Abstract
Introduction
Test whether physicians can validly and reproducibly diagnose diabetic sensorimotor polyneuropathy (DSPN).
Methods
Twelve physicians assessed 24 patients with diabetes mellitus (DM) on consecutive days (576 examinations) with physical features and voice disguised. Results were compared to gold standard 75% group diagnosis and a nerve conduction score (Σ 5 NC nds).
Results
Masking of patients was achieved. Reproducibility measured by the kappa coefficient and compared to Σ 5 NC nd varied considerably among physicians: median and ranges: signs 0.8 (0.32 to 1.0); symptoms 0.79 (0.36 to 1.0) and diagnoses 0.47 (0.33 to 0.84) – both low and high scores indicating poor performance. There was substantial agreement between 75% group dx and confirmed NC abnormality. As compared to Σ 5 NC, individual physicians’ clinical diagnosis was excessively variable and frequently inaccurate.
Discussion
Study physician diagnosis from signs and symptoms were excessively variable, often over-estimating DSPN. Specific approaches to improving proficiency should be tested.
Keywords: diabetic sensorimotor polyneuropathy (DSPN), proficiency of clinical examinations, neurologic signs and symptoms, nerve conduction, quantitative sensation and quantitative autonomic tests
INTRODUCTION
Despite its common occurrence, diabetic sensorimotor polyneuropathy (DSPN) is not as sensitively and reliably diagnosed or its severity as adequately estimated as desirable. This conclusion is based mainly on three observations: 1) the great variability in the reported prevalence of DSPN –ranging from a few percentage points to more than 50%;1 2) in cohort and epidemiology surveys, with some exceptions,2 severity is usually not even evaluated; and 3) there is demonstrated variability of measured endpoints in therapeutic trials.3 Llewelyn and coworkers attribute prevalence variability to three reasons: “differences in defining diabetic neuropathy, the tests used to assess neuropathy, and the type of patient population studied”.1 Among the additional reasons is the possible lack of accuracy and reproducibility of clinical endpoint assessments by medical personnel. This issue of physician proficiency is studied here.
Neurologic signs, symptoms, and electrophysiologic measurements are the most commonly used instruments to diagnose DSPN.2,4–6 Neurologic signs commonly used are decrease or loss of ankle reflexes or vibration sensation of feet, but also increasingly used are composite scores of neurologic signs (e.g., NIS[LL]).2 For neuropathy symptoms, individual or composite scores are used. Clinical neurophysiologic abnormalities used are nerve conduction (NC), quantitative sensation tests (QST) or autonomic tests (QAT).2,7,8 Two histologic studies of biopsied tissue have been used: morphometric studies of biopsied nerve or intra-epidermal nerve fiber densities.9,10 Consensus panels reviewing published data on DSPN found the endpoints listed above are useful in diagnosis and characterization of DSPN.7,11–13 In cohort studies or therapeutic trials, a statistically significant association has been shown between neuropathic symptoms or signs and neuropathy tests, but the r2 of the statistical association between the two varied considerably among studies. They usually accounted for only approximately 5 to 25% of the variability of the data.3,5,14
Reproducibility, especially of the clinical evaluation of polyneuropathy (for its occurrence or severity), has been studied but usually only for individual (or at most for 2 or 3) physicians. With agreement on diagnostic criteria and approaches to be used, high degrees of agreement have been recorded.3,14–16 Attributes of nerve conduction have been found to be variably reproducible measures; some (e.g., F-waves of lower limb nerves) show high degrees of reproducibility.17,18 This attribute, however, may not be an ideal measurement for DSPN, and other attributes may be more representative.5
To assess whether expert physicians can validly and reproducibly diagnose DSPN based only on elicitation of symptoms and signs and without preliminary agreement on criteria and reference values, we judged that a prospective study was needed. In such a study, a panel of physicians, without instruction or consensus development, would examine a representative group of patients with DM and with and without DSPN. They would compare their evaluation of signs, symptoms and diagnosis to a gold standard group diagnosis (the percentage to be set by an expert advisory panel) and to a confirmed (from 2 of 2 or 2 of 3 evaluations) abnormality of NC as measured by a composite score of nerve conduction abnormality.
