Abstract
Objective
To investigate the efficacy, potential limitations, and biological mechanisms of UV-A1 phototherapy for skin sclerosis due to collagen deposition disorders.
Design
Before-and-after trial of UV-A1 irradiation of sclerotic skin; in vivo biochemical analyses after UV-A1 irradiation of normal skin.
Setting
Academic referral center.
Participants
Patients with morphea/scleroderma or sclerodermoid graft-vs-host disease and volunteers without skin disease.
Intervention
Sclerotic skin was treated with high-dose (130 J/cm2; n=12) or medium-dose (65 J/cm2; n=6) UV-A1 phototherapy 3 times per week for 14 weeks; normal skin was treated with UV-A1 irradiation at various doses and frequencies, with biopsies performed afterwards.
Main Outcome Measures
In sclerotic skin, induration was clinically assessed using a scoring scale. In normal skin, quantitative polymerase chain reaction was used to assess antifibrotic responses, defined as decreased type I and type III procollagen and increased matrix metalloproteinase levels.
Results
In patients with sclerotic skin treated with high-dose UV-A1 irradiation, clinical scores for induration modestly decreased. To investigate what factors prevented further improvement (ie, complete clearance), normal skin with light pigmentation was exposed to UV-A1 irradiation (70–150 J/cm2) and was assessed for antifibrotic responses. A single high-dose exposure (110–150 J/cm2) elicited substantial antifibrotic responses and induced skin darkening. This skin darkening attenuated responses to subsequent UV-A1 exposures and was dose dependent. Thus, to minimize skin darkening, additional patients with sclerotic skin were treated with medium-dose UV-A1 phototherapy, which was no less effective than high-dose therapy.
Conclusion
Clinical responses of sclerotic skin to UV-A1 phototherapy were modest because of UV-A1–induced skin darkening, which is photoprotective and attenuates antifibrotic responses.
Scleroderma is a connective tissue disorder characterized by skin thickening (fibrosis). 1,2 Affected skin may appear inflamed initially, but it ultimately becomes bound down, hard, and devoid of inflammation. Sclerodermoid graft-vs-host disease (GVHD) is also characterized by skin fibrosis and occurs as a late consequence of allogeneic hematopoietic stem cell transplantation.3 Topical and systemic treatments, some with dangerous adverse effects, have had limited efficacy for scleroderma and sclerodermoid GVHD.4,5
The pathogenesis of sclerotic skin diseases is not well understood but may involve environmental exposures and abnormal immunity.1,3,4,6 The end result is skin fibrosis secondary to increased collagen deposition in the extracellular matrix of the dermis, the layer of skin below the outer epidermis. 1,4 Indeed, dermal fibroblasts in sclerotic skin demonstrate increased synthesis of type I and type III collagen.7 In addition, a contributing factor may be decreased levels of matrix metalloproteinases (MMPs),8 which degrade collagen in the skin.
UV irradiation promotes degradation of structural components of the dermis.4,9 For example, exposure to UV-A1 (340 to 400-nm) irradiation robustly induces MMP-1 (collagenase 1) in normal human skin and inhibits synthesis of type I and type III collagen in dermal fibroblasts.10–12 Because of its longer wavelengths, UV-A1 irradiation also is less erythemogenic and penetrates deeper into the dermis than UV-A2 (320 to 340-nm) or UV-B (290 to 320-nm) irradiation.13
Based on these observations, investigators have used UV-A1 irradiation to treat sclerotic skin. Since 1995, approximately 20 studies have examined the efficacy of UV-A1 irradiation for localized scleroderma (morphea), systemic scleroderma (which can affect internal organs), and sclerodermoid GVHD.3,4,13 Despite varying phototherapy regimens, these studies reported clinical improvement in most patients. Several studies3,14–22 have reported dramatic improvement or complete clearance of lesions.
In this study, we initially treated sclerotic skin with 130 J/cm2 of UV-A1 irradiation, referred to as “high dose.” However, clinical improvement in these patients was more modest than in published studies.21 Because of the difficulty of obtaining biopsy samples from sclerotic skin, which often heals poorly, we undertook a series of studies in normal skin to better understand UV-A1–induced antifibrotic responses. We measured messenger RNA (mRNA) expression of MMP-1, MMP-3 (stromelysin 1), and MMP-9 (gelatinase B) after UV-A1 irradiation. These proteases are collectively capable of degrading the entire dermal extracellular matrix, and their gene expression correlates with protein expression and enzymatic activity. 11 Because collagen is initially synthesized as a soluble precursor (procollagen), we also measured mRNA expression of type I and type III procollagen. We then correlated MMP and procollagen changes with skin pigmentation. Based on our data, we conclude that a major limitation of UV-A1 phototherapy is skin darkening.
METHODS
PARTICIPANTS
Studies were approved by the University of Michigan institutional review board. Participants provided written informed consent. For patients with scleroderma/GVHD, age younger than 10 years, pregnancy or lactation, history of photosensitivity, topical corticosteroid use within 2 weeks, and use of systemic immunosuppressive medications were exclusionary. Because some patients with GVHD required immunosuppressive drugs to maintain transplant engraftment, these patients were included if such medications had not previously improved their lesions. For subjects with normal skin, age younger than 18 years, use of systemic corticosteroids within 4 weeks or nonsteroidal anti-inflammatory drugs within 2 weeks, and history of photosensitivity were exclusionary. To rule out photosensitivity, provocation testing (60, 100, and 130 J/cm2 of UV-A1) was performed on the buttock skin of each subject before enrollment.
CLINICAL STUDIES
Studies were open clinical trials conducted in the Program for Clinical Research in the Department of Dermatology at the University of Michigan Medical School. For studies on sclerotic skin, 1 or 2 physicians (L.A.G., S.C., R.K., or S.K.) examined each patient at weeks 0, 1, 2, 4, 6, 10, and 14 of UV-A1 phototherapy.
UV-A1 PHOTOTHERAPY
In patients with sclerotic skin, irradiation was administered using a full-bed device (output, 340–420 nm; peak, 380 nm) (Sellamed 24 000; Sellas Medizinische Geräte GmbH, Gevelsberg-Vogelsang, Germany). Bedding was used to shield skin that was not being treated. In subjects with normal skin, buttock skin was irradiated using a portable unit (Sellamed 2000; Sellas Medizinische Geräte GmbH). Skin was shielded with aluminum foil templates (University of Michigan Printing Services) that allowed irradiation to 2.5-cm2 areas of skin surface. Irradiance was verified by using a spectroradiometer (Sola-Scope 2000; Solatell Ltd, Croydon, England) and by an independent contractor (Rapid Precision Testing Laboratory, Cordova, Tennessee). No topical substances besides emollients were allowed during therapy.
