group and simvastatin control group may have been related to the fact that the tPA and simvastatin experiments were two separate experiments performed separately in time, and the fact that the volume and route of administration of control solutions in each experiment were markedly different. The tPA control animals received approximately 3 mL of solution IV over a 30 minute time period, while simvastatin control animals received a significantly smaller volume of control solution intraperitoneally (about 0.06 mL, on the order of a 100 times less). We have data from two prior experiments performed using this model that appears to suggest that volume of fluid administered may have been at play. In those experiments, the mean (±SD) lesion volumes in the control groups were 123.6 ±148.1 mm3 and 137.8 ±107.4 mm3, respectively, in animals that received approximately 0.5 mL of control solution IV very shortly after stroke (unpublished data). A proportional overview of control data we have thus far, including this study, reveals mean lesion volumes of 94.1 mm3, 123.6 mm3, 137.8 mm3, and 301.8 mm3 in animals that received approximately 0.06 mL, 0.5 mL, 0.5 mL, and 3 mL of fluid, respectively, which strongly suggests a correlation with the amount of control solution fluid given. There is human clinical trial data to corroborate this kind of effect on outcome. Hypervolemic hemodilution (consisting of rapid infusion of large volumes of intravenous fluid) was a promising therapeutic modality that was carefully studied in humans as a treatment of acute ischemic stroke until about the late 1990's, when it was found that there was no clear benefit. Furthermore, one study revealed that 5.5% of patients given this treatment died within the first 5 days, compared with only 1.6% of control patients (No author, 1987). In an animal study of focal cerebral ischemia in which the effects of varying degrees of hydration status on cerebral brain water (edema) were studied, the infusion of larger volumes of intravenous saline resulted in significantly increased amount of edema in the infarcted hemisphere Paczynski 2000). Relatively rapid administration of large volumes of fluid may dilute the oxygen-carrying capacity of the blood, possibly worsening ischemia (von Kummer 1989). A careful, systematic review of the totality of the literature regarding hypervolemic hemodilution therapy for acute ischemic stroke in humans at the time concluded that, while not statistically significant, there was a trend towards increased early mortality (Asplund 2002). The difference in baseline lesion volume seen between the two experiments in our study can readily be accounted for by the difference in fluid amount administered. Since the treatment results of each experiment were based on a comparison with a specific control group dedicated to that experiment, the comparison of net treatment effects seen within each experiment is very robust, even if the absolute values of lesion volumes may not be easily compared between experiments.
The lack of effect seen with simvastatin in our study may be related to several factors. First of all, we evaluated simvastatin in a model in which, like the natural disease process, autolysis and spontaneous reperfusion were capable of affecting outcome (as outlined above). In the other post-ischemic statin animal studies mentioned in the introduction (Sironi 2003, Prinz 2008, Elewa 2009), a treatment effect on this tendency was disallowed by the use of a non-biological occlusion, and in these studies outcome must have rested solely on neuroprotective or other effects on neuronal populations downstream. It may be that the main therapeutic effects of statins are neuronal or possibly microvascular, and in our study, a modest neuroprotective effect of simvastatin downstream may have been obscured by the more profound effects of variable spontaneous recanalization upstream.