RESEARCH DESIGN AND METHODS
The design and conduct of the Cl vs NPhys Trial is outlined in more detail in a separate communication.19 In brief, we recruited a representative cohort of 24 volunteers with DM. Some did not have DSPN, and some had varying severities of DSPN. They were recruited from the Rochester Diabetic Neuropathy Study (RDNS) cohort, a cross-sectional and longitudinal study of DM, diabetic microvessel complications and their risk covariates in Olmsted County, MN.15,20 Patients were examined twice by each of the 12 study physicians (especially recruited for these prospective studies) and evaluated twice (or a third time) by NC, QST and QAT (heart rate variation with deep breathing [HRdb] and sudomotor axon reflex test [QSART]). Study physicians (neurologists and diabetologists) were experts in DSPN and came from Canada, Denmark, England, Wales and the USA. Each study physician evaluated each of the 24 patients on November 24 and again on November 25, 2008. We chose the largest number of patients who could reasonably be examined by 12 physicians in one day and then again on the second day, and who could have clinical neurophysiologic tests done within three weeks of the clinical evaluation. For disguise, patients wore surgical clothes, surgical caps, masks, and dark glasses, and their voices were electronically altered at first examination (Fig. 1). Patients provided information only about symptoms related to neuropathy and not about type or duration of DM, known complications of DM, or treatment. Physicians examined patients as they saw fit without direction from study personnel but were asked to record presence or absence of specific signs (decreased knee or ankle reflexes, decreased or absent sensation of touch pressure, vibration, joint position or movement, and pin-prick), muscle weakness, neuropathy symptoms (“asleep numbness,” “prickling,” “burning” or “stabbing pain”), decreased reflexes and whether patients had DSPN or not.
Figure 1.
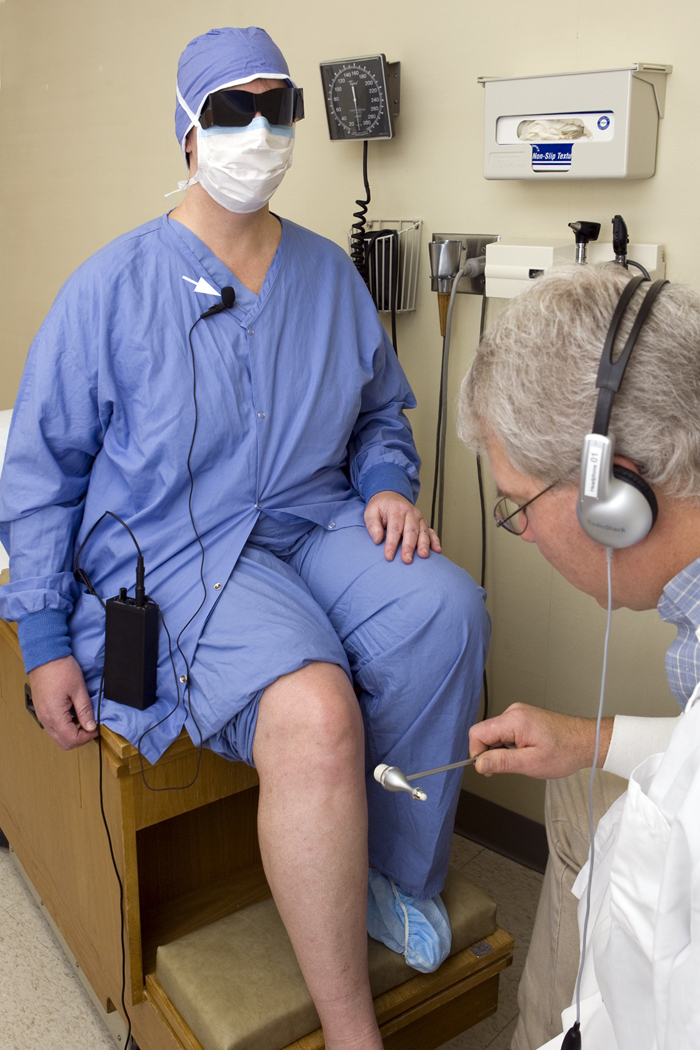
Members of the surveillance team illustrate the dress and electronic voice distortion used at first examination to allow masked assessment of reproducibility on a subsequent evaluation the next day. This is described in more detail in text.
All clinical neurophysiological tests were done twice (and a third time if results were discordant for the diagnosis of DSPN). Tests were performed by different technologists and instruments within the pre-determined 6 week period. The attributes included in Σ 5 NC were chosen because they are among the most representative of nerve conduction abnormalities in DSPN, i.e., peroneal motor nerve conduction velocity, compound muscle action potential amplitude and distal latency, tibial nerve motor distal latency and sural sensory nerve action potential nerve amplitude. QST results were assessed with CASE IVb (WR Medical Electronics, Stillwater, MN). Heart rate deep breathing (HRdb) and Quantitative Sudomotor Axon Reflex Testing (QSART) were assessed by previously described approaches.8 Each neurophysiologic evaluation was done without reference to clinical or previous test information.
Individual study physician’s evaluation of signs and symptoms were compared to 75% group dx (the criterion set by the Expert Advisory Panel)19 and to Σ 5 NC nds abnormality,21 judging agreement, and under and over diagnosis. Additionally, 75% group signs, symptoms, and diagnosis were compared for agreement with Σ 5 NC abnormality. For reproducibility, we employed the kappa coefficient. Descriptive statistics were also used for other purposes.
RESULTS
Choice of percentage for group diagnosis of DSPN
Of the 40 Expert Advisory Panel canvassed, 16 physicians chose different percentage responses for decreased or absent ankle reflexes, vibration loss of toes, leg weakness and neuropathy symptoms. On average, 3% chose 25%, 17.2% chose 50%, 43.8% chose 75%, and 36% chose 95%. Therefore we accepted 75% as the level to be used for group signs, symptoms and diagnosis.
