Abstract
Co-infection of human immunodeficiency virus (HIV) with malaria is one of the pandemic problems in Africa and parts of Asia. Here we investigated the impact of PYR and two other clinical anti-malarial drugs (chloroquine [CQ] or artemisinin [ART]) on HIV-1 replication. Peripheral blood mononuclear cells (PBMCs) or MT-2 cells were infected with HIVNL4.3 strain and treated with different concentrations of the anti-malarial drugs. HIV-1 replication was measured using p24 ELISA. We show that 10 μM CQ and ART inhibited HIV-1 replication by 76% and 60% in PBMCs, respectively, but not in MT-2 cells. In contrast, 10 μM PYR enhanced HIV-1 replication in MT-2 cells by >10-fold. A series of molecular mechanism studies revealed that PYR increased intracellular HIV gag proteins without affecting the promoter or the reverse transcriptase activity. The effect of PYR was independent of HTLV-1 produced by MT-2 cells. Of interest, PYR treatment led to S-phase accumulation and increased AZT and d4T antiviral activity by ~4-fold. Taken together, we show that PYR significantly enhances HIV-1 replication by affecting the cellular machinery. Our results could be relevant for the management of malaria and HIV particularly in regions where HIV-1 and malaria epidemics overlap.
Keywords: Pyrimethamine, DHFR, anti-malaria, S-phase, HIV-1 replication, nucleoside analog
Introduction
Human immunodeficiency virus (HIV) and malaria are two of the leading causes of mortality and morbidity in sub-Saharan Africa and parts of Asia (Herrero et al., 2007; Redmond et al., 2007). In these regions, the HIV epidemic overlaps with that of malaria (Skinner-Adams et al., 2008), and this has been described as two elephants colliding (Whitworth and Hewitt, 2005), giving the impression of a dramatic interaction causing massive destruction (Laufer and Plowe, 2007). Plasmodium falciparum, the protozoan parasite that causes the most lethal form of human malaria (Oguariri et al., 2001; Oguariri et al., 2003) has been controlled principally by two safe, affordable drugs—chloroquine (CQ) and sulfadoxine-pyrimethamine (SP). CQ remains the anti-malarial drug most widely used. In areas where CQ-resistant P. falciparum strains develop, a combination of pyrimethamine (PYR) and sulfadoxine (S) becomes the anti-malarial drug of choice (Rogerson, 2003). PYR is a competitive inhibitor of dihydrofolate reductase (DHFR) enzyme, leading to a deficiency of thymidylate monophosphate (dTMP) (Gangjee et al., 2007), thus causing inhibition of DNA biosynthesis and cell growth. In addition to its anti-malaria property, PYR has been reported as the drug of choice in combination with sulfadiazine or clindamycin for the treatment of acute Toxoplasmic encephalitis (Dannemann et al., 1992; Haverkos, 1987; Klinker et al., 1996a; Klinker et al., 1996b; Leport et al., 1989; Leport et al., 1988). Artemisinin (ART), another widely used antimalaria drug, and its derivatives have been used against multidrug-resistant P. falciparum strains (Meshnick, 2002). It has been reported that the anti-malaria activity of artemisinin is due to iron-mediated cleavage of its peroxidase bridge and generation of three free radicals (Romero et al., 2005; Zhang et al., 1992). With the aim of controlling parasitaemia, patients, including those co-infected with HIV, are given these antimalaria drugs. The effect of such anti-malarial treatment on HIV disease progression due to increased viral replication is important but poorly understood. In general, perspective studies that assess the impact of malaria infection on HIV progression are limited. In the present study, we investigated the impact of pyrimethamine (PYR), an antifolate, in addition to two other clinical anti-malarial drugs on HIV-1 replication in PBMCs, HTLV-1 transformed cell lines (MT-2 and MT-4), and in latently infected U1 cells, and show that PYR significantly enhances HIV-1 replication in MT-2 cells by mediating S-phase accumulation. Interestingly, a combination of PYR and thymidylate nucleoside analogs, AZT or d4T, increased HIV-1 susceptibility to these analogs. An understanding of the biological process responsible for PYR-mediated increase in HIV-1 replication in MT-2 could reveal new drug targets.
Materials and Methods
Cells and reagents
Peripheral blood mononuclear cells (PBMCs) were purified from healthy donors by ficoll-hypaque centrifugation as previously described (Fakruddin et al., 2007) and CD4+ T cells were isolated from the purified PBMCs using CD4 Micro beads (Miltenyi, Auburn, CA). PBMC or CD4+ cells were stimulated with phytohemagglutinin (PHA) (Sigma-Aldrich, St. Louis, MO) with 20 U/ml of IL-2 (Roche Molecular Biochemical, Indianapolis, IN), and maintained in RPM1-1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT), 10 mM of L-glutamine, 100 U/ml of penicillin, and 100 ug/ml of streptomycin (Quality Biologics Inc., Gaithersburg, MD). MT-2 (Haertle et al., 1988; Harada et al., 1985) and MT-4 cells (Larder and Kemp, 1989; Pauwels et al., 1987), and a latently infected cell line, U1 (Folks et al., 1989; Folks et al., 1987) were obtained from the AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases (Rockville, MD) and maintained in completed RPMI-1640. Choroquine (CQ), artemisinin (ART), pyrimethamine (PYR), sulfadoxine, aphidicolin, methotrexate (MTX), zidovudine (AZT), stavudine (d4T), zalcitabine (ddC), and didanosine (ddI) were purchased from Sigma-Aldrich. Lamiduvine (3TC) was obtained from the AIDS Research and Reference Reagent Program, NIAID.
HIV infection and replication assays
HIV-1 stocks were prepared using plasmids encoding the full length of HIVNL4.3 (X4-HIV) (Adachi et al., 1986) as previously described (Imamichi et al., 2003; Oguariri et al., 2007). HIV-1 infection and replication were determined as previously described (Fakruddin et al., 2007; Oguariri et al., 2007). Viral replication was monitored by using a p24 antigen capture assay kit (Perkin-Elmer, Shelton, CT) for HIV-1 or a p19 antigen ELISA kit (Zeptometrix, Buffalo, NY) for HTLV-1. For the effect of PYR on the antiviral activity of antiretroviral drugs (nucleoside analogs), MT-2 cells or PBMCs were infected with an HIVNL4.3 strain and cultured in the presence or absence of increasing concentrations of AZT (0–100 nM), d4T (0–100 nM), 3TC (0–1000 nM), ddC (0–100 nM), ddI (0–1000 nM) alone or in combination with PYR. Viral replication was monitored on day 7.