Another possibility underlying our results may rest with unknown effects on the bioavailability of the simvastatin based on its preparation and route of administration. The bioavailability simvastatin we gave may have been affected by the intraperitoneal route of administration we used in the study. Prinz et al found that, in order to have an effect on outcome in experimental stroke, post-ischemic rosuvastatin had to be given much earlier and at significantly higher doses when given intraperitoneally than when given intravenously (Prinz 2008). We might have found a therapeutic effect had the simvastatin been given intravenously, although Sugawara found that a single 20mg/kg IP dose reduced vasospasm from subarachnoid hemorrhage (Sugawara 2008), and Lapchak found that it reduced post-thrombolysis intracranial hemorrhage (Lapchak & Han 2009), which suggests that, even though these endpoints are not comparable to those assessed in our study, there is pharmacological activity at this dose via this route. Lapchak also found that simvastatin given to rabbits at a dose of 20mg/kg subcutaneously within an hour of experimental ischemic stroke improved neurobehavioral outcome (Lapchak & Han 2010), further suggesting pharmacological activity at this dose, even though there are differences between rabbit and rodent models of stroke that may affect outcome in this regard (Lapchak & Han 2010). The other consideration involves how the simvastatin we used was prepared, in comparison to other studies showing an effect. Sironi chemically activated simvastatin by means of alkaline hydrolysis prior to subcutaneous administration (Sironi 2003), and Asahi prepared simvastatin in a PBS (pH 7.4) solution containing 10% ethanol and chemically activated it by alkaline hydrolysis prior to administration (Asahi 2005). The manner in which we prepared simvastatin for administration (dissolution in 100% DMSO without alkaline hydrolysis activation) may have had an effect its pharmacological activity. However, Sugawara administered un-activated simvastatin in a 10% ethanol solution and found a cerebrovascular effect (Sugawara 2008), and Lapchak found that un-activated simvastatin administered subcutaneously in a dose and formulation identical to ours had therapeutic effects in a rabbit model of ischemic stroke (Lapchak & Han 2009, Lapchak & Han 2010), even though the effects on endpoints in a rabbit may be slightly different than those seen in a rodent model (Lapchak & Han 2010). There is a significant amount of data that reveals that un-activated simvastatin has a pharmacological effect when administered to rodents via an intravenous or intraperitoneal route, whether dissolved in 10% ethanol and 0.9% NaCL (Merx 2004, Merx 2005, Almeida 2008), saline alone (Giusti-Paiva 2004), or in DMSO (Wayman 2003, Nesić 2006, Erkkilä 2005, Bitto 2008, Slotta 2009). Even though it is a consideration in this regard, the fact that we did not activate simvastatin by alkaline hydrolysis is by no means a definitive cause of the lack of activity we noted.
Other considerations underlying our findings may involve the dose and timing of administration. The dose we used in this study was significantly higher on a per-kilogram basis than that currently being used in humans; it was equivalent to giving a 1000 – 1500 mg dose in a standard-sized adult, over an order-of-magnitude higher than the pharmacologically active 5 to 80 mg dose recommended in the package insert from Merck (ZOCOR®(simvastatin) product sheet, accessed from URL http://www.merck.com/product/usa/pi_circulars/z/zocor/zocor_pi.pdf). It is possible, however, that statins have an inverted U-shaped curve for neuroprotection and/or thrombomodulation, with benefit only seen at moderate doses. It may even be possible that the dose we used had undetected toxic effects. In the NeuSTART trial, a dose-finding study using an adaptive design to evaluate doses of lovastatin at 1, 3, 6, 8 and 10 mg/kg/day given orally for 3 days after acute ischemic stroke in humans, increasing doses were associated with elevated liver enzymes and dose-related decreases in blood pressure, and the final model-based toxicity was established at 8 mg/kg (Elkind 2009). In our study we used a different statin given by a different route in a rodent model of stroke, and did not measure blood pressure or liver function tests, and so we cannot be sure if comparable effects were at play. It is also possible that the major determinant of effect may be timing of administration, as opposed to dosage, and our results might have been different had we administered simvastatin at a later time-point, or in repeated doses. Sironi, for example found a therapeutic effect when a statin was administered for 3 consecutive days after permanent MCAO (Sironi 2003). Sugiura found that a statin administered 2 days after transient MCAO diminished the extent of infarct expansion seen days later (Sugiura 2007). Additional studies evaluating the timing of post-ischemic administration and dose-response curves would be needed to answer the dose and timing questions. Finally, the therapeutic efficacy of simvastatin may just be less robust on administration as a single dose post-ischemically than it is given in a more prolonged prophylactic regimen pre-ischemically.
In conclusion, post-ischemic administration of simvastatin did not have an effect on acute experimental thrombembolic stroke in a model with characteristics reflective of the clinical presentation of the disease in humans. The administration of tPA resulted in notable positive effects in all of the acute therapy endpoints studied. The model demonstrated baseline clinical characteristics and a response to standard thrombolytic therapy that were reflective of that seen in humans, and is a clinically relevant tool by which to assess potential therapies with pleitropic effects. More research needs to be performed to elucidate the role for a hyperacute post-ischemic administration of statins in stroke, and determine if there is an efficacious route of administration, timing, and dosing regimen associated with it. Additional experiments evaluating dose-response curves for statins may be in order.
4. Experimental Procedure
All study procedures were approved by the Institutional Use and Care of Animals Committee of the University of California, San Diego, and were performed in accordance with accepted standards of animal care. We used male Fisher 344 rats weighing approximately 300 – 350 grams, obtained from Harlan Sprague Dawley, and allowed them to acclimatize, assume normal sleep wake cycles, and feed ad libitum prior to procedures.
