Abstract
Lewis recipients of orthotopic ACI livers had permanent graft acceptance induced with 3 doses of i.m. FK506 in the early postoperative period. They were studied 100 and 300 days posttransplantation. The recipients rejected ACI as well as Brown Norway (BN) (third-party) skin grafts, and had lymphocytes with substantial reactivity by mixed lymphocyte culture testing against ACI and third-party (BN) alloantigens. Lymphocyte subset redistribution had not occurred in the peripheral blood or spleens of these animals, and there was no evidence of suppressor cell activation by in vitro and in vivo tests. Graft-versus-host reactivity in splenic lymphoid tissues of these recipients was demonstrated with the popliteal lymph node assay. Attempts at adaptive transfer with recipient lymphocytes were unsuccessful. Heart graft acceptance was far more difficult to accomplish than liver graft acceptance, and probably was never permanent. ACI heart graft prolongation in LEW recipients after a brief induction with FK506 lasted for no more than 3 months in most animals. The temporary heart graft acceptance was specific for hearts of the original ACI donor strain but not for ACI skin. Results of studies of lymphocyte subsets and suppressor cell activity were similar to those in the liver recipients. These studies illustrate how poorly graft acceptance is understood and how badly further work is needed to clarify its mechanism.
In a companion study (1), we demonstrated in rats that a 3-day course of FK506 beginning on postoperative day 4 or even later allows the long or permanent survival of heterotopic heart (2, 3) or orthotopic liver homografts. Similar but less-reproducible observations have been made after kidney transplantation in dogs and baboons (4, 5) and liver transplantation in dogs (4). In the past, the induction of long graft survival in these latter species has been seen with a brief course of treatment with other agents such as antilymphocyte serum (6) but this has been uncommon.
The potential immunological mechanisms that have been proposed to explain the prolonged graft acceptance after discontinuance of longer courses of therapy include modulation of graft antigen expression (7-9), depletion of clonally active cells following immune suppression and exposure to the graft (10-12), antigen/antibody blockade of effector cells (13, 14), and the activation of suppressor cells (15-18). Cyclosporine-induced survival of heart allografts has been shown to be the result of early nonspecific immunosuppression, followed by the eventual emergence of active suppression that is specific for the donor organ (19).
In the experiments reported here, we have examined the immunological characteristics in rats that had prolonged heart and liver graft survival following a short course of FK506 administration.
MATERIALS AND METHODS
Animals
Long-surviving graft recipients
Inbred male rats of Lewis (LEW) (RT1l), ACI (RT1a), and Brown Norway (BN) (RT1n) strains and their F1 hybrid (LEW×ACI) F1 were purchased from Harlan Sprague Dawley Inc. (Indianapolis, IN) and Simonsen Laboratory (Gilroy, CA). Most of the animals studied had long-functioning heart or liver allografts following a short-course treatment of intramuacular FK506 as described in a companion study (1). The LEW recipients of ACI heart grafts were given 3 i.m. doses of 1.28 mg/kg FK506 on days 4, 5, and 6 posttransplantation. With this treatment regimen, the median survival of ACI heart grafts was prolonged from a control of 6 to 91.0 days (1). The animals were studied on days 14, 28, and more than 70 days after allografting.
The LEW recipients of ACI liver allografts received 3 or 4 i.m. doses of 1.28 mg/kg FK506 on days 0–2, 2–4, 3–6, or 4–6. The majority of the rats treated at any of these times survived indefinitely, and these long-term survivors were studied at 100 or 300 days after grafting.
Fresh transplants
In some of the special experimental groups, fresh ACI-to-LEW heart or liver transplants were performed with the same surgical techniques used when the chronically surviving animals were originally transplanted. Full-thickness skin transplantation was also performed in a special group (see below).
Tests after cardiac transplantation
Placement of second cardiac allografts
Recipients of ACI hearts were given a second heart from an ACI (original donor strain) or BN (third-party) donor, either 2 or weeks after the primary transplantation. The second heart grafts were transplanted into the subcutis of the ventral neck of recipients using the modified method described by Heron (20). To control for the possibility of a residual FK506 effect from treatment of the primary graft, a control group of LEW rats were given the same 3 doses of FK506 without ACI heart grafting and then grafted with ACI or BN hearts by Heron’s cervical technique 2 or 4 weeks later.