CHROMAMETER
Pigmentation was measured using a chromameter (Minolta CR200; Minolta, Osaka, Japan) under the L* variable (luminescence), which ranges from 0 (pure black) to 100 (pure white).23 For each measurement, 3 readings were averaged. Pigmentation was considered light (L*>65), medium (L*=55–65), or dark (L*<55) based on a scale developed by 1 of the authors (S.K.).
GENE EXPRESSION
Biopsy samples (4–6 mm) were collected after injecting local anesthesia (lidocaine). After mRNA extraction, specific transcripts were quantified by real-time polymerase chain reaction, as described elsewhere.24 The housekeeping gene acidic ribosomal phosphoprotein P0 (36B4) was used as an internal control.
STATISTICAL ANALYSES
Clinical and biochemical end points were compared across dosing regimens using the repeated-measures analysis of variance procedure. Specific comparisons were made using the Dunnett test when comparing treatment groups with a control, the Tukey studentized range test when making all pairwise comparisons, and the paired t test when comparing only 2 groups. When appropriate, logarithmic transformations of the data were performed to achieve normality. Summary data are given as mean (SEM). Comparisons were significant if P<.05 for a 2-tailed hypothesis. Data were analyzed using SAS statistical software version 9.1 (SAS Institute Inc, Cary, North Carolina).
RESULTS
HIGH-DOSE UV-A1 IRRADIATION IMPROVES SCLEROTIC SKIN MODESTLY AND INCREASES SKIN PIGMENTATION SUBSTANTIALLY
High-dose UV-A1 irradiation (130 J/cm2) is reported to be more effective for treating sclerotic skin than low-dose UV-A1 phototherapy (20 J/cm2).21 Therefore, we administered high-dose UV-A1 phototherapy (130 J/cm2) to 12 patients with sclerotic skin (Table 1). Patients were treated 3 times per week for 14 weeks.
Table 1.
Clinical Information for Patients Treated With High-Dose UV-A1 Irradiation (130 J/cm2) 3 Times per Week for 14 Weeksa
| Lesional Induration (Scale, 0–9) |
Lesional Pigmentation (L* Value/Color) |
||||||
|---|---|---|---|---|---|---|---|
| Patient No./ Sex/Age, y |
Diagnosis | Disease Duration, y |
Treatment Location |
Before Therapy |
After Therapy |
Before Therapy |
After Therapy |
| 1/M/43 | Systemic scleroderma | 15 | Left leg | 7 | 3 | 59.4/medium | 49.1/dark |
| 2/F/15 | Scleroderma | 0.75 | Left leg | 8 | 5 | 58.1/medium | 40.3/dark |
| 3/F/74 | Acral scleroderma | 1 | Right middle finger | 7 | 4 | 62.5/medium | 46.1/dark |
| 4/F/42 | Systemic scleroderma | 18 | Left hand | 5 | 4 | 58.2/medium | 49.3/dark |
| 5/M/69 | Acral scleroderma | 28 | Right hand | 8 | 6 | 58.6/medium | 49.3/dark |
| 6/F/66 | Acral scleroderma | 4 | Right hand | 8 | 8 | 52.9/dark | 46.7/dark |
| 7/F/51 | Systemic scleroderma | 5 | Left forearm | 8 | 8 | 63.6/medium | 56.7/medium |
| 8/F/38 | Systemic scleroderma | 5 | Right arm | 6 | 6 | 60.1/medium | 47.4/dark |
| 9/M/48 | Sclerodermoid GVHD | 1 | Left forearm | 6 | 5 | 60.8/medium | 41.7/dark |
| 10/M/65 | Sclerodermoid GVHD | 3 | Left forearm | 6 | 4 | 53.7/dark | 50.5/dark |
| 11/F/45 | Sclerodermoid GVHD | 3 | Left axilla | 6 | 4 | 45.1/dark | 35.6/dark |
| 12/F/65 | Sclerodermoid GVHD | 1 | Left abdomen | 6 | 1 | 50.2/dark | 45.2/dark |
Abbreviation: GVHD, graft-vs-host disease.
The mean patient age was 51.8 years, the mean disease duration was 7.1 years, the mean lesional induration before therapy was 6.8 (after therapy, 4.8), and the mean lesional pigmentation (L* value) before therapy was 56.9 (after therapy, 46.5).
For each patient, 1 circumscribed lesional area was treated. To assess clinical response, skin was palpated for induration (hardness) based on a scale adapted from Rodnan et al.7 Mild induration was categorized as 1 to 3, moderate as 4 to 6, and severe as 7 to 9. Before treatment, the mean (SEM) induration was 6.8 (0.3) (“low severe”) (Figure 1A). By 2 weeks of phototherapy, improvement occurred in 5 of 12 patients. Thereafter, induration scores steadily declined, with significant changes being apparent by 10 weeks (P<.05). At the end of treatment, the mean (SEM) scores were 4.8 (0.6) (“low moderate”) (P<.05). Clinically, target plaques showed modest softening. Improvement was seen in 9 of 12 patients (no improvement occurred in patients 6, 7, and 8). Based on earliest change in induration score of these 9 “responders,” the mean (SEM) time to first improvement was 6.6 (2.1) weeks (approximately 20 sessions). Clearance of lesions did not occur in any patient. Thus, we considered high-dose UV-A1 phototherapy to be moderately effective.
