Abstract
Therapeutic strategies to promote recovery from stroke are now beginning to utilize current knowledge of neural plasticity and the neuromodulatory role of physical rehabilitation. Current interests are also focused on adjuvant therapies that may enhance plasticity associated with recovery and rehabilitation. Amphetamine was one of the earliest pharmacological interventions and continues to show promising results as an adjuvant treatment for recovery of function in pre-clinical animal studies. This drug is a potent modulator of neurological function and cortical excitation, acting primarily through norepinephrine and dopamine mechanisms to enhance arousal and attention, and thus, to facilitate learning of motor skills. Although the results from the pre-clinical studies have been primarily positive, they have not translated well to clinical trials, which have yielded mixed results. This review addresses some of the conflicting evidence from pre-clinical studies conducted between 1982 and 2008 in order to better understand how to optimize the clinical application of amphetamine as an adjuvant therapy for stroke recovery. Among many of the factors that relate to differences in outcome, it is likely that both amphetamine dose and the timing of the intervention with respect to the time of injury affected the outcome.
Keywords: Stroke, recovery, amphetamine, physical therapy, plasticity
1. Introduction
The benefit of behavioral experience after acquired cerebral injuries (stroke, traumatic brain injury) has gained broad acceptance. A recent multi-center clinical trial has demonstrated that significant improvement in motor performance can be achieved in chronic stroke survivors by increased use of the impaired limb for two weeks [46]. To enhance recovery further, basic and clinical scientists have long been interested in the use of various pharmacological agents that might interact with the use-dependent properties of physiotherapy on brain plasticity mechanisms, and thus, potentially result in even greater behavioral benefits. Many pre-clinical and clinical studies over the past 25 years have focused on the psychostimulant dextroamphetamine (d-amphetamine), which is known to increase levels of norepinephrine, serotonin and dopamine in the brain. While the majority of animal studies of brain injury and recovery have reported substantial benefits on motor and cognitive performance, especially when d-amphetamine is combined with behavioral treatment, the results of clinical trials have been mixed. In an effort to understand why this promising treatment approach has, so far, failed to translate effectively from animal to human studies, we highlight some of the main preclinical findings, with emphasis on differences that might yield clues to understanding the results in human stroke trials.
2. Neuromodulation of cortical activity by d-amphetamine
In the central nervous system, d-amphetamine stimulates the release of the catecholamines norepinephrine, dopamine and to a lesser extent, serotonin, from nerve terminals. The stimulant effects of releasing these neurotransmitters is further enhanced by the properties of amphetamine that block reuptake while inhibiting the actions of monoamine oxidase from degrading intracellular accumulation of neurotransmitters. Release of these neurotransmitters has a neuromodulating effect upon motivational and cognitive systems within the brain. The major axonal pathways transporting norepinephrine, dopamine and serotonin ascend from brain stem nuclei (locus coeruleus, the substantia nigra pars compacta and ventral tegmental areas, and raphe nucleus respectively) and modulate cortical excitability relative to motivational states and attentional processes and thus underlie adaptive interaction with the environment. The neuromodulatory effects of these neurotransmitters are thought to function as learning signals [19] responsible for establishing new responses to environmental shifts in behavioral contingencies. In this way amphetamine can have a profound neuromodulatory effect upon behavior due to its amplification of catacholaminergic activity.
3. Pre-clinical studies showing positive outcomes with d-amphetamine after cerebral injury
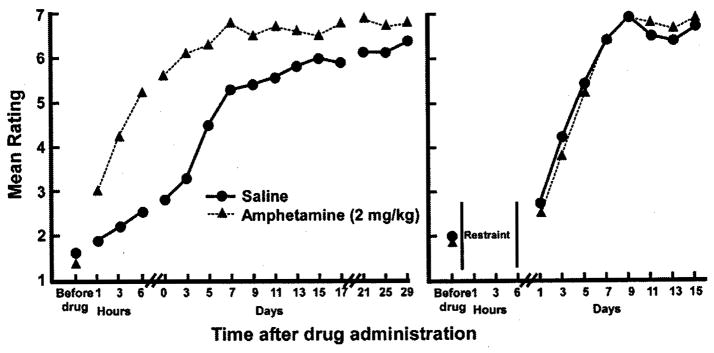
Early interest in using psychostimulants such as amphetamine to promote recovery after brain injury began in earnest in the 1980’s in Dennis Feeney’s laboratory with a seminal paper in which hemiplegic rats were treated with d-amphetamine paired with training on a locomotor task to restore coordinated motor function on a beam-walking task [10]. Injuries were produced by unilateral suction ablation of the somatic sensorimotor cortex. The results from this study indicated that a single dose of d-amphetamine given 24 hr after the injury enhanced the therapeutic effects of behavioral training to restore locomotor skills (Fig. 1). The behavioral improvement was blocked if the animals were restrained, or if d-amphetamine was followed by an injection of the catecholamine antagonist, haloperidol.
Fig. 1.
Original results demonstrating an interactive effect of amphetamine administration and behavioral experience. In male rats, motor cortex was aspirated unilaterally. Twenty-four hours after surgery, animals were given amphetamine or saline. Each animal was evaluated on their ability to traverse a narrow beam to escape white noise and bright light every hour for 6 hours after drug administration. Subsequently, beam-walking was evaluated every other day for 15–29 days. Left: Mean ratings of rat locomotion in amphetamine (triangles) and saline control (circle) groups. In animals that experienced beam-walking during the 6 hours after drug administration. Right: Mean ratings of rat locomotion in animals that were confined to small cages to prevent locomotion during the first 8 hours after drug administration. All animals eventually recovered on the task. However, animals that were given amphetamine followed by the beam-walking experience demonstrated accelerated recovery that was maintained throughout the recovery period. Animals given amphetamine, but restrained after drug administration were identical to saline controls. Redrawn from [10].
Unlike most human rehabilitation trials in which an independent primary outcome measure is used to determine treatment effects, in the Feeney et al. study, and in most pre-clinical studies in animals, the same task that is used for behavioral treatment is also used to track the performance of the animals over time. While the rationale for employing this strategy in animal studies is primarily practical, this issue should be kept in mind when evaluating the translational value of animal studies for predicting outcomes in human trials.