Skin grafts in heart recipients
Skin grafts from either ACI or BN strains were placed on LEW recipients 28 days after cardiac allografts from either ACI or BN donors. Full-thickness 1-cm2 tail skin grafts were sutured to the flanks of the recipients, and a plaster cast was applied for one week. The grafts were inspected daily after removal the casts. Rejection was considered to have occurred when the graft exhibited complete epithelial necrosis as judged grossly.
Influence of splenectomy on heart graft survival
The immunosuppressive effect of CsA was reported to be reduced in rats with splenectomy (21), possibly because of the loss of splenic suppressor cells. Consequently, LEW recipients had splenectomy immediately before they were given an ACI heart and then treated with 1.28 mg/kg of i.m. FK506 on days 4, 5, and 6 posttransplantation.
Lymphocyte subset analysis
Spleen cells were prepared by mincing tissue into RPMI-1640 (Gibco, Grand Island, NY) and then washing twice in the culture medium. Peripheral blood lymphocytes were isolated from heparinized blood by Ficoll-Hypaque density gradient centrifugation (s.g. 1.090) and washed twice in RPMI-1640. Graft-infiltrating cells were obtained from the heart grafts by mincing 1-mm3 pieces in RPMI-1640, and then subjecting the suspension to vigorous mechanical agitation and filtration through a fine nylon mesh (Tetko Inc., Briarcliff Manor, NY).
Surface-associated markers on the lymphocytes were identified using primary antirat monoclonal antibodies with defined specificity (Accurate Scientific, Westbury, NY): W3/25 (CD4; helper/inducer T cells, macrophages), OX8 (CD8; cytotoxic/suppressor T cells, natural killer cells), OX19 (CD5; pan T cell), and OX6 (MHC class II). Test cells (1×106) suspended in 1.0 ml of staining buffer (PBS, pH 7.4, 0.1% sodium azide, 2% FCS) were incubated with primary monoclonal antibodies (1:100 final dilution) for 30 min at 4°C, washed twice and then resuspended with a secondary antibody consisting of a FITC-labeled rat antimouse IgG, heavy- and light-chain–specific (Boehringer-Mannheim, Indianapolis, IN). After a 30-min incubation at 4°C, the cells were washed twice and analyzed for fluorescent staining using the FACSTAR IV flow cytometer (Becton-Dickinson, Lincoln Park, NJ).
Tests after liver transplantation
Skin grafts in liver recipients
The skin grafts from ACI or BN donors were placed on LEW recipients of ACI livers, 100 or 300 days after transplantation. The experimental endpoint was duration of skin graft survival.
Graft-versus-host reactivity
Spleen cells from the “tolerant” liver recipients were tested for the presence of antidonor (ACI) alloreactive T cells, using the popliteal lymph node assay (22). Spleen cells (2×107) from LEW recipients of ACI liver grafts, from normal LEW rats, or from normal F1 rats (ACI×LEW), were suspended in 0.1 ml of medium, and injected subcutaneously into the footpads of young sex-matched (ACI×LEW) F1 recipients. The popliteal nodes were removed and weighed 7 days later. If the injected cells did not contain alloreactive T cells, no modification of the popliteal nodes would be expected.
Mixed lymphocyte reaction and suppressor cells
One-way MLR was performed using mesenteric lymph node cells from LEW liver recipients to which irradiated (2000 rads) spleen cells from donor strain (ACI) or third-party strain (BN) were added. Spleen cells and lymph node cells were prepared by mincing excised tissue into RPMI-1640 culture medium. They were isolated by centrifugation over a Ficoll-Hypaque gradient. Triplicate cultures of 1×106 responder cells and 2×106 stimulator cells were established in a final volume of 0.2 ml of RPMI-1640 culture medium supplemented with 25 mM HEPES buffer, 5×10−6 M 2-mercaptoethanol, penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% heat-inactivated rat serum in round-bottomed microculture plates. Cultures were incubated in a humidified atmosphere of 5% CO2 in air for 5 days at 37°C. Then 16 hr before the termination of the culture, 1 μCi of 3H-thymidine was added to each well. Cultures were harvested with a multiple sample harvester (Skatron Inc., Sterling, VA) and 3H-thymidine uptake was determined by liquid scintillation spectrometry.