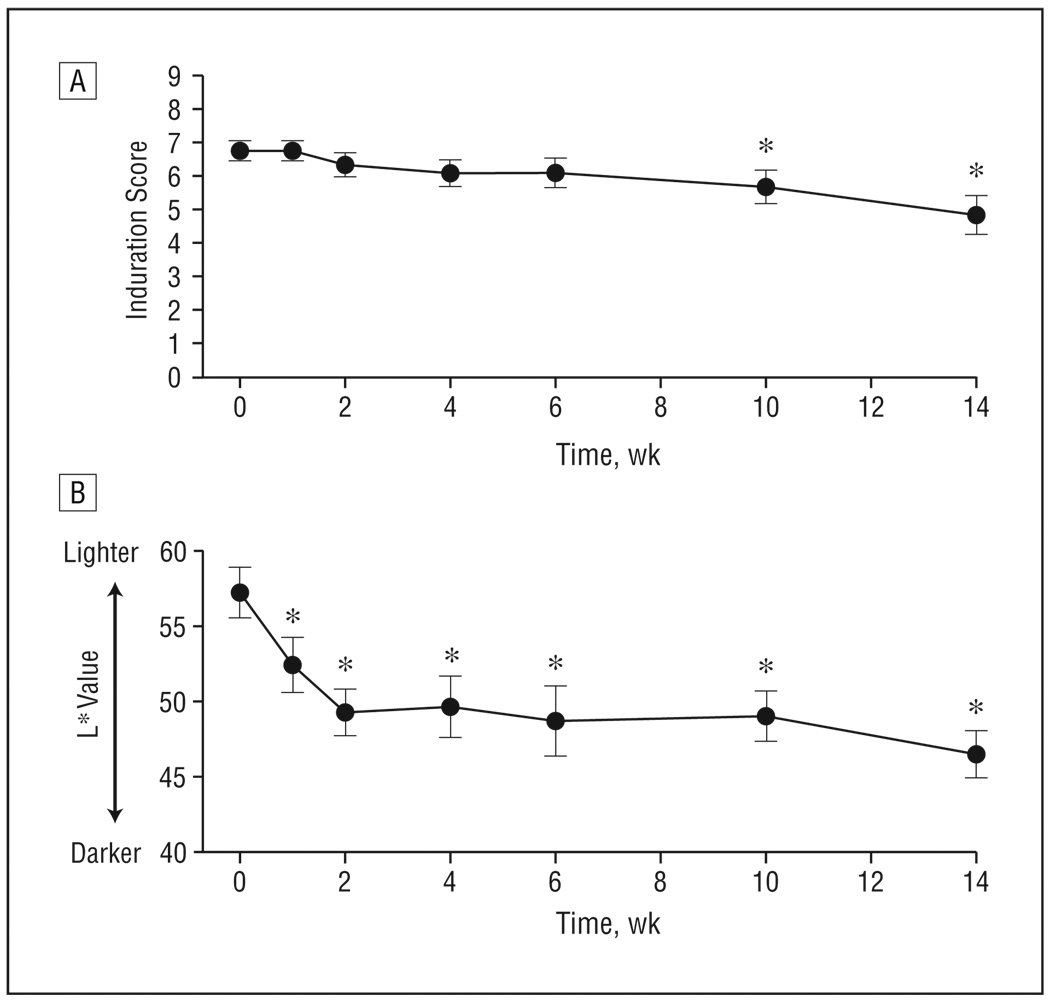
Figure 1.
Mean induration and pigmentation of sclerotic skin in 12 patients treated with high-dose UV-A1 irradiation (130 J/cm2) 3 times weekly. A, Induration was assessed on a scale from 1 (very mild) to 9 (very severe). B, Pigmentation of treated areas was assessed by using a chromameter (L* [luminescence] value). Error bars represent SEM. *P<.05 compared with before therapy (week 0).
All the patients completed therapy without major adverse events. The only adverse effect was darkening of skin color, which was assessed by using a chromameter (L* value).23 At baseline, patients had medium (L*=55–65; n=8) or dark (L*<55; n=4) pigmentation in target plaques, and the mean (SEM) L* value was 56.9 (1.6) (n=12) (Figure 1B). During UV-A1 treatment, target lesions in all the patients became darker (ie, L* values decreased). The greatest decrease in L* values occurred within 1 to 2 weeks of treatment (P<.05), corresponding to the most rapid tanning period. Thereafter, L* values declined gradually such that 11 of the 12 patients had darkly pigmented (L*<55) target lesions by the end of treatment (mean [SEM] L*=46.5 [1.6]; P<.05).
ANTIFIBROTIC RESPONSES GRADUALLY SUBSIDE AFTER A SINGLE HIGH-DOSE UV-A1 EXPOSURE IN LIGHTLY PIGMENTED NORMAL SKIN
Given the relatively modest improvement of patients with scleroderma/GVHD, we investigated what factors may affect response to UV-A1 irradiation. For these studies, we used real-time polymerase chain reaction to assess antifibrotic responses, defined as induction of MMP levels and reduction of procollagen levels. We first examined the duration of antifibrotic responses after a single high-dose UV-A1 exposure (130 J/cm2) in lightly pigmented (L*>65) normal buttock skin (n=8).
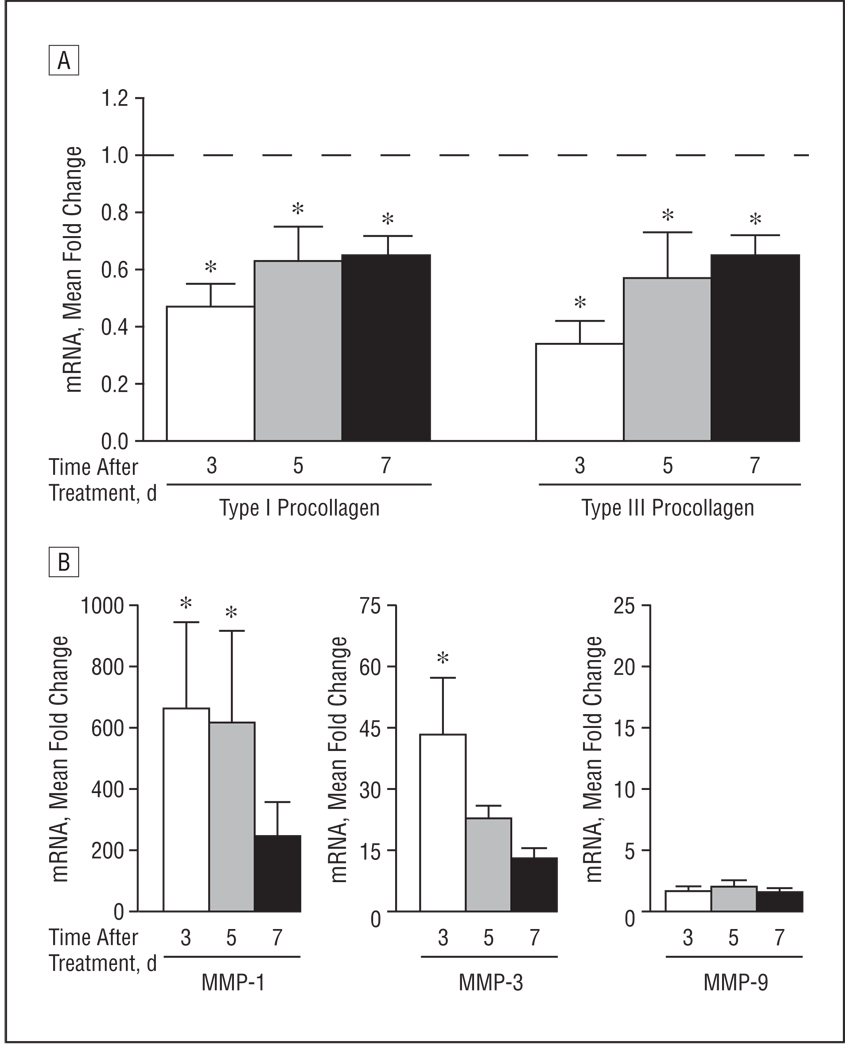
Three days after treatment, mRNA expression of type I and type III procollagen was reduced by 53% and 66%, respectively, relative to untreated skin (P<.05) (Figure 2A). At 5 and 7 days after treatment, expression of type I and type III procollagen mRNA remained significantly suppressed (P<.05). In addition, MMP-1 mRNA expression was significantly upregulated (P<.05) 3 and 5 days after treatment relative to control (Figure 2B). Matrix metalloproteinase 3 expression was induced significantly 3 days after exposure (P<.05). Matrix metalloproteinase 9 expression was not significantly upregulated at any time point (P>.05). These data indicate that a single UV-A1 exposure suppresses procollagen levels for at least 7 days while upregulating MMP-1 and MMP-3 for 3 to 5 days. Thus, antifibrotic responses gradually subside over the period of 1 week after a single UV-A1 exposure.