In this, and many subsequent studies of the interactive effects of d-amphetamine and behavioral experience, assessment of recovery was based on behavioral performance both during the period of acute amphetamine intoxication, and during subsequent time periods. In other studies, behavioral performance was determined in a pre-drug period, and then tracked for several hours after the administration of the drug. Since the half-life of d-amphetamine is approximately 12 hr, not only is it important to provide behavioral experience within several hours of drug administration, but it is equally important to understand the chronic effects of the drug/behavior treatment beyond the 12 hour window. Effects that wane soon after drug intoxication are not as relevant for translating to clinical treatment in human populations. Thus, in the review of the studies that follow, we indicate the time period over which the assessments were made, and emphasize behavioral changes that outlast the injection of the drug by at least 24 hr (Table 1). In the case of the Feeney et al. study, locomotor performance was tracked for 15–29 days after treatment, or up to 30 days after the lesion, and the amphetamine treated group maintained superior performance throughout the follow-up period.
Table 1.
Summary of pre-clinical studies
| Authors | Species | Sex | Lesion model |
Dose (mg/kg) |
1st admin | Drug to behavior time |
Freq. | Last Follow-up |
Behavioral Task (s) |
Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Feeney et al 1982 [10] | Rat | M | SMCa, aspiration | 0.5 1 2 4 |
PLHb 24 | 1 hr | Single | PLD 30 | Beam walking | 0.5 − 1 − 2+ 4 + (at 1 hr PTc) |
| Feeney & Hovda 1983 Exp 1 [11] | Cat | NSd | Frontal (motor) cortex, aspiration | 5(d,l)e 5(d) 8(d) |
PLD 4 | 15 min | PLD 4, 9, 15, 30 | PLD 30 (≤24 hrs PT) | Tactile placing | 5 (d,l) + (>12 hrs PT; waned by 24 hrs) 5, 8 (d) mixed; high mortality |
| Feeney & Hovda 1983 Exp 2 [11] | Cat | NS | Frontal (motor) cortex, undercut | 5(d,l) 5(d) 2.5(d) |
PLD4 | 15 min | Multiple, ≥5 days between doses | PLM 18 (≤24 hrs PT) | Tactile placing | 5 (d,l) + (>12 hrs PT; waned by 24 hrs) 2.5, 5 (d) + but less enduring |
| Hovda & Feeney 1984 exp 1 [21] | Cat | M F |
Frontal cortex, ablation | 5 | PLD10 | 1 hr | Single | PLD 60 | Beam walking | + through 60 days |
| Hovda & Feeney 1984 exp 2 [21] | Cat | M F |
Frontal cortex, ablation | 5 | PLD10 | 1 hr | PLD 10, 14, 18, 22 | PLD 60 | Beam walking | + through 60 days, better than single dose |
| Hovda & Feeney 1985 [20] | Cat | M F |
Visual cortex, extensive ablation | 5 | PLD10 | 3 hrs | PLD 10, 14, 18, 22 | PLD 30 | Visual cliff | + through PLD 30 |
| Feeney & Hovda 1985 [21] | Cat | UNS | Visual cortex, bilateral, ablation | 5 | PLD10 | 1 hr | PLD 10, 14, 18, 22 | PLD 42 | Visual cliff | + through PLD 42 |
| Hovda et al 1987 [22] | Cat | 16M 7F |
Visual cortex, bilateral | 5 | PLD10 | 3 hrs | PLD 10, 14, 18, 22 | PLD 30 | Visual placing | − |
| Tactile placing | + through PLD 30 | |||||||||
| Hovda et al 1989 [23] | Cat | 16M 7F |
Visual cortex, bilateral aspiration | 5 | PLD10 | 3 hrs | PLD 10, 14, 18, 22 | PLD 30 | Visual cliff | + through PLD 30 |
| Sutton et al 1989 [44] | Cat | 5M 6F |
Frontal (motor) cortex, bilateral, aspiration | 5 | PLD 12 | 1 hr | PLD 12, 16, 20 | PLD 30 | Beam walking | + through PLD 30 |
| Goldstein and Davis 1990 [17] | Rat | M | SMC, aspiration | 2.6 | PLH24 | 1 hr | Single | 24 hrs | Beam walking | + through 24 hrs |
| Goldstein and Davis 1990 [15] | Rat | M | SMC, aspiration | 2.6 | PLH 24 | 1 hr | Single | 24 hrs | Beam walking | + |
| Goldstein and Davis 1990 [16] | Rat | M | SMC, aspiration | 2 | PLD1 | 1 hr | Single | 24 hrs | Beam walking | + |
| Hurwitz et al 1991 [24] | Rat | M | Barrel field cortex, photothrombosis | 2 4 |
PLD 4 | 24 hrs | PLD 4, 6, 9, 11 | PLD 35 | T maze, vibrissae stimulation | + through PLD 35 (both doses) |
| Colbourne & Corbett 1992 [7] | Gerbil | F | 3 min CAof | 1 2 |
<1 hr or PLH24 | 15 min (24 hr grp) | Single | PLD 10–11 | Open field activity (spatial mapping) | − |
| Wishart et al 1994 [45] | Gerbil | M | 5 min CAo | 3 | 24 hrs | 48 hrs | Single | PLD 53 | Radial arm maze | − reference memory + working memory |
| Prasad et al. 1995 [34] | Rat | M | Fluid percussion Injury | 2 4 |
PLM10 | 1 day | Single | PLD 14 | Beam walking | + days 1–5 (no dose difference) |
| Morris water maze | + (4 mg/kg only, trial block 5) | |||||||||
| Schmanke et al 1996 [39] Exp 1 | Rat | M | SMC, electrolytic | 2 | PLH24 | Immed | Single | 6 hrs PT | Beam walking | + up to 6 hrs |
| Grid walking | − | |||||||||
| Vibrissa-forelimb placing | − | |||||||||
| Tactile placing | − | |||||||||
| Sticky dot test | − | |||||||||
| Schmanke et al 1996 [39] Exp 2 | Rat | M | SMC, electrolytic | 2 | PLH24 | Immed | PLH 24, PLD 3, 5 | PLD 30–60 (task-specific) | Beam walking | + on PLD 7, 9, 15 |
| Grid walking | − | |||||||||
| Vibrissa-forelimb placing | + (days UNS) | |||||||||
| Tactile placing | +(days UNS) | |||||||||
| Sticky dot test | − | |||||||||
| Schmanke & Barth 1997 [38] | Rat | M | SMC, electrolytic | 2 | PLD 1 | Immed | PLD 1, 3, 5 | PLD 56 | Vibrissa-forelimb placing | + up to PLD 56 |
| Tactile placing | − | |||||||||
| Stroemer et al. 1998 [42] | Rat (SHR)g | M | MCAo | 2 | PLD3 | 1 hr | PLD 3, 6, 13, 16, 19, 22, 25, 28 | PLM 2 | Grid walking | + through 2 mos |
| Morris water maze | − (worse) at 3 days + at 2 mos |
|||||||||
| Brown et al. 2004 [5] | Rat | M | SMC, photothrombosis | 2 | PLH24 | 30 min | Single | PLD 10 | Beam walking | + but group × day interactions not reported; not apparent >PLD 8 |
| Adkins & Jones 2005 [1] | Rat | M | SMC, ET-1 | 1 | PLD 10–14 | 1–2 hrs | Training days 2, 5, 8, 11, 14, 17, 20i | 90 days post-therapy (PLD 100–104) | Pellet retrieval | + through 30 days post-therapy; n.s. at 60 and 90 days |