Transient transfection assays
MT-2 cells were transiently transfected using TransitLT1 (Mirus, Madison, WI) with the previously described HIV-1 LTR luciferase reporter construct (p461) (Oguariri et al., 2007). Transfected cells were cultured in the presence or absence of 10 μM PYR for 48 h. For co-transfection experiments, cells were transiently transfected with Tat expression plasmid (p494) and p461 reporter construct, and cultured in the presence or absence of PYR for 48 h. Cells were assayed for luciferase activity using the Luciferase Assay System (Promega, Madison, WI). Luciferase activity was normalized by total cellular protein measured with a BCA Protein Assay Kit (Pierce, Rockford, IL).
Western immunoblot assays
HIV-1 infected MT-2 cells were treated or untreated with PYR (10 μM) for 2, 4 or 7 days at 37°C. Total cell lysates were obtained as previously described (Oguariri et al., 2007), and protein amount was measured using BCA Protein Assay Kit. Western blotting was performed as previously described (Brann et al., 2006; Oguariri et al., 2007).
Endogenous reverse transcriptase assay
Reverse transcriptase (RT) activity was quantitatively determined using the RT assay kit (Roche Applied Science, Indianapolis, IN). Briefly, HIV-1NL4.3 infected MT-2 cells were cultured for 7 days in the presence or absence of PYR (10 μM). Cell culture supernatants were filtered and the virus particles were pelleted by ultracentrifugation at 10,000 × g for 2 h at 4°C, using sucrose as previously described (Brann et al., 2006). The pelleted virus was lysed in RT assay buffer, and p24 amount was estimated by p24 capture antigen ELISA, respectively. Reverse transcriptase reaction was performed using the lysed virus and the RT activity was determined by the manufacturer's procedure. The specific activity of HIV-1-RT (mU/mg) was normalized with the amount of p24 antigen (Imamichi et al., 2003)
Cell cycle analysis
MT-2 cells were treated with 10 μM PYR, 0.1 μM methotrexate or 100 μM sulfadoxine at 37°C for 48 h. U1 cells, CD4+ T cells and PBMCs were treated with 1 μM PYR at 37°C for 48 h. Cells were washed with PBS and cell cycle analysis was performed as previously described (Imamichi et al., 2003; Oguariri et al., 2007).
Real-time semi-quantitative PCR
MT-2 cells were mock-treated or treated with 10 μM PYR for 48 h at 37°C. Stimulated PBMCs were treated with 1 μM PYR. MT-2 or PBMCs were washed and infected with DNase-treated HIV-1 stock for 2 h at 37°C; then incubated for additional 24 h at 37°C in the presence or absence of PYR. After the 24 h incubation, cells were harvested and genomic DNA was extracted using a QIAamp DNA Mini kit (Qiagen, Valencia, CA) as previously described (Fakruddin et al., 2007). Real-time PCR was performed on the genomic DNA, using an iCycler iQ real-time PCR detection system (Bio-Rad, Hercules, CA) with TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA). The oligonucleotide primers used for the detection of HIV-gag and RNAse P were obtained from TaqMan gene amplification assays (Applied Biosystems). RNase P was used as the reference gene. The amount of real-time quantitative PCR product for HIV-gag was normalized to the amount of RNAse P in the same sample.
Statistical analysis
Statistical analysis was performed using the unpaired t-test of the StatView program (AbacusConcept, Berkeley, CA). p<0.05 was considered as a significant difference.
Results
Pyrimethamine (PYR) enhances HIV-1 replication in HTLV-1 infected MT-2 cells but not in PBMC
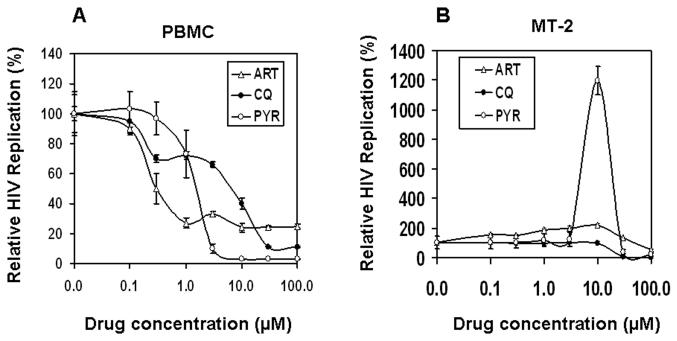
In order to investigate the impact of anti-malarial drugs on HIV-1 replication, we first examined the replication of HIVNL4.3 in normal PBMC exogenously treated with increasing concentrations (0 to 100 μM) of three clinical anti-malarial drugs (ART, CQ or PYR). The results shown in Fig. 1A indicate that 0–1 μM of PYR had no effect on HIV-1 replication in PBMC; although a higher concentration of PYR inhibited HIV, it was due to cytotoxicity, as shown by the trypan blue exclusion assay. On the other hand, ART and CQ at 10 μM each inhibit viral replication by 76% and 60%, respectively. Of note, increase in the drug concentration did not completely inhibit HIV-1 replication. In contrast, in MT-2 cells, whereas treatment with (10 μM) CQ had no effect on HIV-1 replication, compared with the untreated control, treatment with ART (10 μM) or PYR (10 μM) revealed enhancement of HIV-1 replication by 122% and 1200%, respectively (Fig. 1B). More than 10 μM of CQ or 10 μM of PYR demonstrated a strong HIV inhibition, due to a strong cytotoxicity. Based on the surprising enhancement of HIV-1 replication in MT-2 cells by PYR (>10-fold), we then focused our study on PYR and MT-2 cells, using 10 μM of PYR in all subsequent experiments.
Figure 1. PYR enhances HIV-1 replication in MT-2 cells.
The effect of three clinical anti-malarial drugs on HIV-1 replication was assessed in (A) PBMCs or (B) MT-2 cells. HIV-1-infected PBMC or MT-2 cells were cultured in the absence or presence of various concentrations of either chloroquine (CQ), artemisinin (ART), or pyrimethamine (PYR) (Tracqui et al.). Half of the culture media were changed on day 4. HIV replication was monitored on day 7 by p24 antigen capture ELISA. All experiments were performed in triplicates, and data are expressed as percentage of control from mean ± standard deviation.