Figure 2.
Duration of antifibrotic responses after a single high-dose UV-A1 exposure. Eight healthy subjects with lightly pigmented skin (L* [luminescence] >65) were exposed once to high-dose UV-A1 irradiation (130 J/cm2). Biopsies were performed 3, 5, and 7 days later. Real-time polymerase chain reaction was used to assess expression for the indicated messenger RNA (mRNA) transcripts: type I and type III procollagen (A) and matrix metalloproteinases (MMPs) 1, 3, and 9 (B). Data are presented as mean fold change relative to untreated skin (normalized to 1). Error bars represent SEM. In A, the horizontal dashed line indicates fold change of untreated skin. *P<.05 compared with untreated skin.
ANTIFIBROTIC RESPONSES BECOME REFRACTORY TO MULTIPLE HIGH-DOSE UV-A1 EXPOSURES IN NORMAL SKIN
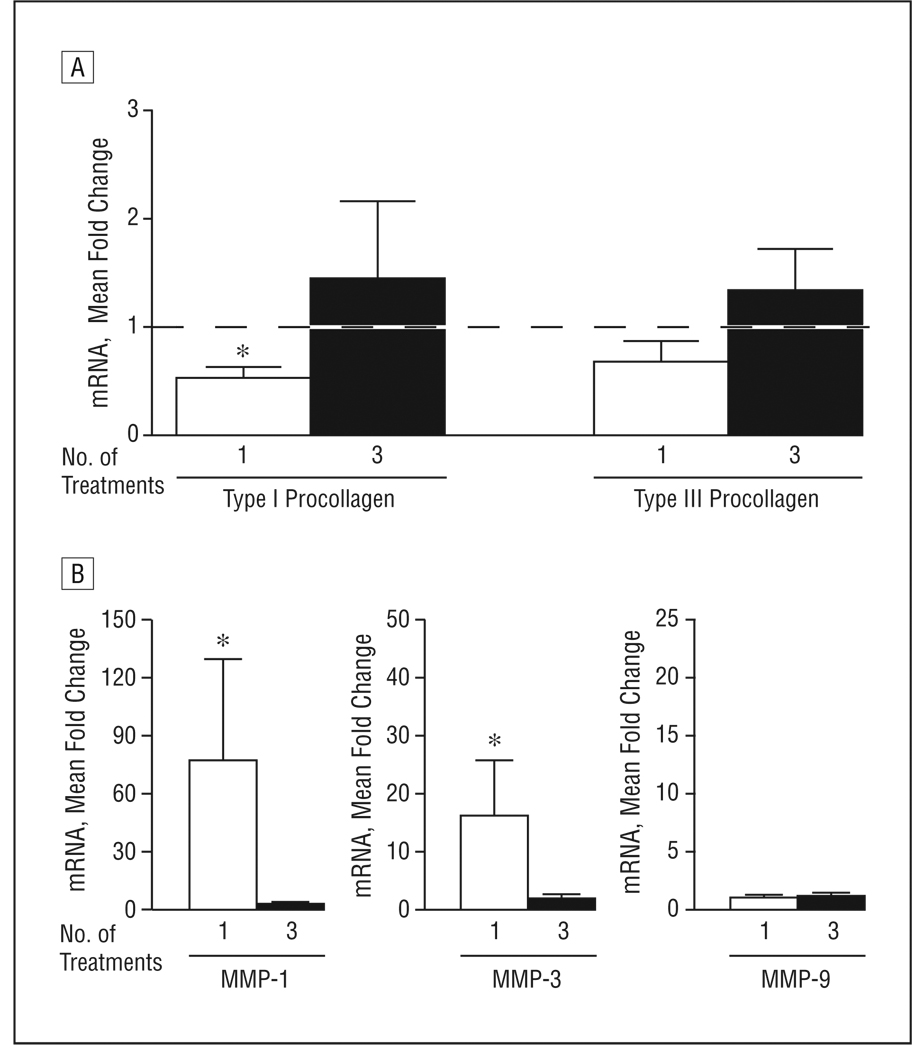
Next, we investigated the biochemical effects of repeated UV-A1 exposures. Eight patients with lightly pigmented (L*>65) normal buttock skin were exposed to high-dose UV-A1 irradiation (110 J/cm2) either 1 time (Friday) or 3 times (Monday, Wednesday, and Friday). A single UV-A1 exposure reduced type I procollagen mRNA expression relative to untreated skin (Figure 3A). However, after 3 exposures, no reduction in type I procollagen levels was observed. In addition, a single UV-A1 exposure significantly upregulated MMP-1 and MMP-3 relative to untreated skin (P<.05) (Figure 3B). However, these MMPs were induced less than 3-fold after 3 exposures. These data indicate that antifibrotic responses become refractory to multiple UV-A1 exposures over the course of 1 week.
Figure 3.
Refractory antifibrotic responses with repetitive high-dose UV-A1 irradiation. Eight healthy subjects with lightly pigmented skin (L* [luminescence] >65) were treated with high-dose UV-A1 irradiation (110 J/cm2) either once (Friday) or 3 times (Monday, Wednesday, and Friday). Four days later (the following Tuesday), all biopsy samples were collected and assessed by real-time polymerase chain reaction for the indicated transcripts: type I and type III procollagen (A) and matrix metalloproteinases (MMPs) 1, 3, and 9 (B). Data are presented as mean fold change relative to untreated skin (normalized to 1). Error bars represent SEM. In A, the horizontal dashed line indicates fold change of untreated skin. *P<.05 compared with untreated skin. mRNA indicates messenger RNA.
ANTIFIBROTIC RESPONSES BECOME REFRACTORY TO HIGH-DOSE UV-A1 PHOTOTHERAPY GIVEN ONCE PER WEEK IN NORMAL SKIN
The previous data demonstrate that a single high-dose UV-A1 exposure substantially induces antifibrotic responses, which subside gradually over 1 week (Figure 2). However, repeated high-dose UV-A1 exposures at intervals shorter than 1 week caused antifibrotic responses to become refractory (Figure 3). Therefore, we treated 10 healthy subjects with lightly pigmented skin (buttock, mean [SEM] L*=71.6 [0.7]) with high-dose UV-A1 irradiation (130 J/cm2) at weekly intervals.