| Gilmour et al 2005 [13] | Rat | M | SMC, ET-1 | 2 | PLD 2 | 1–2 hrs | PLD 2, 5, 8, 11, 14, 17, 20, 23, 26 | PLD 27 | Grid walking | − On drug (1 hr PT) −Off drug(24 hr PT) |
| Pellet retrieval | − worse on drug + off drug |
|||||||||
| Chudasama et al. 2005 [6] | Rat | M | Dorsal prefrontal cortex, bilateral | 0.2 0.4 0.8h |
PLD 11? | UNS | Alternate days | PLD 20 | Combined attention-memory | + attention (0.2 ma/kg only; days not specified) − memory |
| Ramic et al. 2006 [35] | Rat | M | SMC, aspiration lesion | 2 | PLD2 | Immed | PLD 2, 5 | PLW6 | Pellet retrieval | + through PLW6 |
| Ladder walking | + through PLW6 | |||||||||
| Barbay et al 2006 [4] | Sq. m’nk. | 6M 6F |
Motor cortex, electrocoagulation | 0.25 | PLD 10 | 1 hr | Single | PLM3(9 wks post-training) | Pellet retrieval | + through PLM3 |
| Rasmussen et al 2006 [36] | Rat | M | Embolic MCAog | 3.5 | PLD1 | 1 hr | PLD 1, 3, 5, 7 | PLD 28 | Memory test | + only with D-amph alone, − when combined with training |
| Montoya staircase | − | |||||||||
| Alaverdashvill et al 2007 [2] | Rat | F | SMC, pial strip | 1i | PLH24 | 30 min | PLH 24, PLD 4, 7, 10, 13, 16, 19, 22, 42,45 | PLD 47 | Pellet retrieval | − but improved non-reaching movements (locomotion, rearing, turning) |
| Tray reaching | − | |||||||||
| Auriat & Colbourne, 2008 [3] | Rat | M | Intracerebral hemorrhage (striatum) | 2 | PLD 7 | 30 min | PLD 7, 9, 11 | PLD 28 | Montoya staircase | − |
| Tray reaching | j | |||||||||
| Ladder walking | − | |||||||||
| Beam walking | − | |||||||||
| Neurological deficit score | − | |||||||||
| Papadopoulos et al 2008 [33] | Rat | M | MCAo | 2 | PLD2 | 10 min | PLD 2, 5, 8 | PLW 8 | Pellet retrieval | + through PLW8 |
| Ladder walking | + through PLW 8 |
SMC = somatic sensorimotor cortex
PLM = post-lesion minute; PLH = post-lesion hour; PLD = post-lesion day; PMW = post-lesion week; PLM = post-lesion month
PT = post-treatment (after last treatment day)
NS = not specified
Racemic mixture of d- and l-amphetamine. All other studies used d-amphetamine exclusively
CAo = carotid artery occlusion (global ischemia model)
SHR = spontaneously hypertensive rats
All rats received each dose in counterbalanced Latin square design
D-methamphetamine
Excessive dropouts precluded analysis
In contrast, in another early study by Feeney and Hovda, tactile placing (sometimes called forelimb-forelimb placing) was examined in cats after motor cortex ablations [11]. D,l-Amphetamine (racemic mixture) was given on days 4, 9, 15 and 30 post-lesion. Tactile placing was restored within several minutes after the injection, and the improved performance persisted for at least 12 hr. However, improvements were not evident during the pre-treatment testing on each of the treatment days, and the restoration of tactile placing waned by 24 hr after treatment. Thus, at least in this study, the therapeutic effects of amphetamine were only demonstrated during the period of intoxication.
Subsequent studies extended the general findings of an interactive effect of d-amphetamine and behavioral experience in cats, rats and monkeys, and in the recovery of perceptual and cognitive, in addition to motor function (see Table 1). These studies also demonstrated that the therapeutic effects of d-amphetamine could be enduring, as long as 56 days post-lesion [38]. A common finding from these papers is that restoration of function appears to be dose-dependent (multiple doses yield better results) and symptom-related experience in conjunction with the drug is beneficial. A study by Goldstein and Davis [15] indicated that amphetamine alone can have a restorative effect on locomotor skills, but to a lesser extent then when paired with symptom-specific training, implying that there is an additive effect of pairing amphetamine with training.
In early studies, the assumption was that amphetamine restored function by improving metabolic activity in the CNS, thus releasing neurons from remote functional depression associated with brain injury. This explanation for recovery of function has been referred to as resolution of diaschisis and is traditionally associated with spontaneous recovery of function [9]. According to this hypothesis, cerebral injury is associated with both primary and secondary deficits. The primary deficit is associated directly with the site of injury and the secondary deficits are due to suppressed neural activity in otherwise potentially viable areas of the CNS that are functionally related to the primary site of injury. Recovery from cortical injury can be due to a restoration of function to these secondary areas that can occur “spontaneously” or be therapeutically enhanced [22,43].
More recent attempts to explain the effects of d-amphetamine on recovery of function have focused upon neuroanatomical reorganization. Anatomical plasticity had been demonstrated in neocortex following a unilateral infarct in rats [25,26,41]; therefore, it was thought that d-amphetamine could facilitate recovery by enhancing this phenomenon. In one of the first demonstrations of amphetamine-enhanced plasticity, Stroemer et al. made unilateral distal middle cerebral artery (MCA) occlusions in rats, affecting the sensorimotor cortex while sparing subcortical regions. Skilled locomotion and cognitive processing were assessed. The behavioral results showed that multiple injections of d-amphetamine paired with training on a grid-walking task facilitated recovery of skilled forelimb use. A single pairing of d-amphetamine and spatial training on the Morris water maze task did not facilitate recovery. However, d-amphetamine treated rats were able to maintain recovery of spatial memory at two months following training, whereas the saline treated rats did not [42].