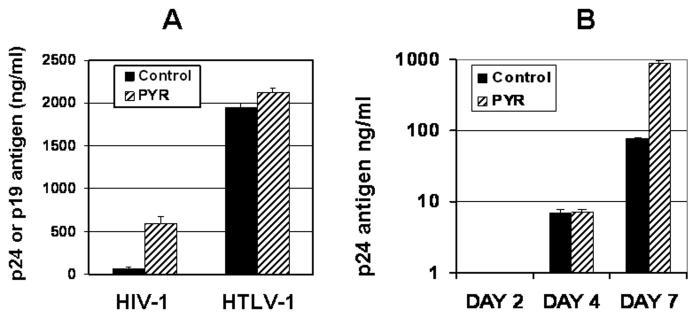
MT-2 cells are HTLV-1 transformed cell lines and spontaneously produce HTLV-1 virions. To demonstrate whether the PYR-mediated increase in viral replication in MT-2 cells was specific to HIV-1, we measured HTLV-1 production from the same culture supernatant collected from MT-2 cells treated or untreated with PYR. Our data shows that PYR enhances HIV-1 replication but has no effect on HTLV-1 production (Fig. 2A), indicating that PYR specifically targets HIV-1 replication in MT-2 cells. To determine whether PYR facilitates HIV replication rate, we performed a viral replication kinetic assay. Culture supernatants from PYR treated or untreated cells were collected on days 2, 4, or 7, and p24 amounts in the supernatant were determined by ELISA. Of interest, PYR-mediated increase in HIV replication was only observed on day 7 post-infection but not on day 4 (Fig. 2B), indicating that PYR may neither facilitate an early-stage of HIV replication nor enhance HIV-1 promoter activity.
Figure 2. The effect of PYR on HTLV-1 production and HIV-1 replication.
(A) PYR has no effect on HTLV-1 production: HIV-infected cells were cultured in the presence or absence of PYR (10 μM). HIV-1 or HTLV-1 production was measured from cell supernatant using p24 or p19 capture antigen ELISA. (B) Kinetic studies of PYR effect on HIV-1 replication in MT-2 cells: HIV-1 infected MT-2 cells were treated or untreated with PYR (10 μM) for 7 days. Virus replication in the supernatant was monitored by using p24 capture antigen ELISA on days 2, 4, and 7.
PYR has no impact on HIV-1 promoter activity and does not facilitate viral budding
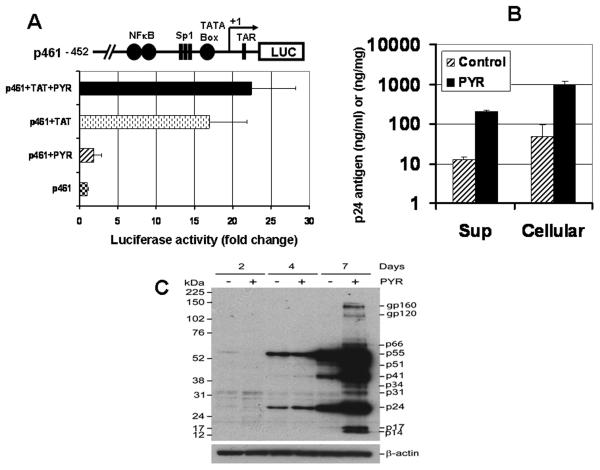
To determine the direct impact of PYR on HIV-1 LTR, a promoter assay was performed. MT-2 cells were transfected with an HIV LTR construct (p461) and then treated or untreated with PYR for 48 h and assayed for luciferase reporter gene activity. As shown in Fig. 3A, PYR had no impact on promoter activity (p = 0.19), even in the presence of TAT expression vector (p = 0.28), suggesting that PYR may be enhancing HIV replication at the posttranscriptional level, rather than in a transcriptional step.
Figure 3. The target of PYR on the HIV-1 life cycle. (A) PYR does not activate HIV-1 LTR promoter activity.
MT-2 cells were transiently transfected with HIV-1 LTR-Luciferase reporter gene (p461) or co-transfected with tat expression plasmid. Cells were cultured in the presence or absence of PYR (10 μM) for 48 hrs and then luciferase activity was measured. The activity was normalized by total cellular protein. The schematic diagram of the plasmid construct containing the HIV-1 LTR-Luciferase reporter gene (p461) is shown.
(B) PYR has no effect on viral budding. HIV infection was performed as above. Intracellular p24 from whole cell extract and extracellular p24 from the cell supernatant were measured by p24 antigen capture assay. Results are representative of three independent experiments.
(C) PYR increases HIV-1 protein expression in MT-2 cells. Whole MT-2 cell extract from days 2, 4, and 7 after HIV-1 infection were prepared with RIPA buffer, and 20 μg of total protein was analyzed by Western blotting. Membrane was probed with HIV-infected patient plasma. The membrane was stripped and subsequently reprobed with anti-β-actin as internal control for equal loading (lower panel).
Having demonstrated that PYR does not substantially activate HIV-1 LTR promoter activity while it enhances p24 production, it was speculated that PYR may facilitate budding rather than impacting any effect on the translational step. To indirectly assess the impact of PYR, we compared intracellular p24 (nanogram p24 per mg total cellular proteins) and extracellular p24 (nanogram p24 per ml). If PYR facilitated budding without increase in translation, the amount of intracellular p24 would be decreased, compared with control. Of interest, as shown in Fig. 3B, not only extracellular p24, but also intracellular p24 was increased by PYR. PYR increased intracellular p24 by 15-fold and extracellular by 17-fold, indicating that PYR does not facilitate viral budding but increased both intra- and extracellular p24.
PYR increases the expression of HIV-1 proteins in MT-2 cells
To define whether the increased intracellular p24 resulted from an accumulated immature Gag precursor, a Western blot was performed, using total cellular proteins collected at three time points (days 2, 4, and 7), and probed with plasma from an HIV-infected patient. As shown in Fig. 3C, HIV proteins, including Gag-pol, Gag, and p24, were enhanced by PYR treatment, as indicated by the accumulation of the protein bands on day 7. Western blot using monoclonal anti-p24 antibody also resulted in a PYR–mediated increase in p24 fragments (data not shown).
PYR does not enhance the reverse transcriptase activity
Since PYR had no impact on HIV promoter activity but increased the intracellular HIV protein on the late stage after infection, it was speculated that PYR may facilitate HIV reverse transcriptase (RT) in virion and increase proviral DNA copy number; thus, the RNA amount may increase. To address the role of PYR on HIV RT activity, we quantitatively compared endogenous reverse transcriptase activity in virus particles from mock- and PYR–treated MT-2 cells. PYR treatment did not increase HIV-1 reverse transcriptase activity (RT units/mg p24) after 7 days of infection (data not shown), thus suggesting that PYR could be acting mainly at the cellular level, rather than affecting the HIV-1 reverse transcriptase step of the viral life cycle.