Suppressor cell reactivity in the MLR was also assayed by mixing lymph node cells obtained from normal (LEW) rats with the lymph node cells from LEW rats that were bearing long-surviving liver grafts; cell ratios were 1:1, 1:3, and 1:10. A total of 2×106 of the mixture of responding cells were stimulated in vitro with 2×106 irradiated ACI or BN spleen cells.
Lymphocyte subset analysis
The fluorescence staining method was the same as in the heart experiments.
Adoptive transfer assay after heart and liver transplantation
The presence of cells with suppressor activity in animals with chronically tolerated heart and liver grafts was determined using an in vivo adoptive transfer assay of spleen cells. Spleens were removed from animals that had heart graft survival for 28 days or >70 days and from liver recipients that had graft survival of 100 or 300 days. Single-cell suspensions were prepared by mincing the recipient spleens into RPMI-1640. A total of 200×106 splenic lymphocytes from these LEW heart or liver recipients was injected intravenously into sublethally irradiated (250 rads) or nonirradiated syngeneic LEW recipients immediately after the placement of a fresh ACI cardiac or liver graft. Survival of the graft was the endpoint of this experiment.
Statistical analysis
Results were analyzed for statistical significance by the unpaired two-tailed Student’s t test, and differences considered statistically significant if P<0.01. Because graft survival results were variable, significance was determined in all survival experiments with the Wilcoxon rank sum test. There was a difference using the 2 techniques only in groups 4 and 5 in Table 4.
Table 4.
Effect of adoptive transfer of splenic lymphocytes from FK506-treated LEW rats with ACI heart grafts on the acceptance of ACI hearts by naive LEW recipients
| Group | Spleocyte donor |
Number of Cells (×10a) |
Irradiation (250 rads) |
n | Survival (days) |
MST (days) |
Pa |
|---|---|---|---|---|---|---|---|
| 1b | — | — | − | 7 | 6, 6, 6, 6, 6, 7, 7 | 6.0 | — |
| 2 | — | — | + | 4 | 6, 7, 7, 8 | 7.0 | NS |
| 3 | Normal LEW | 200 | + | 4 | 6, 6, 7, 10 | 6.5 | NS |
| 4 | FK-treated LEW with ACI heart graft (28 days)c |
200 | + | 8 | 6, 7, 8, 8, 10, 10, 10, 15 | 9.0 | <0.01 (0.02) |
| 5 | FK-treated LEW with ACI heart graft (70 days)c |
200 | + | 6 | 7, 7, 7, 10, 10, 20 | 8.5 | 0.01 (0.07) |
Wilcoxon rank sum test (Student’s t test).
Control values from Table 1.
Spleen cells were pooled from 3 animals, each of which had functioning heart grafts for 28 days and more than 70 days.
RESULTS
Results after cardiac transplantation
Strain specificity of graft acceptance
The test results and controls are summarized in Table 1. In essence, the transplantation of a primary ACI heart to a Lewis recipient with delayed 3-dose FK506 therapy allowed prolonged survival of a second ACI heart transplanted either 2 (group 8) or 4 weeks (group 10) after the transplantation of the first heart. Part of this prolongation in the 2-week but not the 4-week experiments may have been due to residual immunosuppression from the original FK506 therapy, which was demonstrable in control groups 4 and 5 but not groups 6 and 7. Eventually, the second hearts were rejected, usually about when the primary grafts were rejected.
Table 1.
