Abstract
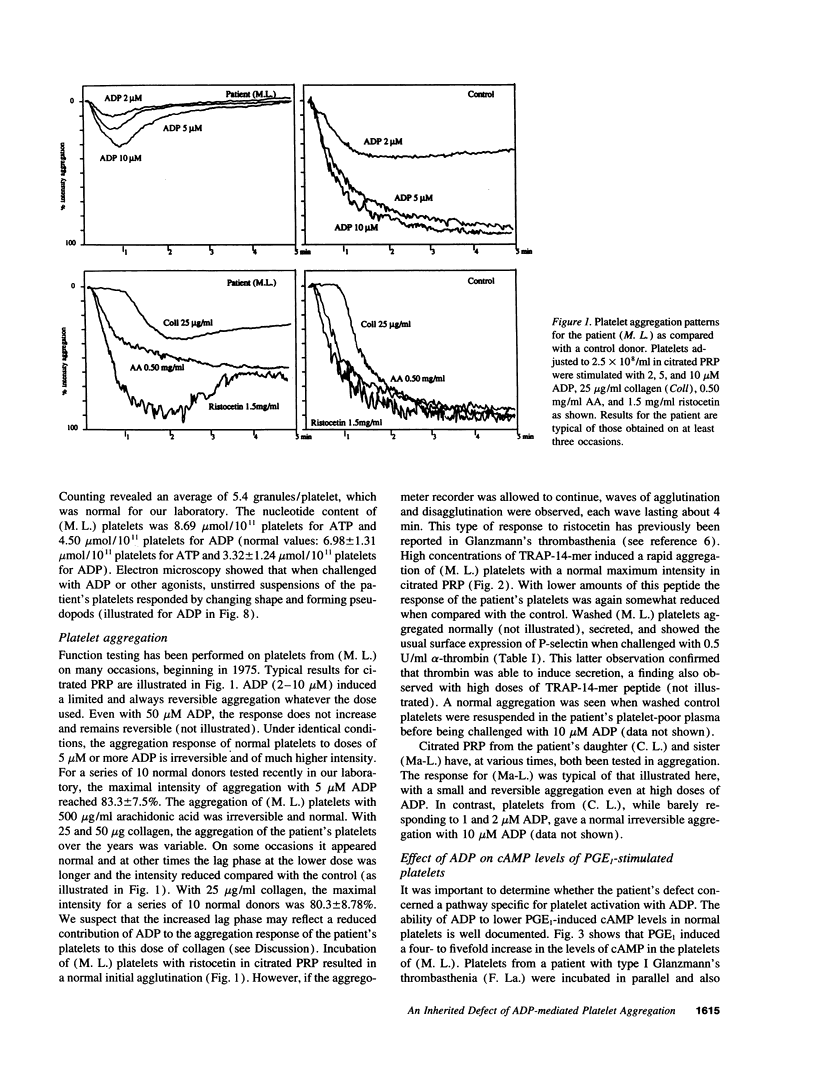