PYR accumulates S-phase and increases proviral copy number
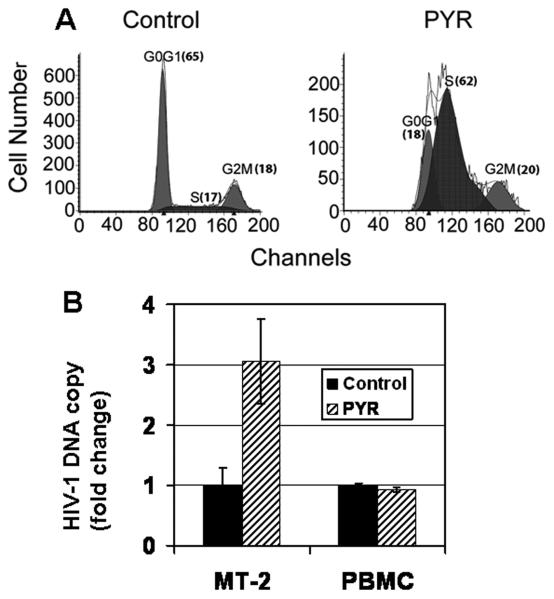
To further investigate the mechanism of PYR-mediated enhancement of HIV-1 replication in MT-2 cells, a cell cycle analysis based on DNA content stained with propidium iodide (PI) was performed. Results demonstrated shifts in the distribution of cells in the G0/G1, S, and G2/M phases of the cell cycle after PYR treatment. A significant accumulation of S-phase was seen in PYR-treated MT-2 cells (Fig. 4A). It was reported that HIV reverse transcriptase (RT) reaction is favored in the S-phase, due to the S-phase–dependent biosynthesis of cellular dNTP, which retroviruses and DNA viruses utilize as substrates during viral replication (Jamburuthugoda et al., 2006), indicating that PYR may increase proviral DNA copy number. To compare the proviral DNA copy number between PYR-treated cells and untreated cells, real-time PCR was performed using 24 h post-infected cells. Our results revealed ~3-fold increase in proviral DNA copy number in the PYR-treated MT-2 cells with accumulated S-phase compared with untreated cells. In contrast, PYR did not increase the copy number of HIV in PBMC (Fig. 4B) suggesting that the effect of PYR may be cell type dependent.
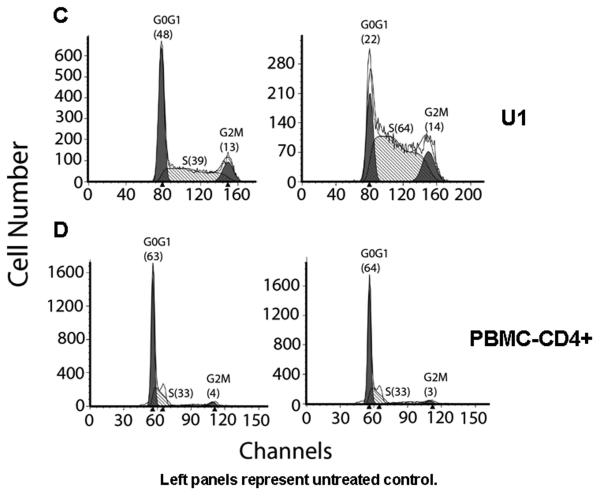
Figure 4. PYR induces S-phase accumulation in MT-2 and U1 cells.
MT-2, U1, and PBMCs were cultured for 48 h in the absence or presence of 1 μM PYR. The cell cycle analysis was studied by propidium iodide staining and evaluated by flow cytometry. In MT-2, (A) the left peaks constitute cells in G0/G1, the right peaks constitute cells in G2/M, and cells in S phase are between the G0/G1 and G2/M peaks. Numbers in parentheses represent the percentage of cells in that phase. The figure is a representative of three independent experiments. (B) PYR increased proviral DNA copy number. MT-2 cells or stimulated PBMCs were infected with HIV-1 for 2 h at 37°C and cultured for an additional 24 h in the absence or presence of 10 μM or 1 μM PYR, respectively. The genomic DNA was isolated and used as a template in quantitative real-time PCR. In each instance, the amount of real-time quantitative PCR product for the gene of interest, HIV-GAG, was normalized to the amount of the reference gene, RNase P, in the same sample. Proviral DNA copy number per106 cells was determined and figure presented as fold change. In U1 cells (C) and CD4+ T-cells in PBMCs (D), the left peaks constitute cells in G0/G1, the right peaks constitute cells in G2/M, and cells in S phase are between the G0/G1 and G2/M peaks. Numbers in parentheses represent the percentage of cells in that phase.
We also evaluated the effect of PYR on HIV-1 replication in latently infected U1 cells. PYR-mediated reactivation of HIV replication was observed in U1 cells by ~5-fold (data not shown). Further analysis reveals that PYR induced S-phase accumulation in chronically infected U1 cells but not in PBMC-derived CD4+ cells (Figs. 4C and D). Taken together, these suggest that S-phase accumulation by PYR may be associated in the mechanism of PYR-mediated enhancement of HIV-1 replication.
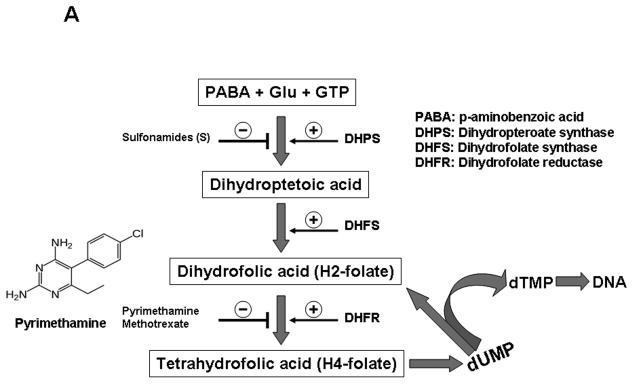
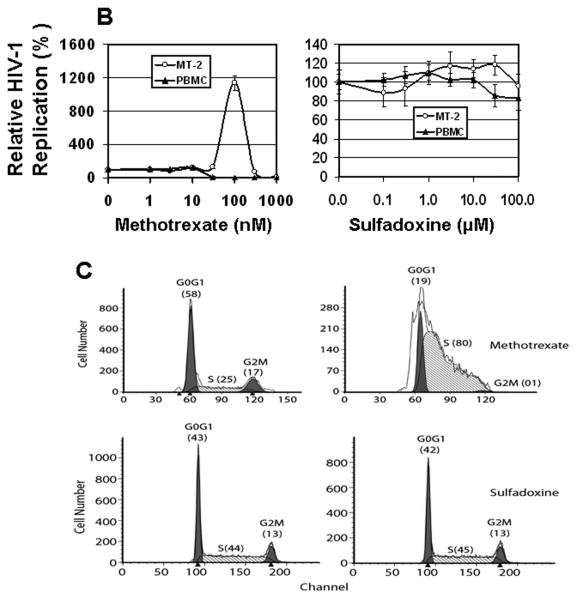
PYR blocks the enzyme dihydrofolate reductase (DHFR) (Fig. 5A), thereby inhibiting thymidylate formation. This imbalance in the concentration of nucleic acid may induce cell cycle arrest. We, therefore, tested the effect of another DHFR inhibitor, methotrexate, on HIV-1 replication and cell cycle in MT-2. Like pyrimethamine, methotrexate enhanced HIV-1 replication along with S-phase accumulation in MT-2 cells (Figs. 5B, left panel and 5C, upper panel), suggesting that agents that inhibit the de novo pyrimidine biosynthetic pathway have the ability to induce S-phase arrest and increase HIV-1 replication in MT-2 cells. Sulfadoxine, a sulfonamide that inhibits the enzyme dihydropteroate synthase (DHPS) is used in combination with pyrimethamine. We determined whether sulfadoxine has any effect on HIV-1 replication. Our data show that unlike PYR, sulfadoxine has no impact on HIV replication or MT2 cell cycle progression (Figs. 5B, right panel and 5C, lower panel), suggesting that DHFR but not DHPS step of the folate metabolic pathway may be the key target in the PYR-mediated effect.