4.1. Preparation of non-autologous clots
Prior to the planned embolization, a donor rat was placed under general inhalational anesthesia using an admixture of isoflurane and 2 Liters/minute of oxygen. Anesthesia induction was carried out with 5% isoflurane, and maintained with 2.5% isoflurane. After sterile preparation of the right neck, approximately 1 cc of venous blood was obtained via transcutaneous venipuncture of the right internal jugular vein with a 23-Guage needle and syringe. The blood was immediately injected into a length of polyethylene catheter (PE-50) so as to fill the lumen completely. This filled catheter was allowed to sit for approximately one minute on a level surface, so as to ensure uniform initial stabilization of clot along the length of the catheter, and was then placed in a 37°C water bath, and allowed to incubate at 37°C from anywhere from one to six hours. At the end of this time period, and immediately prior to use, clot was cut into 40 mm segments, and each segment was “loaded” into a specially modified clot-delivery catheter fashioned out of regular PE-50 tubing, pre-filled with a sterile, pH-neutral, physiological electrolyte solution (Plasmalyte™, Baxter Health Care Corporation, Deerfield IL). At the time of actual embolization, clots were between approximately 2 and 8 hours in age.
4.2. Surgical Technique and Embolization
A rat was placed under general anesthesia with isoflurane as performed above. A rectal temperature probe was placed, and the animal kept euthermic (37°C ± 1°C) using heating pads during the ensuing procedure. After a shave and sterile prep, a midline neck incision was made, and when venous access for treatment infusions was needed, the left external jugular (EJ) vein was exposed and cannulated with a polyethylene catheter. The right internal carotid artery (ICA) was then cannulated with the clot delivery catheter in a manner comparable to that previously described by others (Zhang RL 1997, Zhang Z 1997). Briefly, the right common carotid artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA), as well as the right ptyerygopalatine artery were exposed, the ptyerygopalatine artery tied of with suture ligature, and the ECA tied off, divided, and used as a stump through which to gain access into the ICA. The clot delivery catheter was inserted into the ICA via the ECA, into a depth that approximated the origin of the middle cerebral artery (MCA) intracranially, and the clot was gently injected, together with 0.2 cc of Plasmalyte (so to ensure good seating of the clot). The catheter was then withdrawn, and the ECA arteriotomy closed completely with a suture ligature, maintaining patency of the ICA. The operative incision was then closed and the animal subsequently monitored to recovery off anesthesia.
4.3. Neurological deficit scoring
Upon full recovery from anesthesia (i.e. upon full normalization of righting reflexes, normalization of responses to auditory, visual and tactile stimuli on the normal side, normalization of motor function where unaffected by stroke, and being apparently clear of the discernable influences anesthesia and having an overall “fully awake” appearance – all of which typically occurred at the most about 30 minutes after being taken off anesthesia), and again at defined intervals (such as after treatment and at 24 hours), the degree of neurological deficit was assessed in each animal by an investigator completely blinded to treatment assignment, using a multi-element neurological deficit scoring (neuroscore) paradigm. The paradigm was a modification of that used by Nedelman (Nedelmann 2007). Nedelman described the neuroscore as an evolutionary development from prior work (Bedersen 1986, Longa 1989, Menzies 1992, Zausinger 2000) and found it correlated with infarct volume on hematoxylin and eosin staining. To determine neuroscore in our study, nine items were assessed and scored separately, and then totaled to derive the overall neuroscore: 1) the presence or absence of forelimb extension (in which the animal was suspended gently by the tail and observed for forelimb flexion), 2) the presence or absence instability to lateral push from right (in which the animal was pushed laterally on a graspable surface and assessed to instability or weakness), 3) the presence or absence of stereotyped torso twisting while suspended gently by the tail, 4) the presence or absence of a gait preference while walking a level surface, 5) the presence or absence whisker movements, 6) the normalcy of consciousness 7) hearing, 8) sensory (to left-sided touch), and 9) the presence or absence of a left-sided hemianopia (reaction to visual stimuli approaching from left). Each item was scored in a relatively binary fashion (present/absent, normal/abnormal) and assigned a score of 0 or 10 with 10 denoting an abnormal finding, and totaled to a maximum total neuroscore of 90, with a higher neuroscore denoting a more severe neurological deficit. Only animals that had a pre-treatment neurological deficit of 20 or above were included in the study.