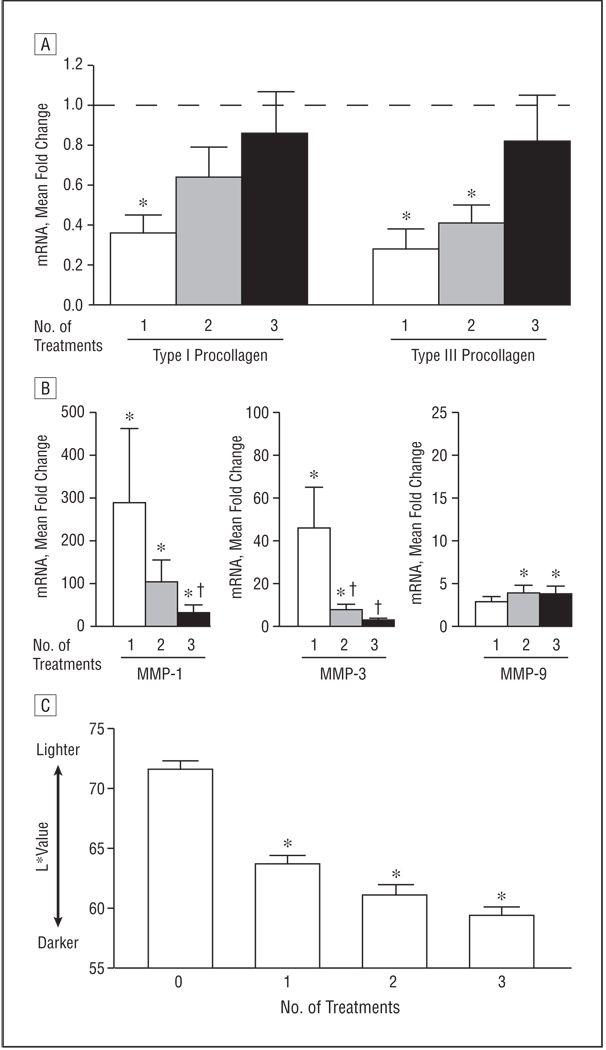
Although type I and type III procollagen mRNA expression was inhibited significantly after the first treatment (P<.05), no statistically significant reductions were observed after the third treatment (Figure 4A). Similarly, upregulation of MMP-1 and MMP-3 was significant after the first treatment (P<.05) but was reduced by 89% (P<.05) and 94% (P<.05), respectively, after the third treatment (Figure 4B).
Figure 4.
Attenuation of antifibrotic responses with weekly high-dose UV-A1 phototherapy. Ten healthy subjects with lightly pigmented skin (L* [luminescence] >65) were treated with high-dose UV-A1 phototherapy (130 J/cm2) once per week. Biopsy samples and skin pigmentation readings were obtained 24 hours after 1, 2, and 3 weekly treatments. A and B, The indicated transcripts (type I and type III procollagen [A] and matrix metalloproteinases [MMPs] 1, 3, and 9 [B]) were assessed by real-time polymerase chain reaction. Data are presented as mean fold change relative to untreated skin (normalized to 1). In A, the horizontal dashed line indicates fold change of untreated skin. C, Skin pigmentation is shown as mean L* value. Error bars represent SEM. *P<.05 compared with untreated skin. †P<.05 compared with a single UV-A1 exposure. mRNA indicates messenger RNA.
Coinciding with these biochemical changes, irradiated skin became darker. Substantial darkening occurred after the first UV-A1 exposure, with L* values decreasing by a mean of 7.9 (P<.05) (Figure 4C). After 3 irradiation sessions, L* values decreased by a mean of 12.2 compared with no treatment (P<.05). Thus, lightly pigmented skin became medium in pigmentation (mean [SEM] L*=59.4 [0.7]) during treatment. These data indicate that antifibrotic responses become refractory to repeated weekly high-dose UV-A1 exposures, which induces substantial skin darkening.
UV-A1 IRRADIATION INDUCES DARKENING OF NORMAL SKIN IN A DOSE-DEPENDENT MANNER
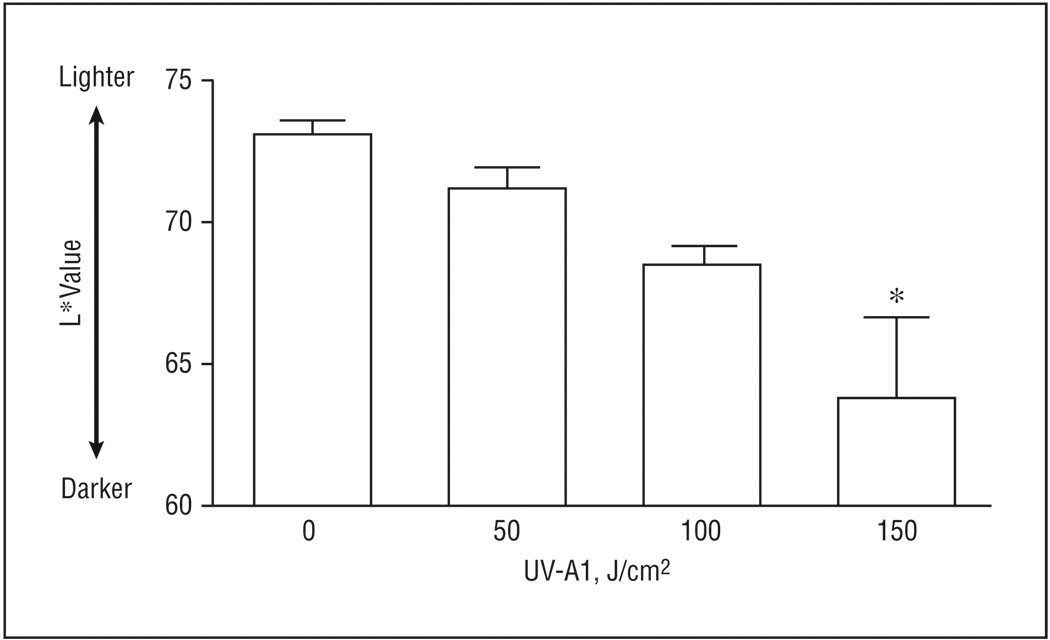
To examine the degree of skin darkening after UV-A1 irradiation, we exposed 8 lightly pigmented healthy individuals (buttock, mean [SEM] L*=73.1 [0.5]) to 3 different doses of UV-A1 and obtained L* values 24 hours later. After exposure to 50 or 100 J/cm2, skin color did not darken to a significant degree (P>.05) (Figure 5). In contrast, exposure to 150 J/cm2 induced a significant reduction in L* values (P<.05) such that skin became medium in pigmentation (mean [SEM] L*=63.8 [2.4]). Thus, darkening of skin color was more intense with high-dose UV-A1 irradiation compared with lower doses.