Conduct of study
The 12 study neuromuscular physicians performed the 576 clinical examinations over a period of two days without need to call on “stand by” physicians or patients, and neurophysiologic tests were performed within the set 6 week time period. At second examination, only a small percentage of patients were recognized by distinctive physical features or medical histories (14.9%) or recall of specific symptoms or findings (7.6%). Thus, independent and masked evaluation was achieved for almost all second clinical evaluations.
Demographic and disease characteristics of the 24 patients
The patients were 17 men and 7 women, whose mean age was 62.5 years (SD 8.7 yr); 10 had type 1, and 14 had type 2 DM for an average duration of 21.3 years (SD 13.3 yr). Their average A1C value over previous longitudinal studies was 7.4% (SD 1.0%). The study cohort for the Cl vs NPhys Trial was chosen so that one-half had and one-half did not have DSPN by a composite score of nerve conduction criteria (for calculation of Σ 5 NC nd see footnote to Table 1). Sixteen had retinopathy (9 mild background, 4 severe background and 2 proliferative). Three patients had nephropathy (2 stage 1 and 1 stage 3).
Table 1.
Frequency of Neuropathy Endpoint Abnormalities of Individual Patients in the Cl vs NPhys Trial*
| Patient | Age | Gender | Clinical Neurologic Exam1 | NC2 | |||
|---|---|---|---|---|---|---|---|
| Signs (no. of 24 eval.) |
Symptoms (no. of 24 eval.) |
Diagnosis (no. of 24 eval.) |
PMCV | Σ 5 NC nds |
|||
| 1 | 68 | F | 16 | 1 | 8 | 0 of 2 | 0 of 2 |
| 2 | 67 | F | 0 | 2 | 0 | 0 of 2 | 0 of 2 |
| 3 | 67 | M | 19 | 1 | 5 | 0 of 2 | 0 of 2 |
| 4 | 63 | M | 18 | 0 | 7 | 0 of 2 | 0 of 2 |
| 5 | 68 | M | 23 | 0 | 13 | 0 of 2 | 0 of 2 |
| 6 | 62 | M | 22 | 12 | 11 | 0 of 2 | 0 of 2 |
| 7 | 62 | M | 24 | 0 | 18 | 0 of 2 | 0 of 2 |
| 8 | 73 | M | 22 | 16 | 7 | 0 of 2 | |
| 9 | 67 | M | 22 | 0 | 15 | 2 of 2 | 0 of 2 |
| 10 | 56 | F | 15 | 3 | 3 | 0 of 2 | 0 of 2 |
| 11 | 53 | M | 22 | 19 | 16 | 0 of 2 | 0 of 2 |
| 12 | 49 | M | 17 | 0 | 12 | 2 of 2 | 0 of 2 |
| 13 | 50 | F | 15 | 2 | 6 | 2 of 2 | 2 of 2 |
| 14 | 65 | M | 23 | 1 | 16 | 2 of 2 | 2 of 3 |
| 15 | 72 | F | 23 | 0 | 14 | 2 of 2 | 2 of 2 |
| 16 | 73 | M | 24 | 3 | 21 | 2 of 2 | 2 of 2 |
| 17 | 55 | M | 24 | 14 | 19 | 0 of 2 | 2 of 2 |
| 18 | 65 | M | 24 | 23 | 23 | 0 of 2 | 2 of 2 |
| 19 | 38 | F | 6 | 0 | 3 | 2 of 2 | 2 of 2 |
| 20 | 66 | M | 24 | 23 | 20 | 2 of 2 | 2 of 2 |
| 21 | 67 | M | 23 | 22 | 23 | 2 of 2 | 2 of 3 |
| 22 | 73 | F | 24 | 22 | 24 | 2 of 2 | 2 of 2 |
| 23 | 58 | M | 24 | 21 | 24 | 2 of 2 | 2 of 2 |
| 24 | 63 | M | 24 | 10 | 22 | 2 of 2 | 2 of 2 |
| N (%) abn. | 18 (75.0) | 6 (25.0) | 9 (37.5) | 12 (50.0) | 12 (50.0) | ||
The full table with inclusion of quantitative sensation and autonomic tests and composite scores is provided as supplementary material.
Signs, symptoms and diagnosis confirmed when ≥ 75% of 12 study physicians diagnosed them on 2 occasions or if ≥ 75% of 24 examinations were so diagnosed. Shaded boxes indicate 75% diagnostic levels of abnormality.
For all clinical neurophysiologic test results, abnormality was ≥ 95th or ≤ 5th percentile based on normative values obtained from RDNS-HS cohort studies56. When composite scores were used, normal deviates values from percentiles, always in the upper tail of the normal distribution, were added and divided by the number of measured variables (e.g., when CMAP is 0, velocity and distal latency cannot be evaluated) and this quotient is then multiplied by the number of measurable variables in the composite score. The 95th percentile lines of the composite scores were estimated in the RDNS-HS cohort56.