Figure 5. PYR and Methotrexate are antifolate drugs.
(A) Schematic representation of folate metabolism and the structure of PYR. Site of action of some antifolate enzymes and their inhibitors are shown. (B) Methotrexate enhances HIV-1 replication in MT-2 cells. HIV-1-infected PBMC or MT-2 cells were cultured in the absence or presence of various concentrations of methotrexate, MTX (0–1000 nM, left panel) or sulfadoxine (0-100 μM, right panel). HIV replication was monitored by p24 antigen capture ELISA on day 7 post-infection. (C) MTX induces S-phase accumulation in MT-2. MT-2 cells were cultured for 48 h in the absence or presence of MTX (100 nM, upper panel) or sulfadoxine (100 μM, lower panel). Cell cycle analysis was studied by propidium iodide staining and evaluated by flow cytometry. The left peaks constitute cells in G0/G1, the right peaks constitute cells in G2/M, and cells in S phase are between the G0/G1 and G2/M peaks. Numbers in parentheses represent the percentage of cells in that phase. Left panels represent untreated control.
Effect of PYR on the antiviral activity of nucleoside analogs in MT-2 cells and primary PBMCs
PYR and methotrexate induced a skewed distribution in cell cycle in MT-2 cell, indicating that the reagents may decrease a concentration of thymidine in the cell (Fig. 5A). Thus, if thymidine analogs e.g., AZT, is administered to the reagent-treated cells, the relative concentration of AZT to thymidine would increase, leading to AZT susceptibility of the treated cells to AZT. To address this hypothesis, we next examined the effect of PYR on five nucleoside analog inhibitors on HIV-1 replication in MT-2 cells or PBMCs. HIV-infected cells were cultured with varying concentrations of the analogs in the presence or absence of 1 or 10 μM PYR. Our data show that in the presence of 10 μM PYR, the antiviral activity of AZT was increased in MT-2 cells by ~3-fold, whereas in PBMC, PYR increased HIV-1 susceptibility to AZT and d4T by >3-fold (Table 1). Based on these results, we further evaluated the effect of PYR on antiviral activity of three additional nucleoside analogues, 3TC, ddC, and ddI, in primary PBMCs. Surprisingly, our data show that PYR enhanced viral resistance to 3TC and ddC, as shown by a 2-fold increase in the IC50. No significant change on the IC50 was observed with ddI (Table 1). These results suggest that treating HIV- and malaria-co-infected individuals with PYR and thymidylate nucleoside analogs like AZT or d4T could confer some clinical benefit.
Table 1.
Impact of PYR on nucleoside analogs and HIV-1 replication
| IC50 (nM)a |
||||||
|---|---|---|---|---|---|---|
| Cell type | PYR Concentration |
AZT | D4T | 3TC | ddC | ddI |
| MT-2 cells | 0 μM | 2.6 ± 0.39 b (100) |
N.D. c | N.D. | N.D. | N.D. |
| 10 μM | 1.0 ± 0.02 (38)* |
N.D. | N.D. | N.D. | N.D. | |
| PBMC | 0 μM | 1.8 ± 0.37 (100) |
7.7 ± 4.67 (100) |
17.3 ± 5.24 (100) |
11.0 ± 3.81 (100) |
256.7 ± 36.60 (100) |
| 1 μM | 0.5 ± 0.10 (28)* |
1.7 ± 1.23 (23)* |
35.0 ± 9.86 (205)* |
23.0 ± 7.10 (210)** |
269.3 ± 34.78 (105) |
|
IC50: the concentration required to inhibit HIV-1 replication by 50%.
Data shown are the mean ± standard error of at least three independent experiments. Values in parentheses represent the percentage difference in IC50s compared to that of 0 μM PYR treated cells.
N.D. stands for not determined.
p<0.01
p<0.05
Discussion
It is reported that co-infections of HIV and malaria are two of the leading causes of mortality and morbidity in sub-saharan Africa and part of Asia (Abu-Raddad et al., 2006; Andrews et al., 2007; Hewitt et al., 2006; Karp and Auwaerter, 2007; Van geertruyden and D'Alessandro, 2007). Despite the fact that malaria may affect HIV progression and transmission, the impact of anti-malaria drugs on HIV replication is poorly understood. In this study, we attempted to assess the effect of clinical anti-malaria drugs (CQ, ART, and PYR) on HIV replication, using several cell types; and we show for the first time that PYR and ART significantly enhance HIV replication in MT-2 cells while they inhibited HIV replication in PBMC. PYR has been used in combination with sulfadoxine as the most widely used anti-malarial therapy for CQ-resistant strains. Several studies on the treatment of Toxoplasmosis using PYR have reported the range of PYR plasma concentration as 2–42 μM (Klinker et al., 1996a; Klinker et al., 1996b; McLeod et al., 1992; Schmidt et al., 2006); while Egeli and Erdogan (Egeli and Erdogan, 1991) reported 0.05 mg/ml or 201 μM as the normal therapeutic plasma level for PYR. Therefore, the 10 μM of PYR used in this study is within a safe clinical dose range.