4.4. Assessment of Clot Burden, TTC staining, and lesion volume
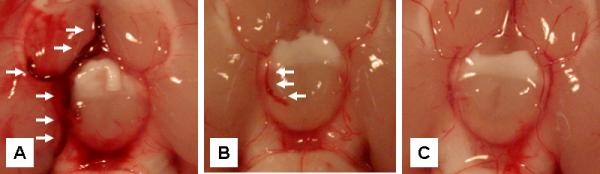
Twenty four hours after clot embolization, each rat was euthanized by transcardiac exsanguination of up to 10 mL of blood while under general inhalational anesthesia and decapitated using a surgical scalpel blade so as to minimize any pressure on the neck region that might result in a significantly supraphysiological rise in the intraluminal pressure of the intracranial vascular tree (thereby minimizing any risk of intravascular clot dislodgement prior to inspection of the vasculature). Each brain with intracranial vessels was removed en-bloc and its ventral surface and circle of Willis examined under a dissecting microscope. The presence or absence, conformation, and total length of residual intravascular clot were noted, and the lengths of all segments of clot measured and totaled. Model development had revealed that at times there will be some residual thrombotic material, which represented residual clot through which the vessel had spontaneously recanalized (see Figure 8 for a typical representation). This type of residual thrombotic material, found in two forms - a partial residual clot or a residual thrombotic “stain” inside the vessel, could obviously not be given the same weight as a solid occlusive clot in an analysis of clot burden, but could also not be completely discounted given the scientific premise of the study. In order to account for these findings in the analysis, the measured length of any residual clot was arbitrarily divided by two (half-weighted), and the length of any residual stain was arbitrarily divided by three.
Figure 8.
The typical appearance of A) a clot in-situ in the middle cerebral artery and adjacent circle of Willis in an embolized animal (white arrows); B) a re-canalized vessel with residual thrombotic material (white arrows) in an embolized animal (see Methods); and C) an embolized animal without residual clot, for comparison.
The brain was then cut into 2mm sections and stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC, from Sigma, St Louis, MO, USA) so as to visualize ischemic lesion area. TTC is a colorless in solution that is reduced by enzymes of functioning mitochondria to yield a deep red formazan, such that infarcted areas of the brain, which do not convert TTC and remain unstained, can be appreciated visually (non-microscopically) (Park 1988, Hatfield 1991). Infarct areas demarcated with TTC correspond closely with those measured with other histological methods (Lundy 1986, Bederson 1986, Osborne 1987, Lin 1993). The TTC slices were then photographed with a digital camera, and imported into an image-analysis program (Image-J, a public domain image-processing program from the National Institutes of Health, http://rsb.info.nih.gov) for an assessment of lesion area. The total lesion volume was calculated by multiplying the lesion area determined on each slice by the slice thickness (2mm), and then adding these products over the total number of slices.
4.5. Model characterization; tissue-plasminogen activator experiment
In order to characterize the performance of the model in the context of a treatment known to have a favorable outcome in acute ischemic stroke, a study using tissue-plasminogen activator (tPA) was performed. Recombinant tPA (Activase® (Alteplase)) was purchased from Genetech Inc (South San Francisco, CA), in the form of a sterile, lyophilized powder, and reconstituted for use as 1mg/mL solution with biological-grade water as per manufacturer instructions. Clot preparation and then experimental procedures for surgery, infusion catheter insertion and clot embolization were carried out as outlined above. An hour after embolization (at which time all animals had full recovery from anesthesia) a neuroscore was performed on each animal as outlined above, and then each randomly received either 10mg/kg of tPA intravenously (with 20% given as an initial bolus, and the remainder (80%) given as an infusion over the remaining 30 minutes), or a comparable weight-based volume of control solution of Plasmalyte (with 20% given as an initial bolus, and the remainder (80%) given as an infusion over the remaining 30 minutes). This tPA dosing regimen was chosen based on prior studies evaluating tPA and experimental stroke (Tejima 2001, Zhang L 2006). An hour after the infusion was initiated (30 minutes after completion), each animal had a second, post-treatment neuroscore. At 24 hours, a third neuroscore was performed, and then clot burden and lesion volume assessments performed as outlined above.