Figure 5.
Rapid darkening of skin with high-dose UV-A1 irradiation. Eight healthy subjects with lightly pigmented skin (L* [luminescence] >65) were treated once with the indicated UV-A1 doses. After 24 hours, chromameter readings (L* values) were obtained. Error bars represent SEM. *P<.05 compared with untreated skin.
INCREASED PIGMENTATION DIMINISHES ANTIFIBROTIC RESPONSES TO A SINGLE UV-A1 EXPOSURE IN NORMAL SKIN
To further investigate the effect of pigmentation on antifibrotic responses, we examined skin with different natural (constitutive) pigmentation. Based on mean (SEM) L* values, we categorized buttock skin of healthy subjects as light (73.3 [1.9]; n=8), medium (61.5 [1.1]; n=8), or dark (40.5 [2.0]; n=12). Each subject was then irradiated with different UV-A1 doses (70, 90, 110, 130, and 150 J/cm2), and biopsies were performed 24 hours later.
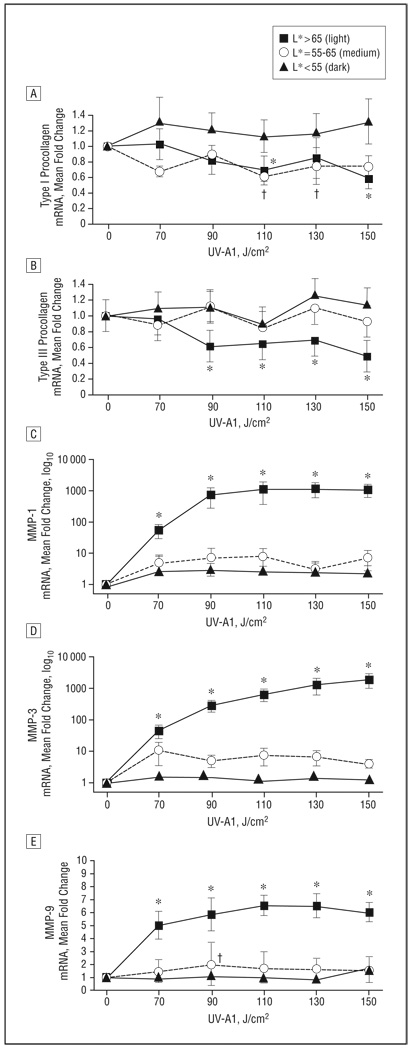
In light skin, the expression of type I procollagen mRNA was reduced dose dependently, with maximal reduction (42%, P<.05) occurring at 150 J/cm2 (Figure 6). Skin with medium pigmentation exhibited a moderate reduction (26%) in type I procollagen at 150 J/cm2. In dark skin, type I procollagen levels did not decrease with any dose. A similar pattern was seen for type III procollagen mRNA in all skin types.
Figure 6.
Abrogation of UV-A1–induced antifibrotic responses in skin with darker pigmentation. Based on L* (luminescence) values, 28 healthy subjects were stratified according to skin pigmentation as light (n=8), medium (n=8), or dark (n=12). Skin was then exposed to the indicated UV-A1 doses. Biopsies were performed 24 hours later, and the indicated transcripts were assessed by real-time polymerase chain reaction: type I procollagen (A), type III procollagen (B), matrix metalloproteinase (MMP) 1 (C), MMP-3 (D), and MMP-9 (E). Data are presented as mean fold change relative to untreated skin (normalized to 1). Error bars represent SEM. *P<.05 for light skin vs untreated skin. †P<.05 for medium skin vs untreated skin.
In light skin, mRNA expression of MMP-1, MMP-3, and MMP-9 was upregulated in a dose-dependent manner, with the greatest changes seen at 130 or 150 J/cm2 (P<.05 for all) (Figure 6). Medium and dark skin demonstrated no substantial induction of MMPs at any dose. These data indicate that a single exposure to UV-A1 irradiation induces antifibrotic responses in light, non-tanned skin but not in pigmented (medium or dark) skin.
MODERATE-DOSE UV-A1 IRRADIATION MODESTLY IMPROVES SCLEROTIC SKIN
The previous data indicate that a single high-dose UV-A1 exposure rapidly induces skin darkening, which, in turn, attenuates antifibrotic responses. This skin darkening is less intense after moderate UV-A1 doses (Figure 5). Therefore, we treated additional patients with scleroderma with moderate-dose UV-A1 irradiation (65 J/cm2) 3 times per week for 14 weeks (Table 2). For each patient, 1 circumscribed lesion was treated.
Table 2.
Clinical Information for Patients Treated With Moderate-Dose UV-A1 Irradiation (65 J/cm2) 3 Times per Week for 14 Weeksa
| Lesional Induration (Scale, 0–9) | |||||
|---|---|---|---|---|---|
| Patient No./ Sex/Age, y |
Diagnosis | Disease Duration, y |
Treatment Location | Before Therapy | After Therapy |
| 13/F/52 | Diffuse scleroderma | 4 | Right arm | 8 | 3 |
| 14/F/15 | Linear morphea | 0.4 | Right arm | 6 | 5 |
| 15/F/63 | Morphea | 1.5 | Medial left breast | 9 | 4 |
| 16/M/67 | Scleroderma | 5 | Right thigh | 6 | 3 |
| 17/M/15 | Morphea | 10 | Upper back | 2 | 1 |
| 18/F/70 | Morphea | 0.5 | Lower left abdomen | 7 | 3 |
The mean patient age was 47.0 years, the mean disease duration was 3.6 years, and the mean lesional induration before therapy was 6.3 (after therapy, 3.2).
Although this cohort had fewer patients (n=6) and more localized sclerotic disease compared with the high-dose group (Table 1), pretreatment induration scores were not significantly different between the 2 groups (P>.05, data not shown). Before treatment, mean (SEM) induration was 6.3 (1.0) (“low severe”) (data not shown). By 1 week of phototherapy, improvement occurred in 2 of 6 patients. Thereafter, induration scores declined steadily, with significant changes observed by the end of treatment (mean [SEM] score, 3.2 [0.5] or “high mild”) (P<.05). Clinically, modest softening occurred, and improvement was seen in all 6 patients (as opposed to 9 of 12 patients in the high-dose group). Based on earliest change in induration score, mean (SEM) time to first improvement was 5.3 (4.8) weeks (approximately 16 sessions), which was not significantly different from that seen with high-dose UV-A1 phototherapy (P>.05, data not shown). Clearance of lesions was not seen in any patient. Overall, we considered this regimen to be moderately effective and similar in efficacy to high-dose UV-A1 irradiation (130 J/cm2).