MT-2 and MT-4 cells are HTLV-1 infected cells. It is known that unlike MT-4 cells, MT-2 cells actively produce a high level of infectious HTLV-1 virions (Koyanagi et al., 1984) and several cytokines (Dhib-Jalbut et al., 1994) as other HTLV-1-transformed cells (Dhib-Jalbut et al., 1994; Hollsberg and Hafler, 1993; Koyanagi et al., 1984). In our study, PYR enhanced HIV replication in MT-2, but not in MT-4 cells (data not shown) without any impact on HTLV-1 production, thus indicating that the increase in HIV replication by PYR is unlikely related to HTLV-1 particles. HIV and HTLV-1 co-infection is reported to have emerged as a worldwide health problem (Casoli et al., 2007) with increasing numbers of co-infections in South America and Africa. The long-term effects of such co-infection, however, are poorly understood (Beilke et al., 2007). Studies in Brazil suggest that co-infection with HTLV-1 and HIV has substantial medical consequences, but as reported by others (Dhib-Jalbut et al., 1994), it remains controversial whether HTLV-1 co-infection leads to progression of HIV-1 in dually infected individuals. Since PYR only increased HIV replication but had no effect on HTLV-1 production, it suggests that PYR may target a processing step in the HIV life cycle.
A series of experiments in this study demonstrated that PYR had no impact on HIV-1 RT, promoter activity, replication, processing, or budding. Cell cycle analysis and real-time PCR assay demonstrated that PYR treatment accumulated S-phase cells and increased proviral DNA copy number in MT-2, respectively. These results suggest that PYR probably enhanced the pre-transcriptional step and facilitated viral RT reaction in the accumulated S-phase, as previously described (Chen and Temin, 1982; Harel et al., 1981; Patki et al., 2000).
As an anti-folate agent, PYR inhibits the enzyme dihydrofolate reductase (DHFR), resulting in decreased intracellular tetrahydrofolate production and interference with thymidylate synthesis (Fig. 5A), thus decreasing the concentration of thymidine. The inhibition or downregulation of DHFR potentially slows the progress of DNA replication required for the progression of cells through S-phase (Sakoff and Ackland, 2000). Another DHFR inhibitor, methotrexate (MTX), also demonstrated increase in HIV replication with S-phase accumulation in MT-2 cells in a similar fashion. Neither reagent had any impact on HIV replication in PBMC but increased the susceptibility to a Thymidine analog HIV RT inhibitor. These results imply that a combination of PYR with AZT may decrease a side effect by the nucleoside analogs. Based on these findings, we hypothesized that although PYR may increase HIV-1 replication, it may increase thymidylate nucleoside analog sensitivity. To support this hypothesis, four additional nucleoside analog antiretroviral drugs (d4T, 3TC, ddC, and ddI) were also investigated. Interestingly, PYR also enhanced the viral susceptibility to d4T, another thymidine analog. A likely explanation could be that the depletion of intracellular dTTP by PYR increased the ratio of the thymidine analogs to dTTP. In contrast, PYR surprisingly increased the viral resistance to two non-thyimidine-pyrimidine analogs, 3TC and ddC. Feedback activation or inhibition of metabolic regulations involved in DNA synthesis has been reported (Kinahan et al., 1979; Moore and Hurlbert, 1966). In our study, it is likely that the presence of PYR induced a feedback regulation of intracellular dTTP and dCTP, whereby an extensive inhibition of dTTP pathway by PYR resulted in an increase in the dCTP pool, thus increasing the dCTP/3TC or ddC ratio that led to their resistance, as observed by an increase in the IC50. Higher concentrations of dNTPs have been reported in tumor cells compared with normal cells. It is, therefore, likely that the different effects of PYR observed in PBMC and MT-2 cells could be related to the different dNTP concentrations found in PBMCs in comparison with MT-2 cells (Smith and Scott, 2006; Traut, 1994). PYR is a DHFR inhibitor that inhibits cellular DNA synthesis by interfering with the de novo thymidine synthesis pathway. Therefore, the inability of PYR to inhibit HIV replication is perhaps surprising. A possible explanation could be that blockage of the de novo pathway by PYR results in increase in the synthesis of dTMP via the salvage pathway, thereby allowing the virus to bypass the inhibitory effect of PYR.
This is the first report that PYR, an anti-malarial drug, enhances HIV-1 replication by facilitating S-phase accumulation in some cell types and potentiate the susceptibility of HIV to anti-retroviral therapy. Overall our data suggest that PYR could have a potential as a comedication with nucleosidic thymidylate analogs. This observation could be relevant for the management of malaria and HIV in dual infected individuals, particularly in the treatment of malaria during pregnancy, and in regions where HIV and malaria epidemics overlap.
Acknowledgments
The authors thank H. C. Lane for supporting this project and providing guidance, R. Dewar for providing the patients' plasma; T. Brann, C. Watkins and C. Yeager for technical support, A. Troxell for assistance with supplies and M. Grau for critical reading of the manuscript.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported [in part] by the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Part of this data was presented in part at the Conference on Retroviruses and Opportunistic Infections (CROI) 2009, Montreal, Canada, February, 8-11, 2009.
Disclosure: The authors declare no competing interests.