The most novel finding in the Stroemer et al. study was that d-amphetamine paired with training enhanced expression of GAP-43 and synaptophysin in both the intact and damaged hemispheres, presumably indicative of synaptogenesis and axonal sprouting. A later study [35] provided more direct evidence of amphetamine-induced axonal sprouting using the anterograde tracer biotinylated dexran amine (BDA). In this study, rats received a unilateral aspiration lesion to remove sensorimotor cortex corresponding to the forelimb motor areas. Motor performance was assessed with a pellet retrieval task and a ladder-walking task. Post-infarct training consisted of placing rats in an enriched environment designed to engage rats in the skilled use of the forelimbs such as climbing a vertical rope, an inclined ladder and a vertically oriented, cylindrical wire grid. The results showed that d-amphetamine paired with environmental enrichment significantly improved skilled forelimb use compared to training alone, amphetamine alone and non-treated rats. However, amphetamine treatment not paired with training also had a significant effect. All treatment groups showed some axonal sprouting from the intact sensorimotor cortex to the deafferented bulbar region of the brainstem. The joint treatment of d-amphetamine paired with training resulted in significantly more BDA labeled corticobulbar fibers crossing midline. A later study [33] extended these findings using an MCAo stroke model, demonstrating a significant increase in axonal sprouting from the intact cortex to the denervated red nucleus and cervical spinal cord.
Pellet retrieval tasks to assess sensorimotor cortex damage have become more common in recent studies in rats and monkeys [1,2,4,13,35]. The popularity of such tasks stems from the reliability of the performance measures, the sensitivity of the task after even relatively small cortical lesions, and, depending upon the size of the lesion, the ability to detect deficits weeks after the injury. Benefits from combined treatment with amphetamine and pellet retrieval training have been demonstrated up to eight weeks post-treatment in rats [33] and nine weeks post-treatment in monkeys [4].
4. Pre-clinical studies with negative outcomes
While the positive results of these preclinical studies have received considerable attention, a quick scan of Table 1 reveals that d-amphetamine has not always been beneficial in animal studies after injury. As discussed above, some of these negative findings occurred when animals were tested after the period of amphetamine intoxication, demonstrating only a transient benefit [11]. In a more recent study by Alaverdashvili et al. in rats given pial strip lesions followed by amphetamine treatment, training facilitated recovery for all rats, but there was no benefit to coupling training with d-amphetamine at either the acute or chronic periods [2]. Also, even though there were no differences between d-amphetamine treated and non-treated rats at the end of training, amphetamine slowed the rate of recovery on a pellet retrieval task. The only benefit observed with amphetamine was that it facilitated certain non-reaching movements, such as locomotion, rearing and turning.
There were a few design idiosyncracies in the Alaverdashvili study that may account for differences in outcome. The amphetamine used was d-methamphetamine. While d-methamphetamine and d-amphetamine are pharmacologically similar, d-amphetamine may be a more potent stimulant of locomotor activity, and more potent than methamphetamine for enhanced working memory [40]. In addition, the drug was delivered orally, administered to the rats in food. Differences in behavioral activation between d-amphetamine and methamphetamine may depend upon the route of administration since methamphetamine is metabolized into amphetamine with peripheral administration (oral or intraperitoneal) which can alter interpretation of dose/behavior relationships [31]. Finally, female rats were used in this experiment. In fact, this is the only study to date using combined amphetamine-behavioral experience treatment that examined female rats. The rationale for the use of female rats in this study was based upon evidence suggesting that d-amphetamine metabolism in humans is more similar to that seen in female rats compared to male rats [30].
Some studies have found that the beneficial effect of combining amphetamine and rehabilitative training after brain injury is task-specific. Gilmour et al. [13] found positive outcomes pairing amphetamine with pellet retrieval training but did not find any benefit when pairing amphetamine with a grid-walking task. Schmanke et al. [39] also found negative results when pairing amphetamine with a grid-walking task even though the combined treatment facilitated recovery on a beam-walking task. These authors speculated that amphetamine could have interfered with training by increasing locomotion and impulsive placement of the forelimb while traversing the grid, which is counterproductive to developing adaptive motor skills necessary to make coordinated placements of the forelimbs to avoid missteps. These results differ from previous studies showing that pairing amphetamine and grid-walking facilitated recovery on the grid-walking task [42].
In a recent experiment amphetamine was found to be beneficial as the sole treatment, but not when paired with post-infarct training. Rasmussen et al. [36], treated rats with d-amphetamine after an embolic MCAo that resulted in unilateral cortical and subcortical damage. Drug treatment transiently facilitated recovery on a cognitive task, but not when paired with training. On a pellet retrieval task, only post-operative training significantly improved recovery; amphetamine attenuated the benefits of post-infarct training when both treatments were coupled. Amphetamine reduced the number of errors on a T-maze task when given alone (first post-training day only). All rats recovered by post-infarct day 24 (4th day of assessment). The failure of rats to benefit from training only and training paired with amphetamine was attributed to the severity of the infarct during the first week of recovery and to the effects associated with the 3.5 mg/kg amphetamine dose. Rats treated with amphetamine were reported to be constantly engaged in stereotypic behavior. The authors also point out that amphetamine intoxication interfered with pellet retrieval. Saline treated rats were able to retrieve and eat pellets during the post-infarct training whereas the amphetamine treated rats ate no more than one pellet. Therefore, the amphetamine treated rats were not able to participate adequately in pellet retrieval training.
No single factor can be easily discerned to explain the differences in preclinical results, as these studies varied in several aspects, including dosing regimen, number and type of behavioral tasks employed and lesion model. Table 2 summarizes the positive and negative effects on various behavioral tasks in preclinical studies of amphetamine. In general, results have been most consistent using the beam-walking task. Ten of 11 studies employing this task have shown a positive benefit of pairing d-amphetamine and experience on the task. Pellet retrieval (5/6 positive studies), performance on the visual cliff (3/3 positive studies), ladder walking (2/3 positive studies), vibrissae-forelimb placing (2/3 positive studies) and tactile placing (4/6 positive studies) have also been reasonably consistent.
Table 2.
Summary of positive and negative preclinical behavioral resultsa
| Task | # positive studies | # negative studies |
|---|---|---|
| Beam Walking | 10 | 1 |
| Grid walking | 1 | 3 |
| Ladder Walking | 2 | 1 |
| Pellet Retrieval | 5 | 1 |
| Tactile placing | 4 | 2 |
| Visual cliff | 3 | 0 |
| Vibrissae-forelimb placing | 2 | 1 |
Minimum of three studies employing same task.