COMMENT
UV-A1 phototherapy holds promise for treating fibrotic skin diseases.2,4 Investigators3,4,14–22 have treated sclerotic skin using a variety of UV-A1 regimens and have reported good to excellent results. Although this study was limited by the small number of patients, we also report that sclerotic skin improves with UV-A1 phototherapy. However, clinical responses were modest. To explain this observation, we investigated antifibrotic responses to UV-A1 irradiation in normal skin.
High-dose UV-A1 irradiation is reported to be more effective for softening sclerotic skin than low-dose UV-A1 irradiation.21 We first investigated whether the frequency of high-dose UV-A1 phototherapy sessions may affect antifibrotic responses. We found that a single exposure to high-dose UV-A1 irradiation induced substantial antifibrotic responses. However, these antifibrotic responses became refractory to subsequent UV-A1 exposures. The use of widely spaced (once per week) phototherapy sessions did not compensate for the attenuation of biochemical responses.
To investigate mechanisms for this attenuation of antifibrotic responses, we examined the role of pigmentation. This was based, in part, on observations that UV-A1 irradiation stimulates pigmentation (melanogenesis), which is photoprotective.25,26 Based on data from normal skin, we found that a single exposure to high-dose UV-A1 irradiation caused substantial darkening of skin such that lightly pigmented skin became medium colored. In skin with increased constitutional pigmentation (ie, naturally medium- or dark-colored skin), antifibrotic responses were not elicited by UV-A1 treatment. Thus, UV-A1 phototherapy is limited by skin darkening.
These observations may explain the modest clinical responses of patients with scleroderma/GVHD treated with high-dose UV-A1 irradiation (130 J/cm2). All the patients had medium (L*=55–65) or dark (L*<55) pigmentation in target plaques at baseline. After 14 weeks of phototherapy, mean pigmentation in these plaques became dark (L*<55). Hence, preexisting (constitutional) and UV-A1–induced (facultative) pigmentation likely prevented robust clinical responses in these patients.
Because a single exposure to high-dose UV-A1 irradiation causes rapid skin darkening, it is likely that antifibrotic responses in patients with scleroderma/GVHD were attenuated soon after initiating therapy. As such, we speculate that softening of sclerotic skin may be brought about largely by biochemical changes induced with early (acute or single) rather than chronic UV-A1 exposure. These acute biochemical changes may precede clinical changes by a considerable time such that skin softens many weeks later. This is consistent with the observation that significant softening of sclerotic skin occurred several weeks after initiating high-dose UV-A1 therapy.
Together, these data have implications for optimizing UV-A1 treatment regimens. First, these findings indicate that UV-A1 therapy is most likely to be effective in lightly pigmented skin. Therefore, it may be reasonable to restrict UV-A1 phototherapy to lightly pigmented individuals who do not easily tan. Because UV-A1 irradiation does not elicit antifibrotic responses in darker skin (eg, naturally dark or tanned by UV-A1), other types of therapy should be attempted in patients with this skin type.
Second, these data suggest that lower UV-A1 doses stimulate less pigmentation and, therefore, may be effective for eliciting antifibrotic responses. Indeed, we found that improvement of sclerotic skin after moderate-dose UV-A1 irradiation (65 J/cm2) was similar to that after high-dose irradiation (130 J/cm2). In contrast to an earlier study,21 these data indicate that moderate UV-A1 doses may be as effective as higher doses, in part because less tanning occurs. However, we predict that even moderate doses, particularly when given repeatedly, would stimulate substantial pigmentation with time. This potentially explains why moderate-dose UV-A1 phototherapy did not completely clear lesions in patients with scleroderma. In addition, this suggests that low UV-A1 doses (<65 J/cm2) may minimize tanning and demonstrate efficacy.
Third, sporadic exposure to UV-A1 irradiation may have equal or more efficacy compared with frequent, repeated exposures. With further validation, this may translate into time and economic savings for patients and physicians. Indeed, because skin darkening and attenuation of antifibrotic responses occur as early as the second UV-A1 exposure, there may be utility for “pulse” therapy. In this situation, single treatments, given several weeks apart, could minimize the effects of tanning and act as an effective adjunct to other antifibrotic drug therapies. Sporadic use of UV-A1 irradiation may also diminish possible long-term adverse effects, such as UV-induced skin cancers.27,28
In conclusion, we used a hypothesis-driven approach to investigate the biochemical effects of UV-A1 phototherapy. Our findings have implications for the design of UV-A1 regimens and the selection of potential “responders.” We suggest that skin darkening is a limitation to UV-A1 phototherapy. This skin darkening may be reduced, to some degree, with low-dose, sporadic UV-A1 irradiation.
Acknowledgments
Funding/Support: This study was supported by grants 5T32 AR007197 (Dr Wang), 1K24 AR01259 (Dr Kang), and 1R01 AR48077 (Dr Kang) from the National Institutes of Health and by the Babcock Research Endowment, University of Michigan. Sellas Medizinische Geräte GmbH donated the full-bed UV-A1 source, but had no involvement in the design or conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation or review of the manuscript.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00129415
Author Contributions: Drs Wang and Garza contributed equally to this article. Drs Wang, Garza, Fisher, and Kang had access to all of the clinical and biochemical data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Kafi, Mayes, Voorhees, Fisher, and Kang. Acquisition of data: Wang, Garza, Cho, Kafi, Hammerberg, Quan, Ratanatharathorn, Voorhees, Fisher, and Kang. Analysis and interpretation of data: Wang, Garza, Cho, Hamilton, Ratanatharathorn, Voorhees, Fisher, and Kang. Drafting of the manuscript: Wang, Garza, and Fisher. Critical revision of the manuscript for important intellectual content: Wang, Garza, Cho, Kafi, Hammerberg, Quan, Hamilton, Mayes, Ratanatharathorn, Voorhees, Fisher, and Kang. Statistical analysis: Hamilton. Obtained funding: Voorhees, Fisher, and Kang. Administrative, technical, and material support: Kafi, Hammerberg, Mayes, and Kang. Study supervision: Voorhees, Fisher, and Kang.
Financial Disclosure: None reported.
Previous Presentation: This study was presented in part as a poster and an oral presentation at the 2007 Society for Investigative Dermatology Meeting; May 10, 2007; Los Angeles, California.
Additional Contributions: Laura VanGoor, BFA, performed graphic work; Suzan Rehbine, LPN, and Carolyn Petersen, LPN, procured tissue; and Harrold Carter, BS, Rui Wang, MS, and Trupta Purohit, MS, provided technical expertise.