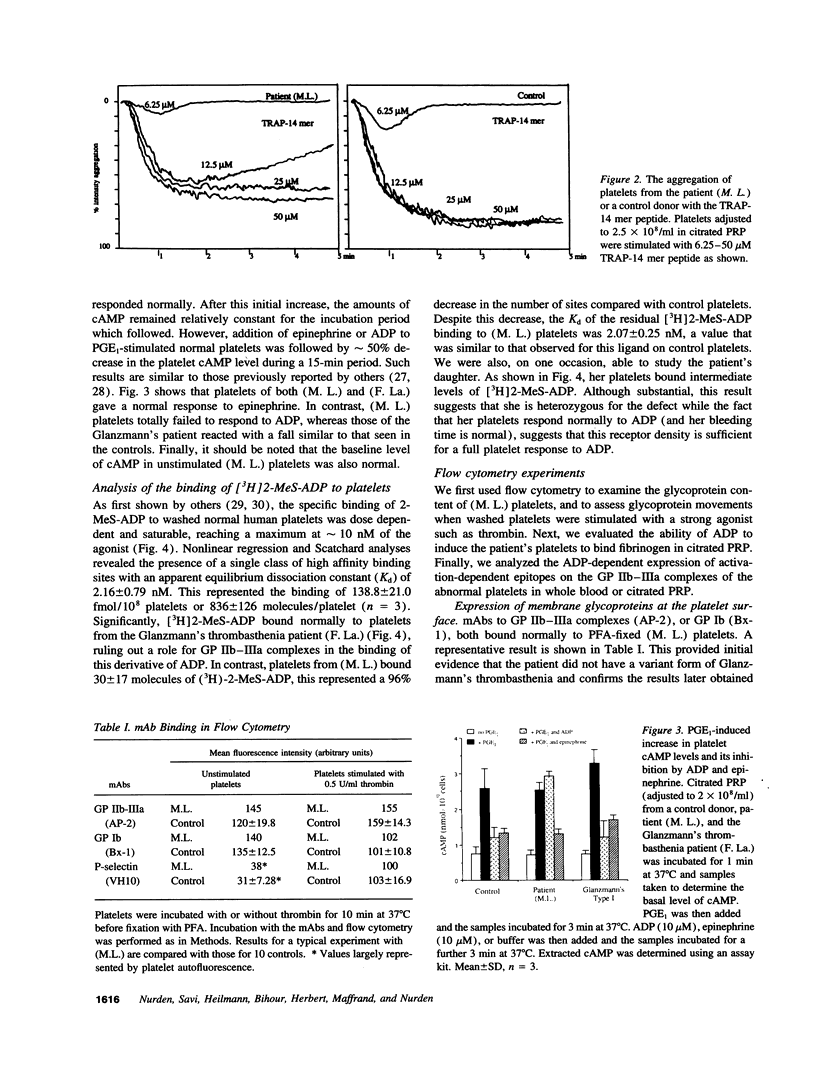
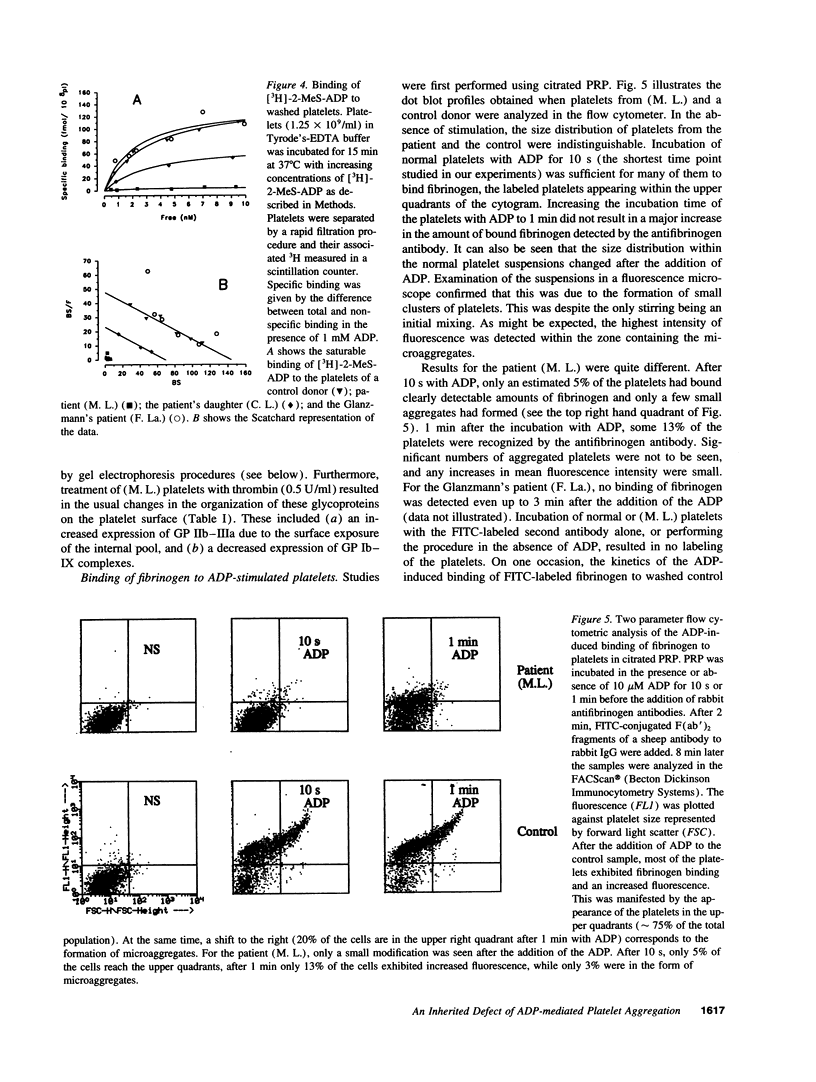