5. Designing preclinical studies combining d-amphetamine with behavioral experience: Factors important for translation to clinical trials
5.1. Summary of clinical findings to date
Since the majority of animal studies have demonstrated positive outcomes on both motor and cognitive assessments when d-amphetamine is combined with behavioral experience, there has been considerable interest in determining whether amphetamine can be used safely and effectively in humans after stroke. A recent Cochrane review of amphetamines for improving recovery after stroke summarized the results of 10 randomized trials involving 287 stroke survivors from papers published through 2006 [28]. In six of these studies, drug administration was followed by rehabilitation within 180 minutes, or within the period of amphetamine intoxication. The main results were that amphetamine: 1) did not reduce death or dependence, 2) increased systolic and diastolic blood pressure, as well as heart rate, and 3) resulted in a better relative change in motor function from baseline to last follow up. The latter result was based on six studies with 176 patients. Also, amphetamine was associated with better language function at follow-up based on one study with 20 patients.
However, examining the Fugl-Meyer assessment of motor performance, the raw scores at follow-up were not better in amphetamine-treated patients. Also, amphetamine was not associated with better activities of daily living scores, depression grade or neurological function at follow-up, nor were relative change scores significant. Thus, the results with amphetamine after stroke are somewhat equivocal. The authors’ conclusion was that too few patients have yet been studied to draw firm conclusions regarding amphetamine and recovery after stroke.
5.2. Time of drug administration
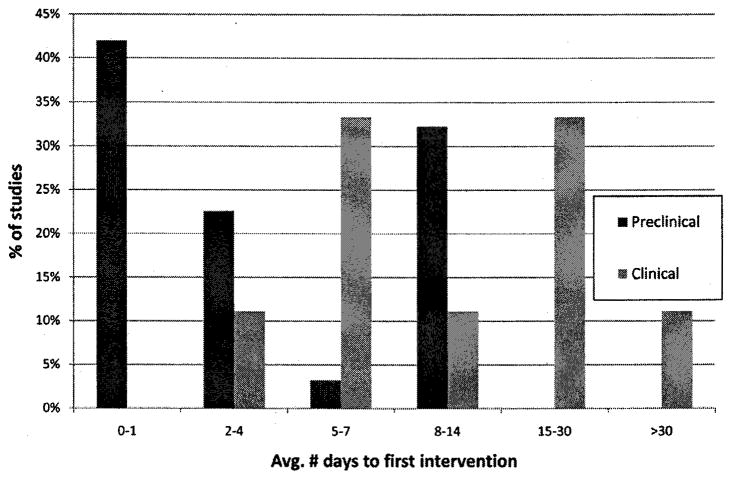
Pre-clinical studies vary greatly in the precise drug administration protocol, especially with respect to time of administration after injury. Figure 2 illustrates that in 40% of the published animal studies, amphetamine was administered within 24 hr after injury. While this time point has been useful to demonstrate proof-of-principle and to investigate the mechanisms underlying the effects of amphetamine after injury, it is not optimal for translation to clinical trials. Nonetheless, the majority of animal studies that have begun administration in later phases of the acute post-injury period (24 hr to one week) as well as the subacute period (one week to 3–4 mos) have demonstrated positive behavioral outcomes (Table 1).
Fig. 2.
Time of administration of d-amphetamine after injury in pre-clinical and clinical studies. Pre-clinical results are based on 31 studies, using a variety of species, injury models, and dosing regimens (Table 1). Clinical results are based on 10 trials summarized in a recent Cochrane review [28].
One of the difficulties in administering amphetamine at later time points in animal models, especially in rats, is that substantial spontaneous recovery occurs using the typically employed injury paradigms. Control animals are often fully recovered by 3–4 weeks after injury. Either more extensive lesion models, more sensitive behavioral assays, or both, are needed in the future to determine if there is a sensitive period for the administration of d-amphetamine after injury.
Figure 2 also illustrates the distribution of average time of first drug administration in published clinical trials included in the 2007 Cochrane review [28]. Though relatively few studies are represented, the distribution is decidedly later compared with animal studies. Very few clinical studies have employed amphetamine administration within the acute time window (<7 days post-stroke), primarily because of autonomic effects. It is possible that administration of amphetamine at later time points after stroke is not as effective, since 2 of 3 studies with average first administration later than one week post-stroke were negative, while 2 of 2 studies with first administration within one week post-stroke were positive. It should be noted though, that in the Gladstone, 2006 study [14], the largest such trial combining amphetamine and physical therapy to date (71 stroke survivors), therapy was begun an average of eight days after stroke, and yet there was negligible effect of the treatment on motor performance scores in the entire cohort. There was a trend toward better recovery in the moderately impaired stroke cohort, compared with the severely impaired cohort, but this trend was not significant. As Martinsson et al. [28] point out, the clinical sample is still too small to draw definitive conclusions at this point.
5.3. Time from amphetamine administration until training
With the exception of a few early studies in cats in which amphetamine administration was followed by behavioral training 3 hrs later, in the vast majority of preclinical studies, the time from drug administration to behavioral training was one hour or less. However, only three of the clinical trials have specified a drug treatment to rehabilitation time of one hr or less. While this factor has not been systematically examined in preclinical studies, it may be important. The pharmacokinetics of amphetamine using various routes of administration are now well-known, but the time of optimal pairing with behavioral training has not been established.
5.4. Dose of amphetamine
In general, preclinical studies have demonstrated a dose-dependent effect of amphetamine-behavioral pairings. That is, multiple dosing regimens appear to be superior to single dosing schedules. However, amphetamine can adversely affect behavior in a dose-dependent manner resulting in hyper-locomotion which transitions into repetitive stereotypical behaviors as dosages increase. This drug-induced behavioral activation has been shown to depend upon increases in dopamine levels in the nucleus accumbens and caudate nucleus resulting in increased locomotion and stereotypy respectively [8,27,32,37]. A systematic review of the literature published by Grilly and Loveland in 2001 [18] characterized the behavioral effects of d-amphetamine in rats at various doses from 55 studies. The common dose of d-amphetamine that resulted in increased locomotion was between 0.4 and 1.0 mg/kg. Doses between 1.0 and 3.0 mg/kg resulted in an intense locomotion, and the beginnings of stereotypy. Doses greater than 3.0 mg/kg induced intense stereotypy.