A column for patients with both signs and symptoms is not provided since it would be identical to the column for symptoms.
Comparative performance between the two gold standard evaluations – 75% group dx and Σ 5 NC abn
In Table 1, we summarize the frequency of endpoint abnormalities for each of the 24 patients, showing numbers of clinical abnormal evaluations of the 12 × 2 = 24 physician examinations of each patient and the number of confirmed nerve test abnormalities (2 of 2 or 2 of 3). We have shaded data boxes meeting ≥ 75% group dx, confirmed Σ 5 NC abn, and other combined test abnormalities.
On visual inspection of the columns marked Diagnosis and Σ 5 NC nds (the two gold standard evaluations) in Table 1, note that 12 of 24 patients were abnormal by Σ 5 NC abn, and 8 of these were also abnormal by 75% group diagnosis and one was abnormal by 75% group diagnosis only. This result suggests a reasonably close agreement between the two gold standard criteria, with Σ 5 NC abn being somewhat more sensitive. However, there is a difference between them. For Σ 5 NC nds abn, there is a more clean separation of normal from abnormal. Thus, for Σ 5 NC abn, all but 2 were abnormal by 2 of 2 abnormalities and for those without NC abn they were so categorized by 0 of 2 abnormalities. By contrast, for 75% group dx, there was a greater overlap of abnormal evaluations between affected and unaffected persons. This lack of clean separation of affected form unaffected patients was also observed for signs and symptoms (Table 1 and in full Tables 2 and 3 – supplemental material). The variability in elicitation of signs and symptoms and diagnosis is discussed in more detail below.
Agreement between the first two gold standard endpoints using the kappa coefficient was 0.83 for Σ 5 NC nds abn and 0.73 for the 75% group dx (excellent and good reproducibility).
From these results it would appear the Σ 5 NC nds abn is more sensitive and perhaps somewhat more reproducible for the diagnosis of DSPN than is 75% group dx.
Comparative performance of 75% group signs as compared to 75% group dx or Σ 5 NC abn
As compared to 75% group dx, agreement was obtained by 75% signs in 9 cases, with over-diagnosis in 9 cases and under-diagnosis in 0 cases (Table 1). As compared to Σ 5 NC nds abn, the figures were 10, 8, and 2 (Table 1). It appears that by either gold standard criterion, 75% signs markedly over-estimate the diagnosis of DSPN when compared to gold standard criteria.
The reproducibility of 75% signs provided a kappa coefficient of 0.56.
Comparative performance of 75% group symptoms as compared to 75% group dx or Σ 5 NC abn
As compared to 75% group dx, 75% group symptoms agreed with this diagnoses in 5 of 9 cases. It was over-diagnosed in 1 case and under-diagnosed in 4 cases (Table 1). For Σ 5 NC nds abnormality, the figures were 5, 1 and 7 cases (Table 1). The data suggests that 75% group symptoms under-diagnosed DSPN as compared to gold standard criteria.
The reproducibility of 75% group symptoms (κ = 0.89) was very high.
Comparative performance of individual investigators as compared to Σ 5 NC abn or 75% group dx
The most striking observation of individual physician performance was the excessive variability of their estimation of signs, symptoms and diagnoses as compared to Σ 5 NC abn or 75% group dx (Tables 1–3 – some of the data in supplementary data). To illustrate this point, we list median values (and ranges) of correct dx at first examination as compared to the gold standard criteria of Σ 5 NC nds ≥ 95th: signs 13 (12 to 19); symptoms 15 (13 to 18); and diagnoses 16 (13–22) (Table 2).
Table 2.
Agreement between Diagnosis Elicited from Two Evaluations of Individual Study Physicians as Compared to Confirmed Σ 5 NC Abnormality*
| Physician | N (of 24) | Agreement Between Visits | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | ||||||||
| Correct Dx |
Under Dx |
Over Dx |
Correct Dx |
Under Dx |
Over Dx |
Kappa | Z | p | |
| Comparison of Individual Physician Dx to Σ 5 NC nds ≥ 95th | |||||||||
| 1 | 17 | 4 | 3 | 17 | 3 | 4 | 0.45 | 2.98 | 0.0015 |
| 2 | 15 | 3 | 6 | 17 | 2 | 5 | 0.49 | 3.16 | 0.0008 |
| 3 | 18 | 2 | 4 | 16 | 3 | 5 | 0.45 | 2.91 | 0.0018 |
| 4 | 17 | 3 | 4 | 14 | 2 | 8 | 0.60 | 4.02 | <0.0001 |
| 5 | 13 | 4 | 7 | 13 | 5 | 6 | 0.58 | 3.91 | <0.0001 |
| 6 | 17 | 2 | 5 | 19 | 2 | 3 | 0.38 | 2.48 | 0.0066 |
| 7 | 14 | 5 | 5 | 18 | 5 | 1 | 0.35 | 2.45 | 0.0072 |
| 8 | 15 | 8 | 1 | 17 | 4 | 3 | 0.33 | 2.15 | 0.0157 |
| 9 | 22 | 2 | 0 | 19 | 2 | 3 | 0.53 | 4.13 | <0.0001 |
| 10 | 14 | 1 | 9 | 12 | 1 | 11 | 0.84 | 4.69 | <0.0001 |
| 11 | 13 | 3 | 8 | 15 | 2 | 7 | 0.40 | 2.50 | 0.0063 |
| 12 | 17 | 3 | 4 | 19 | 2 | 3 | 0.59 | 3.87 | <0.0001 |
| Median | 16.0 | 3.0 | 4.5 | 17.0 | 2.0 | 4.5 | 0.47 | 3.07 | |
| SD | 2.6 | 1.8 | 2.6 | 2.4 | 1.3 | 2.7 | 0.14 | 0.82 | |
| Range | 13 – 22 | 1 – 8 | 0 – 9 | 12 – 19 | 1 – 5 | 1 – 11 | 0.33 – 0.84 | 2.15 – 4.69 | |
The full table with comparison of individual physician signs and individual physician symptoms to Σ 5 NC nds is given in supplementary material.