References
- Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314(5805):1603–6. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59(2):284–91. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews KT, Gatton ML, Skinner-Adams TS, McCarthy JS, Gardiner DL. HIV-malaria interactions: don't forget the drugs. Science. 2007;315(5820):1791. doi: 10.1126/science.315.5820.1791a. author reply 1791. [DOI] [PubMed] [Google Scholar]
- Beilke MA, Traina-Dorge VL, Sirois M, Bhuiyan A, Murphy EL, Walls JM, Fagan R, Winsor EL, Kissinger PJ. Relationship between human T lymphotropic virus (HTLV) type 1/2 viral burden and clinical and treatment parameters among patients with HIV type 1 and HTLV-1/2 coinfection. Clin Infect Dis. 2007;44(9):1229–34. doi: 10.1086/513428. [DOI] [PubMed] [Google Scholar]
- Brann TW, Dewar RL, Jiang MK, Shah A, Nagashima K, Metcalf JA, Falloon J, Lane HC, Imamichi T. Functional correlation between a novel amino acid insertion at codon 19 in the protease of human immunodeficiency virus type 1 and polymorphism in the p1/p6 Gag cleavage site in drug resistance and replication fitness. J Virol. 2006;80(12):6136–45. doi: 10.1128/JVI.02212-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casoli C, Pilotti E, Bertazzoni U. Molecular and cellular interactions of HIV-1/HTLV coinfection and impact on AIDS progression. AIDS Rev. 2007;9(3):140–9. [PubMed] [Google Scholar]
- Chen IS, Temin HM. Establishment of infection by spleen necrosis virus: inhibition in stationary cells and the role of secondary infection. J Virol. 1982;41(1):183–91. doi: 10.1128/jvi.41.1.183-191.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannemann B, McCutchan JA, Israelski D, Antoniskis D, Leport C, Luft B, Nussbaum J, Clumeck N, Morlat P, Chiu J, et al. Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. The California Collaborative Treatment Group. Ann Intern Med. 1992;116(1):33–43. doi: 10.7326/0003-4819-116-1-33. [DOI] [PubMed] [Google Scholar]
- Dhib-Jalbut S, Hoffman PM, Yamabe T, Sun D, Xia J, Eisenberg H, Bergey G, Ruscetti FW. Extracellular human T-cell lymphotropic virus type I Tax protein induces cytokine production in adult human microglial cells. Ann Neurol. 1994;36(5):787–90. doi: 10.1002/ana.410360516. [DOI] [PubMed] [Google Scholar]
- Egeli U, Erdogan G. The clastogenic effect of pyrimethamine (Daraprim) on human chromosomes in lymphocyte cultures. Cell Biol Toxicol. 1991;7(4):347–56. doi: 10.1007/BF00124070. [DOI] [PubMed] [Google Scholar]
- Fakruddin JM, Lempicki RA, Gorelick RJ, Yang J, Adelsberger JW, Garcia-Pineres AJ, Pinto LA, Lane HC, Imamichi T. Noninfectious papilloma virus-like particles inhibit HIV-1 replication: implications for immune control of HIV-1 infection by IL-27. Blood. 2007;109(5):1841–9. doi: 10.1182/blood-2006-02-001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks TM, Clouse KA, Justement J, Rabson A, Duh E, Kehrl JH, Fauci AS. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989;86(7):2365–8. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks TM, Justement J, Kinter A, Dinarello CA, Fauci AS. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987;238(4828):800–2. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Gangjee A, Kurup S, Namjoshi O. Dihydrofolate reductase as a target for chemotherapy in parasites. Curr Pharm Des. 2007;13(6):609–39. doi: 10.2174/138161207780162827. [DOI] [PubMed] [Google Scholar]
- Haertle T, Carrera CJ, Wasson DB, Sowers LC, Richman DD, Carson DA. Metabolism and anti-human immunodeficiency virus-1 activity of 2-halo-2′,3′-dideoxyadenosine derivatives. J Biol Chem. 1988;263(12):5870–5. [PubMed] [Google Scholar]
- Harada S, Koyanagi Y, Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985;229(4713):563–6. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Harel J, Rassart E, Jolicoeur P. Cell cycle dependence of synthesis of unintegrated viral DNA in mouse cells newly infected with murine leukemia virus. Virology. 1981;110(1):202–7. doi: 10.1016/0042-6822(81)90022-2. [DOI] [PubMed] [Google Scholar]
- Haverkos J. Assessment of therapy for Toxoplasma encephalitis. Am J Med. 1987;82:907–914. doi: 10.1016/0002-9343(87)90151-3. [DOI] [PubMed] [Google Scholar]
- Herrero MD, Rivas P, Rallon NI, Ramirez-Olivencia G, Puente S. HIV and malaria. AIDS Rev. 2007;9(2):88–98. [PubMed] [Google Scholar]
- Hewitt K, Steketee R, Mwapasa V, Whitworth J, French N. Interactions between HIV and malaria in non-pregnant adults: evidence and implications. Aids. 2006;20(16):1993–2004. doi: 10.1097/01.aids.0000247572.95880.92. [DOI] [PubMed] [Google Scholar]
- Hollsberg P, Hafler DA. Seminars in medicine of the Beth Israel Hospital, Boston. Pathogenesis of diseases induced by human lymphotropic virus type I infection. N Engl J Med. 1993;328(16):1173–82. doi: 10.1056/NEJM199304223281608. [DOI] [PubMed] [Google Scholar]
- Imamichi T, Murphy MA, Adelsberger JW, Yang J, Watkins CM, Berg SC, Baseler MW, Lempicki RA, Guo J, Levin JG, Lane HC. Actinomycin D induces high-level resistance to thymidine analogs in replication of human immunodeficiency virus type 1 by interfering with host cell thymidine kinase expression. J Virol. 2003;77(2):1011–20. doi: 10.1128/JVI.77.2.1011-1020.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamburuthugoda VK, Chugh P, Kim B. Modification of human immunodeficiency virus type 1 reverse transcriptase to target cells with elevated cellular dNTP concentrations. J Biol Chem. 2006;281(19):13388–95. doi: 10.1074/jbc.M600291200. [DOI] [PubMed] [Google Scholar]
- Karp CL, Auwaerter PG. Coinfection with HIV and tropical infectious diseases. II. Helminthic, fungal, bacterial, and viral pathogens. Clin Infect Dis. 2007;45(9):1214–20. doi: 10.1086/522180. [DOI] [PubMed] [Google Scholar]
- Kinahan JJ, Otten M, Grindey GB. Evaluation of ribonucleoside and deoxyribonucleoside triphosphate pools in cultured leukemia cells during exposure to methotrexate or methotrexate plus thymidine. Cancer Res. 1979;39(9):3531–9. [PubMed] [Google Scholar]