Large doses of amphetamine (≥3 mg/kg) administered after sensorimotor cortical ablations in cats have been shown to transiently restore visual tactile placing [11,29], as well as promote a more enduring recovery of tactile placing [22]. These effects are believed to be due to a resolution of diaschisis by amphetamine temporarily reactivating functionally suppressed areas of the brain allowing the tactile placing reflex [43]. However, when applying rehabilitative behavioral training to achieve enduring functional recovery of more complex behaviors beyond the time of amphetamine intoxication, caution should be taken to choose an appropriate dose that will be psychoactive but not deleterious to task specific behaviors. Studies cited in Grilly and Loveland’s review indicate that doses of d-amphetamine ranging from 1 to 3 mg/kg can have deleterious effects upon certain behavioral and cognitive tasks. Therefore the quality of the behavioral experience depends upon the drug/behavior interaction. Tasks that require more attention and refined motor skills may be more vulnerable to the psychoactive effects of larger doses of d-amphetamine.
The behavioral tests now commonly used for rehabilitative training and assessment are beam-walking, grid-walking and pellet-retrieval tasks (Table 1). Dosing regimen has been shown to have variable effects upon participation in these tasks, possibly affecting outcome. Beam-walking has been successfully coupled with high doses of d-amphetamine in cats (5 mg/kg; refs [13,30]) and rats (4 mg/kg [10]), but the common dose for rats has been 1 to 2.6 mg/kg [5,16,17, 34]. As tasks become more complex requiring more skill to complete, such as grid-walking, higher doses seem to interfere. An example is the Schmanke et al. [39] study in which 2 mg/kg facilitated recovery from deficits on beam-walking task but not on the grid-walking task. The authors speculated that amphetamine interfered with skilled placement of forelimb on the grid-walking task based on proprioceptive feedback. This is more obvious in studies in which amphetamine slowed recovery compared to training only. In both the Alaverdashvili [2] study and the Brown [5] study post-infarct treatment with 1 mg/kg d-methamphetamine or 2 mg/kg d-amphetamine slowed rate of recovery. In the Adkins and Jones [1] study the deleterious effect of amphetamine (1 mg/kg) is obvious on each post-infarct training day in which amphetamine was paired with pellet retrieval training (performance dropped on each day of adjuvant training and significantly improved on the following, drug free day). Rasmussen et al. reported that 3.5 mg/kg d-amphetamine produced stereotypic behaviors that interfered with rehabilitative training on both the cognitive T-maze task and the Montoya pellet retrieval (stair case) task.
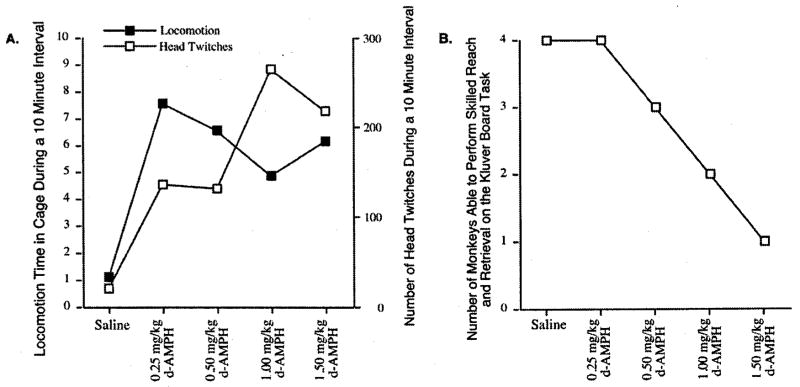
In a recent primate study [4], pilot data was used to not only determine an adequate dose to allow participation in a pellet-retrieval task but a dose that was the least disruptive compared to performance without drugs. Four doses of amphetamine within Grilly and Loveland’s [18] range for “low dose” and “high dose” for rats and humans were selected for comparison in normal squirrel monkeys. All four doses increased locomotion and head movements (Fig. 3). The doses within the low range (0.25 mg/kg) and moderate range (0.50 mg/kg) were the least disruptive to the pellet retrieval task and produced mild psychoactive drug effects (repetitive head movements); doses in the high range (1.0 and 1.5 mg/kg) resulted in more intense head movements and significantly interfered with participation on the skilled reach task (Fig. 3). The lowest dose of amphetamine (0.25 mg/kg) was chosen because it was demonstrated to be psychoactive while producing the fewest effects on the pellet-retrieval task. Larger doses of amphetamine may be more potent for enhanced metabolic activity promoting release from diaschisis, but lower doses may be necessary to promote behavioral learning during rehabilitation.
Fig. 3.
Four experimentally-naïve squirrel monkeys were used to determine the psychostimulant effect of four doses of d-amphetamine upon home cage activity. Experimental sessions were separated by a minimum of one week after saline treatment and two weeks after d-amphetamine treatment. (A) Cage activities (repetitive behavior and locomotion) were monitored during the first hour following drug administration. (B) Motor skill performance was assessed during the second hour using a reach and retrieval task that measured manual skill from a small food-well (5 mm deep, 9.5 mm dia.) which requires restricted use of the digits. Each dose of amphetamine produced a pschoactive effect; however doses within the high dose range (1.0 and 1.5 mg/kg) produced more intense repetitive head movements and interfered with pellet retrieval (Kluver board task).
5.5. Study sample characteristics in preclinical studies: Sex and age
While female animals have been employed in some preclinical studies, there is a distinct bias toward the use of male rats. This is one factor that requires further examination in establishing translational links to human trials. Of the 31 preclinical studies reviewed here, seven used both male and female animals (the six early studies in cats by Feeney and colleagues; one non-human primate study). Two additional rodent studies used only female animals. While it is generally accepted that the use of female animals will introduce greater variability in the results of such studies (principally due to hormonal cycles), from the standpoint of translation of results to human trials, the animal data clearly is biased with regard to sex of the animals. In the clinical trials examined for the Cochrane review [28], both males and females were included, though males generally outnumbered females.
In animal studies, age is usually not provided. However, many of the studies cited in Table 1 indicate that adult animals were used, or it can be assumed that the animals were adults based on reported body weights. However, none of the preclinical studies reviewed here specifically included aged animals. Two reasons for excluding aged animals are typically cited: cost and morbidity. Aged animals often do not survive the anesthesia or the experimental surgical procedures that are required for inducing cerebral injuries. However, given that stroke, in particular, occurs more often in elderly populations (mean age in Cochrane review = 66, ranging from 31 to 91 years), it is critical to understand whether the positive effects of amphetamine in preclinical studies can be extended to aged animal populations.