Specificity of the graft acceptance in LEW recipients after ACI cardiac transplantation with FK506
| Group | First graft |
FK treatmentc |
Second graft |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Donor | MSTa | Pb | n | Timing (weeks)d |
Tissue | Donor | MST | Pb | ||
| 1 | 7 | ACI | 6.0 (6–7) | — | − | — | — | — | — | — | — |
| 2 | 6 | BN | 11.5 (11–13) | — | − | — | — | — | — | — | — |
| 3 | 6 | ACI | 91.0 (23–146) | – | + | — | — | — | — | — | |
| 4 | — | — | — | + | 7 | 2 | Heart | ACI | 10.0 (9–19) | <0.001* — | |
| 5 | — | — | — | + | 7 | 2 | Heart | BN | 34.0 (15–42) | <0.01** — | |
| 6 | — | — | — | + | 5 | 4 | Heart | ACI | 7.0 (6–8) | NS* — | |
| 7 | — | — | — | + | 5 | 4 | Heart | BN | 9.0 (7–20) | NS** — | |
| 8 | 6 | ACI | 63.5 (37–95) | NS*** | + | 6 | 2 | Heart | ACI | 45.0 (15–72) | <0.01* <0.01**** |
| 9 | 5 | ACI | 54.0 (42–79) | NS*** | + | 5 | 2 | Heart | BN | 14.0 (10–17) | NS** <0.01**** |
| 10 | 6 | ACI | 59.5 (48–78) | NS*** | + | 6 | 4 | Heart | ACI | 27.5 (18–42) | <0.01* <0.001**** |
| 11 | 4 | ACI | 56.0 (53–76) | NS*** | + | 4 | 4 | Heart | BN | 10.0 (6–11) | NS** NS**** |
| 12 | 5 | ACI | 36.0 (35–40) | NS*** | + | 5 | 4 | Skin | ACI | 10.0 (9–10) | NS***** |
| 12′ | 5 | ACI | 36.0 (35–40) | NS*** | + | 5 | 4 | Skin | BN | 12.0 (12–14) | NS***** |
Median survival time (range) in days.
P value (Wilcoxon rank sum test) * vs. group 1; ** vs. group 2; *** vs. group 3; **** vs. treated control groups (groups 8 vs. 4, 9 vs. 5, 10 vs. 6, 11 vs. 7); ***** vs. untreated control groups of skin grafting (Table 3).
1.28 mg/kg intramuscularly on days 4, 5, and 6 after first ACI heart allografting.
Challenge with secondary grafting at either 2 weeks or 4 weeks following first grafting.
In contrast, BN hearts transplanted 2 (group 9) and 4 weeks (group 11) after primary ACI grafts had no prolongation whatever, compared with the controls of group 2.
Organ specificity of graft acceptance
ACI and BN skin grafts are normally rejected by LEW recipients in 11 and 14 days, respectively (control data are shown in Table 2). The presence of an ACI heart transplantation 4 weeks earlier with FK506 did not effect the rejection of either ACI or BN skin grafts (groups 12 and 12′, Table 1).
Table 2.
Survival of donor and third-party skin grafts on FK506-treated LEW rats with established ACI liver grafts
| Recipient | Donor | n | Skin graft survival (days) |
MST (days) |
Pa |
|---|---|---|---|---|---|
| LEW (normal) | ACI | 5 | 10, 10, 11, 11, 11 | 11.0 | — |
| BN | 5 | 14, 14, 14, 14, 15 | 14.0 | — | |
| LEW (100 days after ACI liver grafting) |
ACI | 6 | 10, 10, 11, 11, 14, 15 | 11.0 | NS* |
| BN | 6 | 10, 14, 14, 14, 15, 16 | 14.0 | NS** | |
| LEW (300 days after ACI liver grafting) |
ACI | 3 | 14, 14, 14 | 14.0 | NS* |
| BN | 3 | 15, 16, 18 | 16.0 | NS** |
P (Wilcoxon rank sum test): * vs. survival of ACI skin grafts in normal LEW rats; ** vs. survival of BN skin grafts in normal LEW rats.
Influence of splenectomy
The results after heart transplantation were not significantly different with or without splenectomy in the animals treated with FK506 (Table 3).
Table 3.
Influence of splenectomy on heart graft survival in FK506-treated animals
| Group | Treatment | n | Graft survival (days) |
MST (days) |
Pa |
|---|---|---|---|---|---|
| 1b | — | 7 | 6, 6, 6, 6, 6, 7, 7 | 6.0 | — |
| 2b | 1.28 mg/kg, days 4, 5, 6 | 6 | 23, 51, 89, 93, 98, 146 | 91.0 | <0.01 |
| 3 | Splenectomy | 3 | 6, 6, 6 | 6.0 | NS |
| 4 | 1.28 mg/kg, days 4, 5, 6 with splenectomyc |
9 | 30, 33, 37, 38, 40, 41, 136, 167d, 173d |
40.0 | <0.001 |
P value (Wilcoxon rank sum test) group 2 vs. 4: NS.
Control values from Table 1.
Splenectomy was performed on the day of grafting.
Animal was sacrificed with pulsating graft.