- Klinker H, Langmann P, Richter E. Plasma pyrimethamine concentrations during long-term treatment for cerebral toxoplasmosis in patients with AIDS. Antimicrob Agents Chemother. 1996a;40(7):1623–7. doi: 10.1128/aac.40.7.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinker H, Langmann P, Richter E. Pyrimethamine alone as prophylaxis for cerebral toxoplasmosis in patients with advanced HIV infection. Infection. 1996b;24(4):324–7. doi: 10.1007/BF01743370. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y, Hinuma Y, Schneider J, Chosa T, Hunsmann G, Kobayashi N, Hatanaka M, Yamamoto N. Expression of HTLV-specific polypeptides in various human T-cell lines. Med Microbiol Immunol. 1984;173(3):127–40. doi: 10.1007/BF02123761. [DOI] [PubMed] [Google Scholar]
- Larder BA, Kemp SD. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT) Science. 1989;246(4934):1155–8. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- Laufer MK, Plowe CV. The Interaction between HIV and Malaria in Africa. Curr Infect Dis Rep. 2007;9(1):47–54. doi: 10.1007/s11908-007-0022-3. [DOI] [PubMed] [Google Scholar]
- Leport C, Bastuji-Garin S, Perronne C, Salmon D, Marche C, Bricaire F, Vilde JL. An open study of the pyrimethamine-clindamycin combination in AIDS patients with brain toxoplasmosis. J Infect Dis. 1989;160(3):557–8. doi: 10.1093/infdis/160.3.557. [DOI] [PubMed] [Google Scholar]
- Leport C, Raffi F, Matheron S, Katlama C, Regnier B, Saimot AG, Marche C, Vedrenne C, Vilde JL. Treatment of central nervous system toxoplasmosis with pyrimethamine/sulfadiazine combination in 35 patients with the acquired immunodeficiency syndrome. Efficacy of long-term continuous therapy. Am J Med. 1988;84(1):94–100. doi: 10.1016/0002-9343(88)90014-9. [DOI] [PubMed] [Google Scholar]
- McLeod R, Mack D, Foss R, Boyer K, Withers S, Levin S, Hubbell J. Levels of pyrimethamine in sera and cerebrospinal and ventricular fluids from infants treated for congenital toxoplasmosis. Toxoplasmosis Study Group. Antimicrob Agents Chemother. 1992;36(5):1040–8. doi: 10.1128/aac.36.5.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshnick SR. Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32(13):1655–60. doi: 10.1016/s0020-7519(02)00194-7. [DOI] [PubMed] [Google Scholar]
- Moore EC, Hurlbert RB. Regulation of mammalian deoxyribonucleotide biosynthesis by nucleotides as activators and inhibitors. J Biol Chem. 1966;241(20):4802–9. [PubMed] [Google Scholar]
- Oguariri RM, Borrmann S, Klinkert MQ, Kremsner PG, Kun JF. High prevalence of human antibodies to recombinant Duffy binding-like alpha domains of the Plasmodium falciparum-infected erythrocyte membrane protein 1 in semi-immune adults compared to that in nonimmune children. Infect Immun. 2001;69(12):7603–9. doi: 10.1128/IAI.69.12.7603-7609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguariri RM, Brann TW, Imamichi T. Hydroxyurea and interleukin-6 synergistically reactivate HIV-1 replication in a latently infected promonocytic cell line via SP1/SP3 transcription factors. J Biol Chem. 2007;282(6):3594–604. doi: 10.1074/jbc.M608150200. [DOI] [PubMed] [Google Scholar]
- Oguariri RM, Mattei D, Tena-Tomas C, Uhlemann AC, Kremsner PG, Kun JF. Recombinant Duffy binding-like-alpha domains of Plasmodium falciparum erythrocyte membrane protein 1 elicit antibodies in rats that recognise conserved epitopes. Parasitol Res. 2003;90(6):467–72. doi: 10.1007/s00436-003-0884-8. [DOI] [PubMed] [Google Scholar]
- Patki AH, Zielske SP, Sieg SF, Lederman MM. Preferential S phase entry and apoptosis of CD4(+) T lymphocytes of HIV-1-infected patients after in vitro cultivation. Clin Immunol. 2000;97(3):241–7. doi: 10.1006/clim.2000.4940. [DOI] [PubMed] [Google Scholar]
- Pauwels R, De Clercq E, Desmyter J, Balzarini J, Goubau P, Herdewijn P, Vanderhaeghe H, Vandeputte M. Sensitive and rapid assay on MT-4 cells for detection of antiviral compounds against the AIDS virus. J Virol Methods. 1987;16(3):171–85. doi: 10.1016/0166-0934(87)90002-4. [DOI] [PubMed] [Google Scholar]
- Redmond AM, Skinner-Adams T, Andrews KT, Gardiner DL, Ray J, Kelly M, McCarthy JS. Antimalarial activity of sera from subjects taking HIV protease inhibitors. Aids. 2007;21(6):763–5. doi: 10.1097/QAD.0b013e328031f41a. [DOI] [PubMed] [Google Scholar]
- Rogerson S. HIV-1, antiretroviral therapy, and malaria. Lancet. 2003;362(9389):1008–9. doi: 10.1016/S0140-6736(03)14447-9. [DOI] [PubMed] [Google Scholar]
- Romero MR, Efferth T, Serrano MA, Castano B, Macias RI, Briz O, Marin JJ. Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “in vitro” replicative system. Antiviral Res. 2005;68(2):75–83. doi: 10.1016/j.antiviral.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Sakoff JA, Ackland SP. Thymidylate synthase inhibition induces S-phase arrest, biphasic mitochondrial alterations and caspase-dependent apoptosis in leukaemia cells. Cancer Chemother Pharmacol. 2000;46(6):477–87. doi: 10.1007/s002800000164. [DOI] [PubMed] [Google Scholar]
- Schmidt DR, Hogh B, Andersen O, Hansen SH, Dalhoff K, Petersen E. Treatment of infants with congenital toxoplasmosis: tolerability and plasma concentrations of sulfadiazine and pyrimethamine. Eur J Pediatr. 2006;165(1):19–25. doi: 10.1007/s00431-005-1665-4. [DOI] [PubMed] [Google Scholar]
- Skinner-Adams TS, McCarthy JS, Gardiner DL, Andrews KT. HIV and malaria co-infection: interactions and consequences of chemotherapy. Trends Parasitol. 2008;24(6):264–71. doi: 10.1016/j.pt.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Scott WA. The influence of natural substrates and inhibitors on the nucleotide-dependent excision activity of HIV-1 reverse transcriptase in the infected cell. Curr Pharm Des. 2006;12(15):1827–41. doi: 10.2174/138161206776873572. [DOI] [PubMed] [Google Scholar]
- Tracqui A, Mikail I, Kintz P, Mangin P. Nonfatal prolonged overdosage of pyrimethamine in an infant: measurement of plasma and urine levels using HPLC with diode-array detection. J Anal Toxicol. 1993;17(4):248–50. doi: 10.1093/jat/17.4.248. [DOI] [PubMed] [Google Scholar]
- Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140(1):1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- Van geertruyden JP, D'Alessandro U. Malaria and HIV: a silent alliance. Trends Parasitol. 2007;23(10):465–7. doi: 10.1016/j.pt.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Whitworth JA, Hewitt KA. Effect of malaria on HIV-1 progression and transmission. Lancet. 2005;365(9455):196–7. doi: 10.1016/S0140-6736(05)17752-6. [DOI] [PubMed] [Google Scholar]
- Zhang F, Gosser DK, Jr., Meshnick SR. Hemin-catalyzed decomposition of artemisinin (qinghaosu) Biochem Pharmacol. 1992;43(8):1805–9. doi: 10.1016/0006-2952(92)90713-s. [DOI] [PubMed] [Google Scholar]