4.6. Simvastatin experiment
A generous gift of pharmaceutical-grade (United States Pharmacopoeia) simvastatin was obtained from Chong Kun Dang Pharmaceutical Co. Ltd. (Seoul, South Korea), in the form of a lyophilized powder, and reconstituted for use as 100mg/mL solution with 100% HPLC grade dimethyl sulfoxide (DMSO, from Fisher Scientific). Clot preparation and then experimental procedures for surgery, infusion catheter insertion and clot embolization were carried out as outlined above. An hour after embolization (upon full recovery from anesthesia) a neuroscore was performed on each animal, after which each randomly received either simvastatin 20mg/kg intraperitoneally as a one-time dose or a comparable weight-based volume of vehicle solution. This simvastatin dose was chosen based on that used in other studies revealing a cerebrovascular effect when given post-ictally (Sironi 2003, Asahi 2005, Sugawara 2008, Lapchak & Han 2009). At 24 hours, a second neuroscore was performed, and then clot burden and lesion volume assessments performed as outlined above.
4.7. Statistical analyses
All embolization procedures and assessments (neuroscore, clot burden, lesion volume) were carried out by a single investigator (KZG) completely blinded to treatment assignment. Data was presented as means with standard deviations, and box-plotted as medians with interquartile ranges. Chi-square was used to proportionally analyze variables with categorical values. A non-parametric Mann-Whitney U or Wilcoxon Rank-Sum Test was used for numerical data that was not normally distributed. Analyses were performed with NCSS 2001 from Number Cruncher Statistical Systems (Kaysville, UT). Differences with a p-value < 0.05 were considered statistically significant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE REFERENCES
- Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59(6):862–867. doi: 10.1212/wnl.59.6.862. [DOI] [PubMed] [Google Scholar]
- Almeida JL, Sampietre SN, Mendonça Coelho AM, Trindade Molan NA, Machado MC, Monteiro da Cunha JE, Jukemura J. Statin pretreatment in experimental acute pancreatitis. JOP. 2008;9:431–439. [PubMed] [Google Scholar]
- Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, Moskowitz MA. Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke. 2001;32:980–986. doi: 10.1161/01.str.32.4.980. [DOI] [PubMed] [Google Scholar]
- Asahi M, Huang Z, Thomas S, Yoshimura S, Sumii T, Mori T, Qiu J, Amin-Hanjani S, Huang PL, Liao JK, Lo EH, Moskowitz MA. Protective effects of statins involving both eNOS and tPA in focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:722–729. doi: 10.1038/sj.jcbfm.9600070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA, Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- Asplund K. Haemodilution for acute ischaemic stroke. Cochrane Database Syst Rev. 2002;(4):CD000103. doi: 10.1002/14651858.CD000103. [DOI] [PubMed] [Google Scholar]
- Balduini W, Mazzoni E, Carloni S, De Simoni MG, Perego C, Sironi L, Cimino M. Prophylactic but not delayed administration of simvastatin protects against long-lasting cognitive and morphological consequences of neonatal hypoxic-ischemic brain injury, reduces interleukin-1beta and tumor necrosis factor-alpha mRNA induction, and does not affect endothelial nitric oxide synthase expression. Stroke. 2003;34:2007–2012. doi: 10.1161/01.STR.0000080677.24419.88. [DOI] [PubMed] [Google Scholar]
- Baracchini C, Manara R, Ermani M, Meneghetti G. The quest for early predictors of stroke evolution: can TCD be a guiding light? Stroke. 2000;31:2942–2947. doi: 10.1161/01.str.31.12.2942. [DOI] [PubMed] [Google Scholar]