In Table 2, we show the number of correct, under- and over-diagnoses of each of the 12 study physicians at first and second evaluations and as compared to Σ 5 NC abn (Table 2) or as compared to 75% group abn (Table 3). Tables 2 and 3 also provide kappa coefficients for each investigator. It is important to note that the 2 physicians who had complete agreement (1.0; physicians 5 and 11, Table 2) obtained this score by over-diagnosing DSPN by both gold standard criteria.
Table 3.
Agreement between Diagnosis Elicited from Two Evaluations of Individual Study Physicians as Compared to Confirmed 75% Clinical Dx Abnormality*
| Physician | N (of 24) | Agreement Between Visits | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | ||||||||
| Correct Dx |
Under Dx |
Over Dx |
Correct Dx |
Under Dx |
Over Dx |
Kappa | Z | p | |
| Comparison of Individual Physician Dx to 75 % Dx | |||||||||
| 1 | 16 | 3 | 5 | 18 | 1 | 5 | 0.45 | 2.82 | 0.0024 |
| 2 | 16 | 1 | 7 | 18 | 0 | 6 | 0.41 | 2.22 | 0.0134 |
| 3 | 19 | 0 | 5 | 19 | 0 | 5 | 0.24 | 1.19 | 0.1178 |
| 4 | 20 | 0 | 4 | 15 | 0 | 9 | 0.50 | 2.83 | 0.0023 |
| 5 | 16 | 1 | 7 | 16 | 2 | 6 | 0.48 | 2.81 | 0.0025 |
| 6 | 16 | 1 | 7 | 20 | 0 | 4 | 0.37 | 2.12 | 0.0170 |
| 7 | 17 | 2 | 5 | 21 | 2 | 1 | 0.09 | 0.66 | 0.2560 |
| 8 | 18 | 5 | 1 | 20 | 1 | 3 | 0.08 | 0.58 | 0.2799 |
| 9 | 21 | 1 | 2 | 20 | 0 | 4 | 0.51 | 3.01 | 0.0013 |
| 10 | 13 | 0 | 11 | 11 | 0 | 13 | 0.83 | 4.15 | <0.0001 |
| 11 | 12 | 2 | 10 | 16 | 0 | 8 | 0.37 | 2.12 | 0.0171 |
| 12 | 20 | 0 | 4 | 20 | 0 | 4 | 0.40 | 1.96 | 0.0250 |
| Median | 16.50 | 1.00 | 5.00 | 18.50 | 0.00 | 5.00 | 0.41 | 2.17 | |
| SD | 2.76 | 1.50 | 2.93 | 2.89 | 0.80 | 3.14 | 0.20 | 1.03 | |
| Range | 12 – 20 | 0 – 5 | 1 – 11 | 11 – 21 | 0 – 2 | 1 – 13 | 0.08 – 0.83 | 0.58 – 4.15 | |
The full table with comparison of individual physician signs and individual physician symptoms to confirmed 75% group Dx is given in supplementary material.