Lymphocyte subset analysis 7 days after heart transplantation with FK506
FK506 treatment was done on days 4, 5, and 6. The total number of cells infiltrating the cardiac allografts was markedly reduced after treatment with FK506, compared with the findings in untreated animals (1). In 5 grafts studied on day 7 in animals given 1.28 mg/kg FK506 i.m. on days 4, 5, and 6, the proportion of OX19, OX8, w3/25, and OX6 lymphocytes in the diminished infiltrate was not significantly different from that in the cardiac grafts of untreated controls (data not shown). The subset proportions were similar in the spleen in 3 other similarly treated heart recipients, although there was an increased expression of class II histocompatibility antigens (OX6) in the peripheral blood lymphocytes of these versus untreated animals (P<.0001). Three nonoperated Lewis rats given the same FK506 treatment had no significant changes in lymphocyte subset distribution in their spleens or peripheral blood.
Results after liver transplantation
Organ specificity of graft acceptance
Both ACI and BN skin grafts were rejected in the normal way 100 days or 300 days after ACI-to-LEW liver transplantation (Table 2).
Graft-versus-host reactivity (popliteal lymph node assay)
After the injection of parental strain (LEW) splenic lymphocytes into F1 footpads, draining popliteal lymph nodes (PLN) of F1 hybrids were noted to have significantly increased weight (mean PLN weight: 92.88±1.89 mg), whereas the splenic lymphocytes from the syngeneic F1 hybrid did not induce PLN hypertrophy (5.83±1.6 mg) (Fig. 1). The splenic lymphocytes from FK506-treated LEW recipients with permanent acceptance of ACI liver grafts demonstrated an intermediate GVHD response between the positive and negative controls (PLN weight: 35.81±8.12 mg). This showed that LEW lymphocytes from the spleen of unaltered controls—and, to a lesser extent, from animals with permanent liver graft acceptance—were capable of recognizing the ACI alloreactivity of the F1 test animal.
Figure 1.

Graft-versus-host reactivity of spleen cells from LEW rats with permanently accepted ACI liver grafts, as demonstrated with the popliteal lymph node assay in F1 hybrid (ACI×LEW) test animals.
Mixed lymphocyte reactivity and suppressor cells
Mesenteric lymph node cells from 6 LEW recipients of permanently accepted ACI liver grafts responded to irradiated ACI and BN lymphocytes (Fig. 2). There were no exceptions, although the degree of proliferation stimulated by the ACI alloantigens was more variable than with BN.
Figure 2.
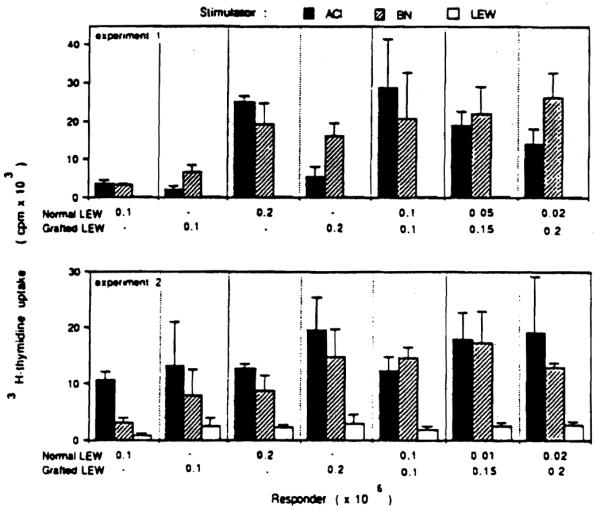
Mixed lymphocyte reaction of lymph node cells taken from LEW rats bearing permanently accepted ACI liver grafts. Note the substantial antidonor (anti-ACI) reactivity.
In the same MLR system, suppressor cell activity was looked for in lymph node cells from the LEW rats bearing ACI livers. Lymph node cells from the grafted rats failed to suppress the MLR when lymphocytes from unmodified LEW donors were exposed to irradiated ACI or BN lymphocytes (Fig. 2), indicating that the liver graft acceptance was not associated with suppressor cell activity.
Lymphocyte subset distribution
In 3 LEW recipients 100 days after liver grafting, lymphocytes from the spleen and peripheral blood did not have a changed proportion of OX19, OX8, w3/25, and OX6 lymphocytes compared with normal nonoperated controls (data not shown).
Adoptive transfer after Mart and liver transplantation
Suppressor cell activity in spleens in FK506-treated recipients of either cardiac or liver allografts was looked for in adoptive transfer experiments.