- Barber PA, Davis SM, Infeld B, Baird AE, Donnan GA, Jolley D, Lichtenstein M. Spontaneous reperfusion after ischemic stroke is associated with improved outcome. Stroke. 1998;29:2522–2528. doi: 10.1161/01.str.29.12.2522. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski HM. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Bitto A, Minutoli L, Altavilla D, Polito F, Fiumara T, Marini H, Galeano M, Calò M., Lo Cascio P, Bonaiuto M, Migliorato A, Caputi AP, Squadrito F. Simvastatin enhances VEGF production and ameliorates impaired wound healing in experimental diabetes. Pharmacol Res. 2008;57:159–169. doi: 10.1016/j.phrs.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Bowler JV, Wade JP, Jones BE, Nijran KS, Steiner TJ. Natural history of the spontaneous reperfusion of human cerebral infarcts as assessed by 99mTc HMPAO SPECT. J Neurol Neurosurg Psychiatry. 1998;64:90–97. doi: 10.1136/jnnp.64.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino M, Balduini W, Carloni S, Gelosa P, Guerrini U, Tremoli E, Sironi L. Neuroprotective effect of simvastatin in stroke: a comparison between adult and neonatal rat models of cerebral ischemia. Neurotoxicology. 2005;26:929–933. doi: 10.1016/j.neuro.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Dangas G, Badimon JJ, Smith DA, Unger AH, Levine D, Shao JH, Meraj P, Fier C, Fallon JT, Ambrose JA. Pravastatin therapy in hyperlipidemia: effects on thrombus formation and the systemic hemostatic profile. J Am Coll Cardiol. 1999;33:1294–1304. doi: 10.1016/s0735-1097(99)00018-2. [DOI] [PubMed] [Google Scholar]
- Elewa HF, Kozak A, El-Remessy AB, Frye RF, Johnson MH, Ergul A, Fagan SC. Early atorvastatin reduces hemorrhage after acute cerebral ischemia in diabetic rats. J Pharmacol Exp Ther. 2009;330:532–540. doi: 10.1124/jpet.108.146951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind MS, Sacco RL, Macarthur RB, Peerschke E, Neils G, Andrews H, Stillman J, Corporan T, Leifer D, Liu R, Cheung K. High-dose lovastatin for acute ischemic stroke: results of the phase I dose escalation neuroprotection with statin therapy for acute recovery trial (NeuSTART) Cerebrovasc Dis. 2009;28:266–275. doi: 10.1159/000228709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1998 Jul 21;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M. Statins and stroke. J Cereb Blood Flow Metab. 2005 Sep;25:1093–1110. doi: 10.1038/sj.jcbfm.9600116. [DOI] [PubMed] [Google Scholar]
- Erkkilä L, Jauhiainen M, Laitinen K, Haasio K, Tiirola T, Saikku P, Leinonen M. Effect of simvastatin, an established lipid-lowering drug, on pulmonary Chlamydia pneumoniae infection in mice. Antimicrob Agents Chemother. 2005;49:3959–3962. doi: 10.1128/AAC.49.9.3959-3962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essig M, Nguyen G, Prie D, Escoubet B, Sraer JD, Friedlander G. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors increase fibrinolytic activity in rat aortic endothelial cells. Circ Res. 1998;83:683–689. doi: 10.1161/01.res.83.7.683. [DOI] [PubMed] [Google Scholar]
- Fuentes B, Martínez-Sánchez P, Díez-Tejedor E. Lipid-lowering drugs in ischemic stroke prevention and their influence on acute stroke outcome. Cerebrovasc Dis. 2009;27:126–133. doi: 10.1159/000200450. [DOI] [PubMed] [Google Scholar]
- Goldstein LB, Suarez JI. The quest for the “Holy Grail” of ischemic stroke cytoprotection: Statins may not be the answer. Neurology. 2009;73:2058–9. doi: 10.1212/WNL.0b013e3181c6785c. [DOI] [PubMed] [Google Scholar]
- Hatfield RH, Mendelow AD, Perry RH, Alvarez LM, Modha P. Triphenyltetrazolium chloride (TTC) as a marker for ischaemic changes in rat brain following permanent middle cerebral artery occlusion. Neuropathol Appl Neurobiol. 1991;17:61–67. doi: 10.1111/j.1365-2990.1991.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Han MK. The 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor simvastatin reduces thrombolytic-induced intracerebral hemorrhage in embolized rabbits. Brain Res. 2009;1303:144–150. doi: 10.1016/j.brainres.2009.09.064. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Han MK. Simvastatin Improves Clinical Scores In A Rabbit Multiple Infarct Ischemic Stroke Model: Synergism With A ROCK Inhibitor, But Not The Thrombolytic Tissue Plasminogen Activator. Brain Res. 2010 doi: 10.1016/j.brainres.2010.05.035. [Epub ahead of print] In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs U, Gertz K, Huang P, Nickenig G, Böhm M, Dirnagl U, Endres M. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000;31:2442–2449. doi: 10.1161/01.str.31.10.2442. [DOI] [PubMed] [Google Scholar]
- Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lundy EF, Solik BS, Frank RS, Lacy PS, Combs DJ, Zelenock GB, D'Alecy LG. Morphometric evaluation of brain infarcts in rats and gerbils. J Pharmacol Methods. 1986;16:201–214. doi: 10.1016/0160-5402(86)90042-2. [DOI] [PubMed] [Google Scholar]
- Menzies SA, Hoff JT, Betz AL. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery. 1992;31:100–6. doi: 10.1227/00006123-199207000-00014. discussion 106–107. [DOI] [PubMed] [Google Scholar]
- Merx MW, Liehn EA, Janssens U, Lütticken R, Schrader J, Hanrath P, Weber C. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation. 2004;109:2560–2565. doi: 10.1161/01.CIR.0000129774.09737.5B. [DOI] [PubMed] [Google Scholar]
- Merx MW, Liehn EA, Graf J, van de Sandt A, Schaltenbrand M, Schrader J, Hanrath P, Weber C. Statin treatment after onset of sepsis in a murine model improves survival. Circulation. 2005;112:117–124. doi: 10.1161/CIRCULATIONAHA.104.502195. [DOI] [PubMed] [Google Scholar]
- Milionis HJ, Giannopoulos S, Kosmidou M, Panoulas V, Manios E, Kyritsis AP, Elisaf MS, Vemmos K. Statin therapy after first stroke reduces 10-year stroke recurrence and improves survival. Neurology. 2009;72:1816–1822. doi: 10.1212/WNL.0b013e3181a711cb. [DOI] [PubMed] [Google Scholar]
- Montaner J, Chacón P, Krupinski J, Rubio F, Millán M, Molina CA, Hereu P, Quintana M, Alvarez-Sabín J. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J Neurol. 2008;15:82–90. doi: 10.1111/j.1468-1331.2007.02015.x. [DOI] [PubMed] [Google Scholar]
- Nedelmann M, Wilhelm-Schwenkmezger T, Alessandri B, Heimann A, Schneider F, Eicke BM, Dieterich M, Kempski O. Cerebral embolic ischemia in rats: correlation of stroke severity and functional deficit as important outcome parameter. Brain Res. 2007;1130:188–196. doi: 10.1016/j.brainres.2006.10.087. [DOI] [PubMed] [Google Scholar]
- Nesić Z, Todorović Z, Stojanović R, Basta-Jovanović G, Radojević-Skodrić S, Velicković R, Chatterjee PK, Thiemermann C, Prostran M. Single-dose intravenous simvastatin treatment attenuates renal injury in an experimental model of ischemia-reperfusion in the rat. J Pharmacol Sci. 2006;102:413–417. doi: 10.1254/jphs.sce06002x. [DOI] [PubMed] [Google Scholar]
- Niessen F, Hilger T, Hoehn M, Hossmann KA. Differences in clot preparation determine outcome of recombinant tissue plasminogen activator treatment in experimental thromboembolic stroke. Stroke. 2003;34:2019–2024. doi: 10.1161/01.STR.0000080941.73934.30. [DOI] [PubMed] [Google Scholar]
- Multicenter trial of hemodilution in acute ischemic stroke. I. Results in the total patient population. Scandinavian Stroke Study Group. Stroke. 1987;18:691–699. doi: 10.1161/01.str.18.4.691. (No author) [DOI] [PubMed] [Google Scholar]
- Osborne KA, Shigeno T, Balarsky AM, Ford I, McCulloch J, Teasdale GM, Graham DI. Quantitative assessment of early brain damage in a rat model of focal cerebral ischaemia. J Neurol Neurosurg Psychiatry. 1987;50:402–410. doi: 10.1136/jnnp.50.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CK, Mendelow AD, Graham DI, McCulloch J, Teasdale GM. Correlation of triphenyltetrazolium chloride perfusion staining with conventional neurohistology in the detection of early brain ischaemia. Neuropathol Appl Neurobiol. 1988;14:289–298. doi: 10.1111/j.1365-2990.1988.tb00889.x. [DOI] [PubMed] [Google Scholar]
- Paczynski RP, Venkatesan R, Diringer MN, He YY, Hsu CY, Lin W. Effects of fluid management on edema volume and midline shift in a rat model of ischemic stroke. Stroke. 2000;31:1702–1708. doi: 10.1161/01.str.31.7.1702. [DOI] [PubMed] [Google Scholar]
- Prinz V, Laufs U, Gertz K, Kronenberg G, Balkaya M, Leithner C, Lindauer U, Endres M. Intravenous rosuvastatin for acute stroke treatment: an animal study. Stroke. 2008;39:433–438. doi: 10.1161/STROKEAHA.107.492470. [DOI] [PubMed] [Google Scholar]
- Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- Toni D, Fiorelli M, Zanette EM, Sacchetti ML, Salerno A, Argentino C, Solaro M, Fieschi C. Early spontaneous improvement and deterioration of ischemic stroke patients. A serial study with transcranial Doppler ultrasonography. Stroke. 1998;29:1144–1148. doi: 10.1161/01.str.29.6.1144. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T, American Heart Association. American Stroke Association Council on Stroke. Council on Cardiovascular Radiology and Intervention. American Academy of Neurology Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- Shabanzadeh AP, Shuaib A, Wang CX. Simvastatin reduced ischemic brain injury and perfusion deficits in an embolic model of stroke. Brain Res. 2005;1042:1–5. doi: 10.1016/j.brainres.2005.01.105. [DOI] [PubMed] [Google Scholar]
- Slotta JE, Laschke MW, Wang Y, Schilling MK, Menger MD, Thorlacius H. Inhibition of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase reduces leukocyte recruitment and hepatocyte apoptosis in endotoxin-induced liver injury. Investig Med. 2009;57:645–649. doi: 10.2310/JIM.0b013e3181a23cb0. [DOI] [PubMed] [Google Scholar]
- Sugiura S, Yagita Y, Sasaki T, Todo K, Terasaki Y, Ohyama N, Hori M, Kitagawa K. Postischemic administration of HMG CoA reductase inhibitor inhibits infarct expansion after transient middle cerebral artery occlusion. Brain Res. 2007;1181:125–129. doi: 10.1016/j.brainres.2007.08.069. [DOI] [PubMed] [Google Scholar]
- Sironi L, Cimino M, Guerrini U, Calvio AM, Lodetti B, Asdente M, Balduini W, Paoletti R, Tremoli E. Treatment with statins after induction of focal ischemia in rats reduces the extent of brain damage. Arterioscler Thromb Vasc Biol. 2003;23:322–327. doi: 10.1161/01.atv.0000044458.23905.3b. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Ayer R, Jadhav V, Chen W, Tsubokawa T, Zhang JH. Simvastatin attenuation of cerebral vasospasm after subarachnoid hemorrhage in rats via increased phosphorylation of Akt and endothelial nitric oxide synthase. J Neurosci Res. 2008;86:3635–3643. doi: 10.1002/jnr.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejima E, Katayama Y, Suzuki Y, Kano T, Lo EH. Hemorrhagic transformation after fibrinolysis with tissue plasminogen activator: evaluation of role of hypertension with rat thromboembolic stroke model. Stroke. 2001;32:1336–1340. doi: 10.1161/01.str.32.6.1336. [DOI] [PubMed] [Google Scholar]
- von Kummer R, Back T, Scharf J, Hacke W. Stroke, hemodilution, and mortality. Stroke. 1989;20:1286–1287. doi: 10.1161/01.str.20.9.1286. [DOI] [PubMed] [Google Scholar]
- Wayman NS, Ellis BL, Thiemermann C. Simvastatin reduces infarct size in a model of acute myocardial ischemia and reperfusion in the rat. Med Sci Monit. 2003;9:BR155–159. [PubMed] [Google Scholar]
- Yrjänheikki J, Koistinaho J, Kettunen M, Kauppinen RA, Appel K, Hüll M, Fiebich BL. Long-term protective effect of atorvastatin in permanent focal cerebral ischemia. Brain Res. 2005;1052:174–179. doi: 10.1016/j.brainres.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Zausinger S, Hungerhuber E, Baethmann A, Reulen H, Schmid-Elsaesser R. Neurological impairment in rats after transient middle cerebral artery occlusion: a comparative study under various treatment paradigms. Brain Res. 2000;863:94–105. doi: 10.1016/s0006-8993(00)02100-4. [DOI] [PubMed] [Google Scholar]
- Zanette EM, Roberti C, Mancini G, Pozzilli C, Bragoni M, Toni D. Spontaneous middle cerebral artery reperfusion in ischemic stroke. A follow-up study with transcranial Doppler. Stroke. 1995;26:430–433. doi: 10.1161/01.str.26.3.430. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Liu X, Hozeska A, Stagliano N, Riordan W, Lu M, Chopp M. Treatment of embolic stroke in rats with bortezomib and recombinant human tissue plasminogen activator. Thromb Haemost. 2006;95:166–173. [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang RL, Jiang Q, Raman SBK, Cantwell L, Chopp M. A new rat model of thrombotic focal cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:123–135. doi: 10.1097/00004647-199702000-00001. [DOI] [PubMed] [Google Scholar]