DISCUSSION
The prevalence of DM appears to be increasing.22,23 Whether microvascular complications, including DSPN, are also increasing, needs to be tested. For this purpose, accurate estimation of the occurrence and severity of DSPN is needed. It is also needed to test putative therapies. Like retinopathy and nephropathy, the complication of DSPN may begin insidiously over long times and with few or no symptoms.2,3,20
When DSPN develops to an overt and expressed degree, physicians presumably have little difficulty recognizing its presence by characteristic symptoms (“asleep-numbness,” “prickling,” “burning,” “sticking,” “stabbing,” or “deep aching pain” or loss of sensation in characteristic distal lower limb distributions), neurologic signs (decreased or absent ankle or knee reflexes, distal lower limb sensory loss, sudomotor loss and distal muscle weakness in more severe cases). However, it may be difficult and in some cases impossible to recognize physiologic degrees of DSPN by clinical approaches, because a variable percentage of cases are asymptomatic and clinical methods of assessment are not sufficiently expressed, valid or reproducible to recognize this minimal degree of involvement. The recently proposed case definition of distal polyneuropathy (including DSPN) developed by an AAN consensus committee and based on review of the medical literature might be considered by whether it would serve as the minimal criteria for DSPN. This consensus case definition states that the “highest likelihood of polyneuropathy occurs with a combination of multiple symptoms, multiple signs, and abnormal electrodiagnostic studies”.13 Whereas this case definition is intuitively correct, it probably does not serve well as a specific minimal criterion for the diagnosis of DSPN for the following reasons: 1) specificity is emphasized over sensitivity, e.g., patients with unequivocal electrophysiologic or other valid test abnormalities but who do not have signs and symptoms would be excluded from the diagnosis; 2) the emphasis on both “multiple signs” and “multiple symptoms” would exclude patients from diagnosis when they had only symptoms or signs or had single symptoms or signs; 3) the specific signs or symptoms or how they are to be graded as abnormal are not defined; and 4) specific criteria for how electrodiagnostic criteria are to be judged and by what reference criteria are not given.
In this study, we assessed the comparative performance of individual neuromuscular physicians’ proficiency to accurately and reproducibly judge neurologic signs, symptoms, and diagnosis of DSPN as compared to gold standard 75% group dx or Σ 5 NC abn. From its unique design features and from elicited responses from study physicians, it appears that the study was sufficiently rigorous to assess validity and reproducibility. It is important to also ask whether the chosen gold standard evaluations are themselves adequate to recognize DSPN. In support of 75% group dx as a gold standard criterion, we list the historical use of symptoms and signs for this purpose, the conclusions of published consensus panels, and the judgment of our expert advisory panel. Likewise, for use of nerve conduction abnormality, we list historical precedence and extensive correlative studies (reviewed in Introduction). We deliberately used a composite score of nerve conduction to ensure that multiple attributes of several nerves and functions and in an appropriate anatomical region (the leg) were tested and that results were corrected for applicable variables from study of a large and appropriate reference cohort of healthy subjects drawn from the same population as were the patients. That the two chosen gold standard endpoints are appropriate endpoints also came from the close association between diagnoses based on the two criteria.
This study has shown that the 75% group dx correlates closely with Σ 5 NC abn but apparently detects a less severe degree of DSPN. That abnormality of nerve conduction is a more sensitive diagnostic criterion has previously been demonstrated (see Introduction). Although the 75% group dx is useful for the diagnosis of DSPN for this study, it is of course impractical for use in epidemiologic surveys, controlled therapeutic trials, or medical practice.
How accurate, invariant, and reproducible are individual physicians’ evaluations? Although the reproducibility of the judgments about DSPN by individual physicians ranged from good to very good, there was considerable and excessive variability among physician judgment of signs, symptoms, and diagnosis. Some of the best kappa scores of reproducibility were at the expense of over-calling abnormality, giving a wrong indication of polyneuropathy. By either gold standard criterion, the judgment of physicians about neuropathic signs, symptoms, and diagnoses was excessively variable. The problem was especially problematic for signs which were markedly over-estimated. This over-estimation of neurologic signs is not readily explained.
The study also provides information on the use of QSTs, HRdb and QSART for the diagnosis of DSPN. In this cohort, none of these measures performed as well as did Σ 5 NC nds – an observation also found in our RDNS cohort study of persons with DM in Olmsted County.3,14,20 These results, therefore, suggest that small fiber measures, i.e., QSTs of small sensory fibers, HRdb and QSART, may not be as sensitive as large fiber measures for the detection of DSPN in community patients with DSPN.
If clinical approaches are to be used (and we think they should be), neurologic assessments and judgments of clinical signs, symptoms, and diagnosis need to be improved.
Assuming that our study physicians are among the best clinical examiners in medical practice, the excessive variability and over-diagnoses especially of signs, is of considerable concern because the problem may be even greater in general medical practice. Recognizing that individual physicians tend to over-diagnose neuropathic findings, symptoms, and diagnosis and that there is excessive variability of their judgments suggest that changes need to be made to improve clinical assessments. The following approaches might be considered for this purpose: 1) Adoption of the rule that physicians’ grading of signs and symptoms should aim to be a direct indication of polyneuropathy – not an indication of physiologic abnormality, e.g., due to age, gender or other anthropomorphic variables; 2) Physicians might grade signs and symptoms, aiming for comparable degrees of specificity and sensitivity; 3) Physicians might school themselves to identify abnormality of their clinical evaluations with reference to NC or other clinical neurophysiologic test abnormalities; 4) Prior to protocol studies, instruction sessions, consensus development, and certification might precede conduct of the study; 5) Physicians who have schooled themselves by NC, QST, and QAT standards might provide peer clinical evaluations of patients compared to which study physicians could judge their performance. Such proficiency testing is extensively used by the American College of Pathologists;24 and 6) More standard and validated tests with reference values could be developed.