Splenic lymphocytes from cardiac recipients
Control LEW rats subjected to 250 rads total-body irradiation alone or irradiation plus transfer of 200×106-splenic lymphocytes from normal LEW rats rejected ACI heart grafts with an MST of 7.0 days (group 2) and 6.5 days (group 3), respectively (Table 4). Transfer of 200×106 lymphocytes from recipients with surviving heart grafts for 28 days or more than 70 days did not consistently prolong graft survival (groups 4 and 5), although there were more examples of long graft survival than in the controls. The mean prolongation was not significant with the Student’s t tests (group 4, P=.02; group 5, P=.07) but was with the Wilcoxon rank sum test (P<0.01).
Splenic lymphocytes from liver recipients
Splenic lymphocytes 100 and 300 days after liver grafting did not alter the survival of ACI liver grafts transplanted into naive LEW recipients (Table 5).
Table 5.
Effect of adoptive transfer of splenic lymphocytes from FK506-treated LEW rats with ACI liver grafts on the acceptance of ACI livers by naive LEW recipients
| Group | Splenocyte donor |
Num- ber of cells (×10a) |
n | Graft survival (days) |
MST (days) |
Pa |
|---|---|---|---|---|---|---|
| 1 | — | — | 10 | 9, 9, 9, 9, 10, 10, 10, 11, 12, 13 |
10.0 | — |
| 2 | Normal LEW | 200 | 5 | 9, 9, 9, 10, 10 | 9.0 | NS |
| 3 | FK-treated LEW with ACI liver graft (100 days)b |
200 | 7 | 8, 8, 10, 10, 11, 12, 12 | 10.0 | NS |
| 4 | FK-treated LEW with ACI liver graft (300 days)b |
200 | 4 | 9, 10, 10, 11 | 10.0 | NS |
Wilcoxon rank sum test.
Splenocytes were pooled from 4 and 2 animals, each of which had functioning liver grafts for 100 and more than 300 days.
DISCUSSION
The predictability and relative ease with which permanent liver graft acceptance could be achieved made it possible to study a large group of animals the clinical—and presumably immunologic—status of which was stable after 100 or 300 days. These rats have been followed for as long as 1½ years. They were not immunologically depressed. They were healthy, vigorously rejected third-party or even donor-specific skin grafts, and possessed lymphocytes that were stimulated strongly in mixed lymphocytes culture of third-party alloantigens, and more weakly by donor-specific antigens. Not only could the recipient lymphoid tissue be shown to retain antidonor reactivity following allotransplantation, but weak graft-versus-host reactivity was identified in the recipient spleen.
The conclusion was that long-term survival of the liver allografts was not due solely to classic tolerance. There was no evidence of a change in the lymphocyte population or lymphocyte subsets in the spleen or peripheral blood, and neither was there evidence of a crucial role of suppressor cells, either with in vitro or in vivo (MLR, splenectomy, and adoptive transfer) experiments. Nevertheless, it is intriguing that the frequency of heart graft prolongation was greater in the splenectomized animals, although not significantly so, and that there was a modest but significant prolongation of heart grafts after adoptive transfer. The experiments do not necessarily disprove accepted mechanisms of tolerance induction—but, taken together, they do not strongly support any of the monolithic theories of graft acceptance, including clonal deletion, active enhancement, suppressor cell activation, or antigen-antibody blockade of effector cells. The findings are not even fully compatible with a concatenation hypothesis (23) in which multiple immunologic changes could playa variable but reinforcing role.
One possibility in a concatenation hypothesis that has not been ruled out is that the liver graft itself undergoes a change. The crucial experiment to determine this—namely, orthotopic retransplantation of a well tolerated ACI liver to a Lewis recipient—has not been done and may not be technically feasible. However, it is known that the entire Kuppfer cell (macrophage) system of the accepted liver changes rather quickly to that of the recipient in well-tolerated human grafts, while the hepatocytes, vascular endothelium, and carrier lymphoid tissue remain those of the donor (24). The switch to recipient status of the dendritic cell–rich macrophage system could be such a subtle event that its significance in liver graft acceptance has been overlooked. Other ways in which a graft could be modified may be suggested by the classic experiments on thyroid transplantation by Woodruff and Woodruff (25).