REFERENCES
- 1.Charles C, Clements P, Furst DE. Systemic sclerosis: hypothesis-driven treatment strategies. Lancet. 2006;367(9523):1683–1691. doi: 10.1016/S0140-6736(06)68737-0. [DOI] [PubMed] [Google Scholar]
- 2.Denton CP, Black CM. Targeted therapy comes of age in scleroderma. Trends Immunol. 2005;26(11):596–602. doi: 10.1016/j.it.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Breuckmann F, Gambichler T, Altmeyer P, Kreuter A. UVA/UVA1 phototherapy and PUVA photochemotherapy in connective tissue diseases and related disorders: a research based review. BMC Dermatol. 2004;4(1):11. doi: 10.1186/1471-5945-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher GJ, Kang S. Phototherapy for scleroderma: biologic rationale, results, and promise. Curr Opin Rheumatol. 2002;14(6):723–726. doi: 10.1097/00002281-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Denton CP, Black CM, Abraham DJ. Mechanisms and consequences of fibrosis in systemic sclerosis. Nat Clin Pract Rheumatol. 2006;2(3):134–144. doi: 10.1038/ncprheum0115. [DOI] [PubMed] [Google Scholar]
- 6.Sakkas LI. New developments in the pathogenesis of systemic sclerosis. Autoimmunity. 2005;38(2):113–116. doi: 10.1080/16066350500095415. [DOI] [PubMed] [Google Scholar]
- 7.Rodnan GP, Lipinski E, Luksick J. Skin thickness and collagen content in progressive systemic sclerosis and localized scleroderma. Arthritis Rheum. 1979;22(2):130–140. doi: 10.1002/art.1780220205. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Hatamochi A, Ueki H, Nakata M, Oishi Y. Decreased collagenase expression in cultured systemic sclerosis fibroblasts. J Invest Dermatol. 1994;103(3):359–363. doi: 10.1111/1523-1747.ep12394936. [DOI] [PubMed] [Google Scholar]
- 9.Scharffetter K, Wlaschek M, Hogg A, et al. UVA irradiation induces collagenase in human dermal fibroblasts in vitro and in vivo. Arch Dermatol Res. 1991;283(8):506–511. doi: 10.1007/BF00371923. [DOI] [PubMed] [Google Scholar]
- 10.Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379(6563):335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 11.Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 12.Mempel M, Schmidt T, Boeck K, et al. Changes in collagen I and collagen III metabolism in patients with generalized atopic eczema undergoing medium-dose ultraviolet A1 phototherapy. Br J Dermatol. 2000;142(3):473–480. doi: 10.1046/j.1365-2133.2000.03359.x. [DOI] [PubMed] [Google Scholar]
- 13.Kreuter A, Hyun J, Stucker M, Sommer A, Altmeyer P, Gambichler T. A randomized controlled study of low-dose UVA1, medium-dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. J Am Acad Dermatol. 2006;54(3):440–447. doi: 10.1016/j.jaad.2005.11.1063. [DOI] [PubMed] [Google Scholar]
- 14.Calzavara Pinton P, Porta F, Izzi T, et al. Prospects for ultraviolet A1 phototherapy as a treatment for chronic cutaneous graft-versus-host disease. Haematologica. 2003;88(10):1169–1175. [PubMed] [Google Scholar]
- 15.Kreuter A, Breuckmann F, Uhle A, et al. Low-dose UVA1 phototherapy in systemic sclerosis: effects on acrosclerosis. J Am Acad Dermatol. 2004;50(5):740–747. doi: 10.1016/j.jaad.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Kreuter A, Gambichler T, Avermaete A, et al. Combined treatment with calcipo-triol ointment and low-dose ultraviolet A1 phototherapy in childhood morphea. Pediatr Dermatol. 2001;18(3):241–245. doi: 10.1046/j.1525-1470.2001.018003241.x. [DOI] [PubMed] [Google Scholar]
- 17.Gruss CJ, Von Kobyletzki G, Behrens-Williams SC, et al. Effects of low dose ultraviolet A-1 phototherapy on morphea. Photodermatol Photoimmunol Photomed. 2001;17(4):149–155. doi: 10.1034/j.1600-0781.2001.170401.x. [DOI] [PubMed] [Google Scholar]
- 18.von Kobyletzki G, Uhle A, Pieck C, Hoffmann K, Altmeyer P. Acrosclerosis in patients with systemic sclerosis responds to low-dose UV-A1 phototherapy. Arch Dermatol. 2000;136(2):275–276. doi: 10.1001/archderm.136.2.275. [DOI] [PubMed] [Google Scholar]
- 19.Kerscher M, Dirschka T, Volkenandt M. Treatment of localised scleroderma by UVA1 phototherapy [letter] Lancet. 1995;346(8983):1166. doi: 10.1016/s0140-6736(95)91843-4. [DOI] [PubMed] [Google Scholar]
- 20.Gruss C, Reed JA, Altmeyer P, McNutt NS, Kerscher M. Induction of interstitial collagenase (MMP-1) by UVA-1 phototherapy in morphea fibroblasts. Lancet. 1997;350(9087):1295–1296. doi: 10.1016/s0140-6736(05)62472-5. [DOI] [PubMed] [Google Scholar]
- 21.Stege H, Berneburg M, Humke S, et al. High-dose UVA1 radiation therapy for localized scleroderma. J Am Acad Dermatol. 1997;36(6, pt 1):938–944. doi: 10.1016/s0190-9622(97)80277-0. [DOI] [PubMed] [Google Scholar]
- 22.Kerscher M, Volkenandt M, Gruss C, et al. Low-dose UVA phototherapy for treatment of localized scleroderma. J Am Acad Dermatol. 1998;38(1):21–26. doi: 10.1016/s0190-9622(98)70533-x. [DOI] [PubMed] [Google Scholar]
- 23.Westerhof W, Estevez-Uscanga O, Meens J, Kammeyer A, Durocq M, Cario I. The relation between constitutional skin color and photosensitivity estimated from UV-induced erythema and pigmentation dose-response curves. J Invest Dermatol. 1990;94(6):812–816. doi: 10.1111/1523-1747.ep12874671. [DOI] [PubMed] [Google Scholar]
- 24.Kang S, Cho S, Chung JH, Hammerberg C, Fisher GJ, Voorhees JJ. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-κB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166(6):1691–1699. doi: 10.1016/s0002-9440(10)62479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravnbak MH, Wulf HC. Pigmentation after single and multiple UV-exposures depending on UV-spectrum. Arch Dermatol Res. 2007;299(1):25–32. doi: 10.1007/s00403-006-0728-3. [DOI] [PubMed] [Google Scholar]
- 26.Denton CP, Black CM. Scleroderma (systemic sclerosis) In: Wolff K, Goldsmith LA, Katz SI, Gilchrist BA, Paller AS, Leffell DJ, editors. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill Medical Publishing Division; 2008. pp. 1553–1562. [Google Scholar]
- 27.Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanoma. Proc Natl Acad Sci U S A. 1993;90(14):6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staberg B, Wulf HC, Klemp P, Poulsen T, Brodthagen H. The carcinogenic effect of UVA irradiation. J Invest Dermatol. 1983;81(6):517–519. doi: 10.1111/1523-1747.ep12522855. [DOI] [PubMed] [Google Scholar]