Supplementary Material
Acknowledgments
Supported in part by – The Kawamura Fund, the Society for the Support of Neurologic Education and Research of Rochester, MN, and a grant obtained from the National Institutes of Neurological Disorders and Stroke (NS36797).
The authors acknowledge the excellent help of personnel in: Peripheral Neuropathy Research Laboratory: Susanne K. Capelle, JaNean K. Engelstad, and Lawrence V. Witt; EMG Laboratory: Sarah A. Motl, Sherry K. Moyer, Kay A. Spavin, and Andrew J. Zafft; QST Laboratory: Jennifer A. Winkler, B.S. and Karen A. Lodermeier; Autonomic Laboratory: Jade A. Gehrking, Tonette L. Gehrking, Valeria Iodice, M.D., and David M. Sletten; and Section of Engineering: Randall C. Newman, B.S.E.E. and Shaun Herring. Quality assurance of the nerve conductions was performed by William J. Litchy, M.D.; QST by Peter J. Dyck, M.D.; and autonomic results by Kurt Kimpinski, M.D., Ph.D. and Phillip A. Low, M.D. Mary Lou Hunziker prepared the manuscript. These studies could not have been done without the dedicated and expert help of patients in the RDNS.
Abbreviations
- DM
diabetes mellitus
- DSPN
diabetic sensorimotor polyneuropathy
- HRdb
heart rate variation with deep breathing
- NC
nerve conduction
- QST
quantitative sensation tests
- QAT
quantitative autonomic tests
- QSART
quantitative sudomotor axon reflex test
- RDNS
Rochester Diabetic Neuropathy Study
- Σ 5 NC nds
summated 5 attributes of nerve conduction expressed as normal deviates – see legend to Table 1 for denervations
APPENDIX
Cl vs N Phys Trial Authors
Peter J. Dyck, M.D.1; Carol J. Overland1; Phillip A. Low, M.D.1; William J. Litchy, M.D.1; Jenny L. Davies, B.A.1; P. James B. Dyck, M.D.1; and Peter C. O’Brien, Ph.D.2 (Coordinating Committee) and Henning Andersen, M.D.3, James W. Albers, M.D.4, Charles F. Bolton, M.D.5, John England, M.D.6, Christopher J. Klein, M.D.1, Gareth Llewelyn, M.D.7, Michelle L. Mauermann, M.D.1, James W. Russell, M.D.8, Wolfgang Singer, M.D.1, Gordon Smith, M.D.9, Solomon Tesfaye, M.D.10 and Adrian Vella, M.D.11 (Study Neurologists and Diabetologists)
From the 1Peripheral Neuropathy Research Laboratory, 2Division of Biostatistics and 11Division of Endocrinology, Mayo Clinic, Rochester, MN, USA; 3Aarhus University Hospital, Aarhus, Denmark; 4University of Michigan, Ann Arbor, MI; 5Queen’s University, Kingston, Ontario, Canada; 6Louisiana State University, New Orleans, LA; 7University Hospital of Wales, Cardiff, Wales, UK; 8University of Maryland, Baltimore, MD; 9University of Utah, Salt Lake City, UT; and 10Royal Hallamshire Hospital, Sheffield, UK.
References
- 1.Llewelyn JG, Tomlinson DR, Thomas PK. Diabetic Neuropathies. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. Fourth Edition. Philadelphia: Elsevier; 2005. pp. 1951–1992. [Google Scholar]
- 2.Dyck PJ. Detection, characterization, and staging of polyneuropathy: assessed in diabetics. Muscle Nerve. 1988;11:21–32. doi: 10.1002/mus.880110106. [DOI] [PubMed] [Google Scholar]
- 3.Dyck PJ, Norell JE, Tritschler H, Schuette K, Samigullin R, Ziegler D, et al. Challenges in design of multicenter trials: end points assessed longitudinally for change and monotonicity. Diabetes Care. 2007;30:2619–2625. doi: 10.2337/dc06-2479. [DOI] [PubMed] [Google Scholar]
- 4.Mulder DW, Lambert EH, Bastron JA, Sprague RG. The neuropathies associated with diabetes mellitus: a clinical and electromyographic study of 103 unselected diabetic patients. Neurology. 1961;11:275–284. doi: 10.1212/wnl.11.4.275. [DOI] [PubMed] [Google Scholar]
- 5.LaMontagne A, Buchthal F. Electrophysiological studies in diabetic neuropathy. Journal of Neurology, Neurosurgery & Psychiatry. 1970;33:442–452. doi: 10.1136/jnnp.33.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pirart J. Diabetes mellitus and its degenerative complications: A prospective study of 4,400 patients observed between 1947 and 1973. Diabetes Care. 1978;1(Part 2):252–263. [Google Scholar]
- 7.Quantitative sensory testing: a consensus report from the Peripheral Neuropathy Association. Neurology. 1993;43:1050–1052. doi: 10.1212/wnl.43.5.1050. [DOI] [PubMed] [Google Scholar]
- 8.Low PA, Sletten DM. Laboratory Evaluation of Autonomic Failure. In: Low PA, Benarroch EE, editors. Clinical Autonomic Disorders. Third Edition. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 130–163. [Google Scholar]