It has been much harder to induce heart graft acceptance and the details of dose timing are more critical (1). The experiments showing limited privilege for a second heart from the same (but not a third-party) donor strain were carried out much earlier (14–28 days) after the primary transplantation than in the liver experiments. In these experiments, the fate of the first and second hearts from the same donor strain was linked. When rejection occurred, it simultaneously effected both hearts as if the grafts were equal in the eyes of the immune system. It is possible that induction of heart graft acceptance is more difficult than that of liver because there is not a replacement of its dendritic cell–rich macrophage components. No studies of this important question are available to our knowledge.
More work will be necessary before graft acceptance after short-term therapy, or failure to achieve this objective, can be understood. Then practical application could be close behind as increasingly powerful immunosuppressive agents become available for induction. Even in mongrel dogs, it has been possible using short courses of FK506 to have prolonged kidney and liver graft survival (4, 26). In this species, as in the rat, the liver is “easier” than the kidney, but with both organs in outbred dogs an unqualified permanent success is the exception. However, what makes the exception could be the key to a new rule.
Possible reasons for the easier acceptance of liver versus other organ grafts have been discussed elsewhere (27). It is possible that donor-specific HLA antigens that are produced by the new liver (28) could play a role. These HLA antigens appear very promptly after revascularization of human liver grafts (29).
Footnotes
This work was supported by research grants from the Veterans Administration and Project Grant DK 29961 from the National Institutes of Health, Bethesda, MD.
REFERENCES
- 1.Murase N, Kim DG, Todo S, Cramer DJ, Fung J, Starzl TE. Suppression of allograft rejection with FK 506 I: prolonged cardiac and liver survival in rats following short course therapy. Transplantation. 1990;50:186. doi: 10.1097/00007890-199008000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murase N, Todo S, Lee PH, et al. Heterotopic heart transplantation in the rats receiving FK-506 alone or with cyclosporine. Transplant Proc. 1987;19(suppl 6):71. [PMC free article] [PubMed] [Google Scholar]
- 3.Ochiai T, Nakajima K, Nagata M, et al. Studies of the induction and maintenance of long-term graft acceptance by treatment with FK-506 on heterotopic cardiac allotransplantation in the rat. Transplantation. 1987;44:734. doi: 10.1097/00007890-198712000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Todo S, Ueda Y, Demetria AJ, et al. Immunosuppression of canine, monkey, and baboon allografts by FK-506: with special reference to synergism with other drugs and tolerance induction. Surgery. 1988;104:239. [PMC free article] [PubMed] [Google Scholar]
- 5.Todo S, Demetris AJ, Ueda U, et al. Renal transplantation in baboons under FK-506. Surgery. 1989;106:444. [PubMed] [Google Scholar]
- 6.Starzl TE, Marchioro TL, Porter KA, Iwasaki Y, Cerilli GJ. The use of heterologous antilymphoid agents in canine renal and liver transplantation and in human renal homotransplantation. Surg Gynecol Obstet. 1967;124:301. [PMC free article] [PubMed] [Google Scholar]
- 7.Hart DNJ, Winearls CG, Fabre JW. Graft adaptation: studies on possible mechanisms in long-term surviving rat renal allografts. Transplantation. 1980;30:73. [PubMed] [Google Scholar]
- 8.Lechler RI, Batchelor JR. Mechanisms of reduced immunogenicity of retransplanted rat kidney allografts. Transplant Proc. 1983;15:316. [Google Scholar]
- 9.Silvers WK, Kimura H, Desquenne-Clark L, Miyamoto M. Some new perspectives on transplantation immunity and tolerance. Immunol Today. 1987;8:117. doi: 10.1016/0167-5699(87)90037-5. [DOI] [PubMed] [Google Scholar]