5.6. Injury model
The relevance of various cerebral injury models for translation to human trials has long been a subject of controversy. In one sense, preclinical studies often are designed to illustrate proof-of-principle, and not necessarily mimic human conditions of stroke or traumatic brain injury. Also, the demonstration that amphetamine paired with behavioral experience can accelerate recovery across a variety of injury models provides further credence to the notion that this is a very robust effect. However, it is clear that the majority of preclinical studies to date do not replicate the conditions of acquired cerebral injuries in humans. Most importantly, the majority of animal studies are restricted to cortical injury, while strokes in humans often result in injury to subcortical structures (striatum, internal capsule, thalamus), either alone or in combination with cortical injury. Very few preclinical studies have examined the effect of amphetamine on the types of injuries that most often occur in human stroke.
6. Summary
It is clear from both preclinical and clinical studies that post-injury training is an important element in promoting recovery. The quality of the post-injury experience is crucial to the rate and extent of recovery. Amphetamine, when paired with behavioral experience, appears to facilitate recovery in an additive or interactive way, at least in most preclinical and some clinical studies. Neuromodulation of plasticity is helpful to enhance plasticity, creating a permissive state for learning. This may especially be important after brain injuries that can cause reduction in neuromodulatory catacholamines. Amphetamine may enhance neural signals to maximize sensorimotor integration and maybe resolve issues of diaschisis and enable participation in symptom relevant experience. It would seem that successful pairing of psychostimulants with behavioral training requires a delicate balance between achieving an adequate psychoactive dose and one that does not severely interfere with cognitive or behavioral processes. Many factors related to the optimal design of clinical trials pairing amphetamine and behavioral experience, such as the timing of treatment relative to injury onset, and the timing, quality and quantity of the behavioral experience have not yet been established. Animal models might yet yield important information regarding the most important factors affecting motor and cognitive outcomes, but close attention to study design, with an eye toward the eventual translation to human populations, is important for future studies.
Acknowledgments
This work was supported by a Javits Neuroscience Investigator Award from the National Institutes of Health to RJN (R37NS030853), a Bugher Award to RJN from the American Heart Association, and the Landon Center on Aging.
References
- 1.Adkins DL, Jones TA. D-amphetamine enhances skilled reaching after ischemic cortical lesions in rats. Neurosci Lett. 2005;380:214–218. doi: 10.1016/j.neulet.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 2.Alaverdashvili M, Lim DH, Whishaw IQ. No improvement by amphetamine on learned non-use, attempts, success or movement in skilled reaching by the rat after motor cortex stroke. Eur J Neurosci. 2007;25:3442–3452. doi: 10.1111/j.1460-9568.2007.05594.x. [DOI] [PubMed] [Google Scholar]
- 3.Auriat AM, Colbourne F. Influence of amphetamine on recovery after intracerebral hemorrhage in rats. Behav Brain Res. 2008;186:222–229. doi: 10.1016/j.bbr.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Barbay S, Zoubina EV, Dancause N, Frost SB, Eisner-Janowicz I, Stowe AM, Plautz EJ, Nudo RJ. A single injection of D-amphetamine facilitates improvements in motor training following a focal cortical infarct in squirrel monkeys. Neurorehabil Neural Repair. 2006;20:455–458. doi: 10.1177/1545968306290773. [DOI] [PubMed] [Google Scholar]
- 5.Brown AW, Bjelke B, Fuxe K. Motor response to amphetamine treatment, task-specific training, and limited motor experience in a postacute animal stroke model. Exp Neurol. 2004;190:102–108. doi: 10.1016/j.expneurol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Chudasama Y, Nathwani F, Robbins TW. D-Amphetamine remediates attentional performance in rats with dorsal prefrontal lesions. Behav Brain Res. 2005;158:97–107. doi: 10.1016/j.bbr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Colbourne F, Corbett D. Effects of d-amphetamine on the recovery of function following cerebral ischemic injury. Pharmacol Biochem Behav. 1992;42:705–710. doi: 10.1016/0091-3057(92)90018-b. [DOI] [PubMed] [Google Scholar]
- 8.Conti LH, Segal DS, Kuczenski R. Maintenance of amphetamine-induced stereotypy and locomotion requires ongoing dopamine receptor activation. Psychopharmacology (Berl) 1997;130:183–188. doi: 10.1007/s002130050227. [DOI] [PubMed] [Google Scholar]
- 9.Feeney DM, Baron JC. Diaschisis. Stroke. 1986;17:817–830. doi: 10.1161/01.str.17.5.817. [DOI] [PubMed] [Google Scholar]
- 10.Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 11.Feeney DM, Hovda DA. Amphetamine and apomorphine restore tactile placing after motor cortex injury in the cat. Psychopharmacology (Berl) 1983;79:67–71. doi: 10.1007/BF00433018. [DOI] [PubMed] [Google Scholar]
- 12.Feeney DM, Hovda DA. Reinstatement of binocular depth perception by amphetamine and visual experience after visual cortex ablation. Brain Res. 1985;342:352–356. doi: 10.1016/0006-8993(85)91135-7. [DOI] [PubMed] [Google Scholar]
- 13.Gilmour G, Iversen SD, O’Neill MF, O’Neill MJ, Ward MA, Bannerman DM. Amphetamine promotes task-dependent recovery following focal cortical ischaemic lesions in the rat. Behav Brain Res. 2005;165:98–109. doi: 10.1016/j.bbr.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Gladstone DJ, Danells CJ, Armesto A, McIlroy WE, Staines WR, Graham SJ, Herrmann N, Szalai JP, Black SE. Physiotherapy coupled with dextroamphetamine for rehabilitation after hemiparetic stroke: a randomized, double-blind, placebo-controlled trial. Stroke. 2006;37:179–185. doi: 10.1161/01.STR.0000195169.42447.78. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein LB, Davis JN. Post-lesion practice and amphetamine-facilitated recovery of beam-walking in the rat. Restor Neurol Neurosci. 1990;1:311–314. doi: 10.3233/RNN-1990-1501. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein LB, Davis JN. Beam-walking in rats: studies towards developing an animal model of functional recovery after brain injury. J Neurosci Methods. 1990;31:101–107. doi: 10.1016/0165-0270(90)90154-8. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein LB, Davis JN. Influence of lesion size and location on amphetamine-facilitated recovery of beam-walking in rats. Behav Neurosci. 1990;104:320–327. doi: 10.1037//0735-7044.104.2.320. [DOI] [PubMed] [Google Scholar]