- 9.Greene DA, Arezzo JC, Brown MB. Effect of aldose reductase inhibition on nerve conduction and morphometry in diabetic neuropathy. Zenarestat Study Group. Neurology. 1999;53:580–591. doi: 10.1212/wnl.53.3.580. [DOI] [PubMed] [Google Scholar]
- 10.Hermann GA, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53:1634–1640. doi: 10.1212/wnl.53.8.1634. [DOI] [PubMed] [Google Scholar]
- 11.Diabetic polyneuropathy in controlled clinical trials: Consensus Report of the Peripheral Nerve Society. Ann Neurol. 1995;38:478–482. doi: 10.1002/ana.410380323. [DOI] [PubMed] [Google Scholar]
- 12.American Association of Electrodiagnostic Medicine. Guidelines in Electrodiagnostic Medicine. Muscle Nerve. 1999;22 suppl 8:S3–S300. doi: 10.1002/(SICI)1097-4598(199612)19:12<1626::AID-MUS18>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 13.England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, et al. Distal symmetric polyneuropathy: A definition for clinical research. Report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005;64(2):199–207. doi: 10.1212/01.WNL.0000149522.32823.EA. [DOI] [PubMed] [Google Scholar]
- 14.Dyck PJ, Litchy WJ, Daube JR, Harper CM, Dyck PJB, Davies J, et al. Individual attributes versus composite scores of nerve conduction abnormality: sensitivity, reproducibility, and concordance with impairment. Muscle Nerve. 2003;27(2):202–210. doi: 10.1002/mus.10320. [DOI] [PubMed] [Google Scholar]
- 15.Dyck PJ, Kratz KM, Lehman KA, Karnes JL, Melton LJ, III, O'Brien PC, et al. The Rochester Diabetic Neuropathy Study: design, criteria for types of neuropathy, selection bias, and reproducibility of neuropathic tests. Neurology. 1991;41:799–807. doi: 10.1212/wnl.41.6.799. [DOI] [PubMed] [Google Scholar]
- 16.Valk GD, Grootenhuis PA, van Eijk JT, Bouter LM, Bertelsmann FW. Methods for assessing diabetic polyneuropathy: validity and reproducibility of the measurement of sensory symptom severity and nerve function tests. Diabetes Research & Clinical Practice. 2000;47:87–95. doi: 10.1016/s0168-8227(99)00111-4. [DOI] [PubMed] [Google Scholar]
- 17.Husstedt IW, Evers S, Grotemeyer KH. Reproducibility of different nerve conduction velocity measurements in healthy test subjects and patients suffering from diabetic polyneuropathy. Electromyogr Clin Neurophysiol. 1997;37:359–363. [PubMed] [Google Scholar]
- 18.Kohara N, Kimura J, Kaji R, Goto Y, Ishii J, Takiguchi M, et al. F-wave latency serves as the most reproducible measure in nerve conduction studies of diabetic polyneuropathy: multicentre analysis in healthy subjects and patients with diabetic polyneuropathy. Diabetologia. 2000;43:915–921. doi: 10.1007/s001250051469. [DOI] [PubMed] [Google Scholar]
- 19.Dyck PJ, Overland CJ, Low PA, Litchy WJ, Davies JL, Dyck PJB, et al. Evaluation of Diabetic Polyneuropathy: Design of the Neurologic Examination vs. Clinical Neurophysiology Tests Trial (CL vs NPhys Trial). Chapter 75. In: Dyck PJ, Dyck PJB, Low PA, Klein CJ, Amrami KK, Engelstad JK, Spinner RJ, editors. Companion to Peripheral Neuropathy: 78 Illustrated Cases and New Developments. Philadelphia: Elsevier; 2010. [Google Scholar]
- 20.Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: The Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817–824. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- 21.Dyck PJ, O'Brien PC, Davies J, Klein CJ, Dyck PJB. Nerve Tests Expressed as Percentiles, Normal Deviates, and Composite Scores. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. Fourth Edition. Philadelphia: Elsevier; 2005. pp. 971–984. [Google Scholar]
- 22.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 23.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care. 2006;29(9):2114–2116. doi: 10.2337/dc06-1136. [DOI] [PubMed] [Google Scholar]
- 24.Clinical Laboratory Improvement Amendments of 1988. 1988; 42 U.S.C. 263a PL100-578 [Google Scholar]
- 25.Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, O'Brien PC. Variables influencing neuropathic endpoints: The Rochester Diabetic Neuropathy Study of healthy subjects. Neurology. 1995;45:1115–1121. doi: 10.1212/wnl.45.6.1115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