- 10.Hutchinson IV, Zola H. Antigen-reactive cell opsonization (ARCO): a mechanism of immunological enhancement. Transplantation. 1977;23:464. doi: 10.1097/00007890-197706000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson IV. Antigen-reactive cell opsonization (ARCO) and its role in antibody-mediated immune suppression. Immunol Rev. 1980;49:167. doi: 10.1111/j.1600-065x.1980.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 12.Nossal GJV, Pike BL, Good MF, Miller JFAP, Gamble JR. Functional clonal deletion and suppression as complementary mechanisms in T-lymphocyte tolerance. Curr Top Microbiol Immunol. 1986;126:199. doi: 10.1007/978-3-642-71152-7_24. [DOI] [PubMed] [Google Scholar]
- 13.Stuart FP, Fitch FW, Rowley DA, Biesecker JL, Hellstrom KE, Hellstrom I. Presence of cell-mediated immunity and serum blocking factors in rat renal allograft enhanced by passive immunization. Transplantation. 1971;12:331. doi: 10.1097/00007890-197110000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Stuart FP, Scolland DM, McKearn TJ, Fitch FW. Cellular and humoral immunity after allogenic renal transplantation in the rat V. Appearance of anti-idiotypic antibody and its relationship to cellular immunity after treatment with donor spleen cells and alloantibody. Transplantation. 1976;22:455. doi: 10.1097/00007890-197611000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Hutchinson IF, Shadur CA, Duarte JSA, Strom TB, Tilney NI. Cyclosporin A spares selectively lymphocytes with donor specific suppressor characteristics. Transplantation. 1981;32:210. doi: 10.1097/00007890-198109000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Hall BM, Gurley KE, Pierce NW, Dorsch SE. Specific unresponsiveness in rats with cyclosporine II. Sequential changes in alloreactivity of T-cell subsets. Transplantation. 1989;47:1030. doi: 10.1097/00007890-198906000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Pearce NW, Spinelli A, Gurley KE, Dorsch SE, Hall BM. Mechanisms maintaining antibody-induced enhancement of allografts: II. Mediation of specific suppression by short lived CD4+ T cells. J Immunol. 1989;143:499. [PubMed] [Google Scholar]
- 18.Dorsch SE, Roser B. Recirculating, suppressor T-cells in transplantation tolerance. J Exp Med. 1977;145:1144. doi: 10.1084/jem.145.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagao T, White DJG, Calne RY. Kinetics of unresponsiveness induced by a short course of cyclosporin A. Transplantation. 1982;33:31. doi: 10.1097/00007890-198201000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Heron I. A technique for accessory cervical heart transplantation in rabbits and rats. Acta Pathol Microbiol Scand [A] 1971;79:366. doi: 10.1111/j.1699-0463.1971.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 21.Morris RE, Hoyt G, Baldwin J, Meiser B. Splenectomy antagonized the action by cyclosporine. Transplant Proc. 1988;20:1079. [PubMed] [Google Scholar]
- 22.Cramer DV, Daris BK, Shonnand JW, et al. The graft-versus-host reactivity in AG-B/MLR disparate strain of rats. Transplantation. 1977;23:498. doi: 10.1097/00007890-197706000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Starzl TE, Putnam CW, editors. Experience in hepatic transplantation. Saunders; Philadelphia: 1969. Efforts to mitigate or prevent rejection; p. 229. with the assistance of. Chapter 12. [Google Scholar]
- 24.Porter KA. Pathdogy of the orthotopic homogarft and heart graft. In: Starzl TE, editor. Experience in hepatic transplantation. Saunders; Philadelphia: 1969. p. 464. [Google Scholar]
- 25.Woodruff MFA, Woodruff HG. The transplantation of normal tissue: with special reference to auto- and homotransplants of thyroid and spleen in the anterior chamber of the eye, and subcutaneously in guinea pigs. Philos Trans R Soc Land [Biol] 1950;234:559. [PubMed] [Google Scholar]
- 26.Ueda Y, Todo S, Eiras G, et al. Tolerance induction after dog kidney or liver transplantation. Transplant Proc. 1990;22:80. [PMC free article] [PubMed] [Google Scholar]
- 27.Starzl TE, Demetria AJ. Liver transplantation: a 31-year perspective. Curr Probl Surg. 1990 Feb;27:124, 150. doi: 10.1016/0011-3840(90)90021-v. [DOI] [PubMed] [Google Scholar]
- 28.Davies HS, Pollard SH, Calne RY. Soluble HLA antigens in the circulation of liver graft recipients. Transplantation. 1989;67:524. doi: 10.1097/00007890-198903000-00025. [DOI] [PubMed] [Google Scholar]
- 29.Pollard SG, Davies HS, Calne RY. Perioperative appearance of serum class I antigen during liver transplantation. Transplantation. 1990;49:659. doi: 10.1097/00007890-199003000-00039. [DOI] [PubMed] [Google Scholar]