- 18.Grilly DM, Loveland A. What is a “low dose” of d-amphetamine for inducing behavioral effects in laboratory rats? Psychopharmacology (Berl) 2001;153:155–169. doi: 10.1007/s002130000580. [DOI] [PubMed] [Google Scholar]
- 19.Harley CW. Norepinephrine and dopamine as learning signals. Neural Plast. 2004;11:191–204. doi: 10.1155/NP.2004.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hovda DA, Feeney DM. Haloperidol blocks amphetamine induced recovery of binocular depth perception after bilateral visual cortex ablation in cat. Proc West Pharmacol Soc. 1985;28:209–211. [PubMed] [Google Scholar]
- 21.Hovda DA, Fenney DM. Amphetamine with experience promotes recovery of locomotor function after unilateral frontal cortex injury in the cat. Brain Res. 1984;298:358–361. doi: 10.1016/0006-8993(84)91437-9. [DOI] [PubMed] [Google Scholar]
- 22.Hovda DA, Sutton RL, Feeney DM. Recovery of tactile placing after visual cortex ablation in cat: a behavioral and metabolic study of diaschisis. Exp Neurol. 1987;97:391–402. doi: 10.1016/0014-4886(87)90099-9. [DOI] [PubMed] [Google Scholar]
- 23.Hovda DA, Sutton RL, Feeney DM. Amphetamine-induced recovery of visual cliff performance after bilateral visual cortex ablation in cats: measurements of depth perception thresholds. Behav Neurosci. 1989;103:574–584. doi: 10.1037//0735-7044.103.3.574. [DOI] [PubMed] [Google Scholar]
- 24.Hurwitz BE, Dietrich WD, McCabe PM, Alonso O, Watson BD, Ginsberg MD, Schneiderman N. Amphetamine promotes recovery from sensory-motor integration deficit after thrombotic infarction of the primary somatosensory rat cortex. Stroke. 1991;22:648–654. doi: 10.1161/01.str.22.5.648. [DOI] [PubMed] [Google Scholar]
- 25.Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res. 1992;581:156–160. doi: 10.1016/0006-8993(92)90356-e. [DOI] [PubMed] [Google Scholar]
- 26.Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. J Neurosci. 1994;14:2140–2152. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuczenski R, Segal D. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinsson L, Hardemark H, Eksborg S. Amphetamines for improving recovery after stroke. Cochrane Database Syst Rev. 2007:CD002090. doi: 10.1002/14651858.CD002090.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer PM, Horel JA, Meyer DR. Effects of dl-amphetamine upon placing responses in neodecorticate cats. J Comp Physiol Psychol. 1963;56:402–404. doi: 10.1037/h0049297. [DOI] [PubMed] [Google Scholar]
- 30.Milesi-Halle A, Hendrickson HP, Laurenzana EM, Gentry WB, Owens SM. Sex- and dose-dependency in the pharmacokinetics and pharmacodynamics of (+)-methamphetamine and its metabolite (+)-amphetamine in rats. Toxicol Appl Pharmacol. 2005;209:203–213. doi: 10.1016/j.taap.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Milesi-Halle A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:140–149. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller K, Kunko PM, Whiteside D, Haskett C. Time course of amphetamine-induced locomotor stereotypy in an open field. Psychopharmacology (Berl) 1989;99:501–507. doi: 10.1007/BF00589899. [DOI] [PubMed] [Google Scholar]
- 33.Papadopoulos CM, Tsai SY, Guillen V, Ortega J, Kartje GL, Wolf WA. Motor Recovery and Axonal Plasticity With Short-Term Amphetamine After Stroke. Stroke. 2008 doi: 10.1161/STROKEAHA.108.519769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prasad RM, Dose JM, Dhillon HS, Carbary T, Kraemer PJ. Amphetamine affects the behavioral outcome of lateral fluid perussion injury in the rat. Restor Neurol Neurosci. 1995;9:65–75. doi: 10.3233/RNN-1995-9201. [DOI] [PubMed] [Google Scholar]
- 35.Ramic M, Emerick AJ, Bollnow MR, O’Brien TE, Tsai SY, Kartje GL. Axonal plasticity is associated with motor recovery following amphetamine treatment combined with rehabilitation after brain injury in the adult rat. Brain Res. 2006;1111:176–186. doi: 10.1016/j.brainres.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen RS, Overgaard K, Hildebrandt-Eriksen ES, Boysen G. D-amphetamine improves cognitive deficits and physical therapy promotes fine motor rehabilitation in a rat embolic stroke model. Acta Neurol Scand. 2006;113:189–198. doi: 10.1111/j.1600-0404.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 37.Rebec GV, White IM, Puotz JK. Responses of neurons in dorsal striatum during amphetamine-induced focused stereotypy. Psychopharmacology (Berl) 1997;130:343–351. doi: 10.1007/s002130050249. [DOI] [PubMed] [Google Scholar]
- 38.Schmanke T, Barth TM. Amphetamine and task-specific practice augment recovery of vibrissae-evoked forelimb placing after unilateral sensorimotor cortical injury in the rat. J Neurotrauma. 1997;14:459–468. doi: 10.1089/neu.1997.14.459. [DOI] [PubMed] [Google Scholar]
- 39.Schmanke TD, Avery RA, Barth TM. The effects of amphetamine on recovery of function after cortical damage in the rat depend on the behavioral requirements of the task. J Neurotrauma. 1996;13:293–307. doi: 10.1089/neu.1996.13.293. [DOI] [PubMed] [Google Scholar]
- 40.Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology (Berl) 2003;165:359–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- 41.Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26:2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- 42.Stroemer RP, Kent TA, Hulsebosch CE. Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke. 1998;29:2381–2393. doi: 10.1161/01.str.29.11.2381. discussion 2393–2385. [DOI] [PubMed] [Google Scholar]
- 43.Sutton RL, Hovda DA, Chen MJ, Feeney DM. Alleviation of brain injury-induced cerebral metabolic depression by amphetamine: a cytochrome oxidase histochemistry study. Neural Plast. 2000;7:109–125. doi: 10.1155/NP.2000.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutton RL, Hovda DA, Feeney DM. Amphetamine accelerates recovery of locomotor function following bilateral frontal cortex ablation in cats. Behav Neurosci. 1989;103:837–841. doi: 10.1037//0735-7044.103.4.837. [DOI] [PubMed] [Google Scholar]
- 45.Wishart TB, Ijaz S, Shuaib A. Differential effects of amphetamine and haloperidol on recovery after global forebrain ischemia. Pharmacol Biochem Behav. 1994;47:963–968. doi: 10.1016/0091-3057(94)90304-2. [DOI] [PubMed] [Google Scholar]
- 46.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. Jama. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]