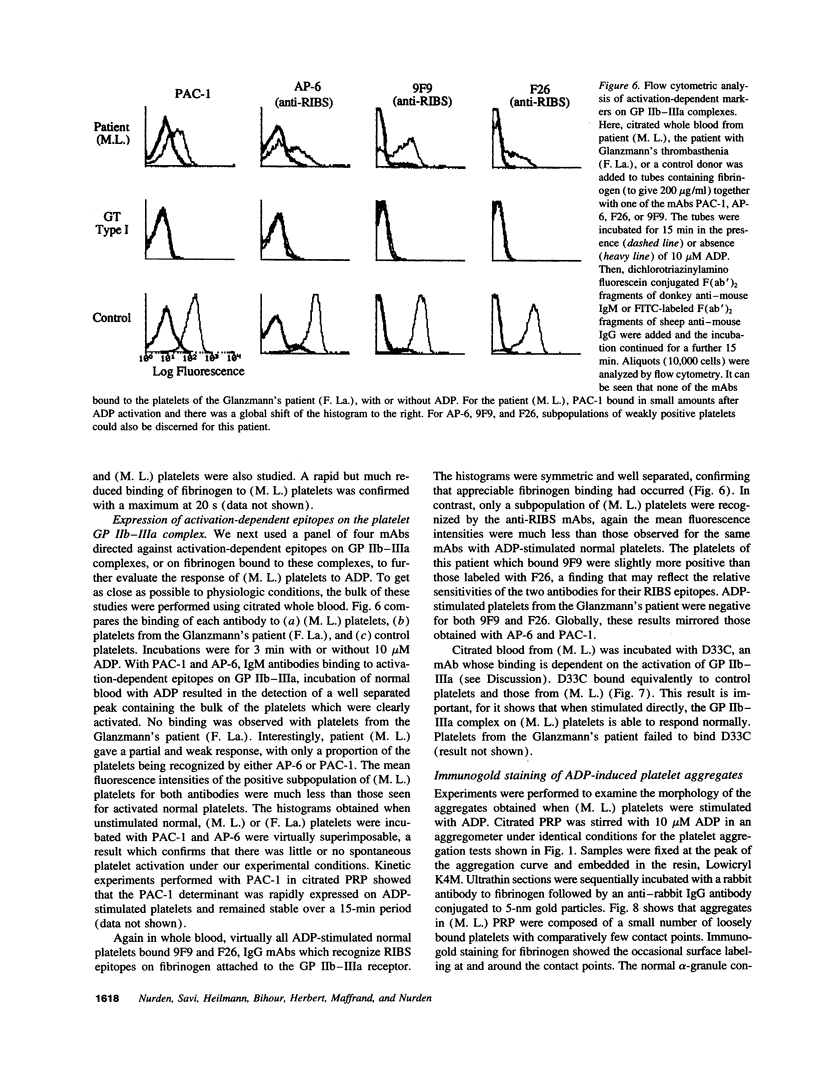
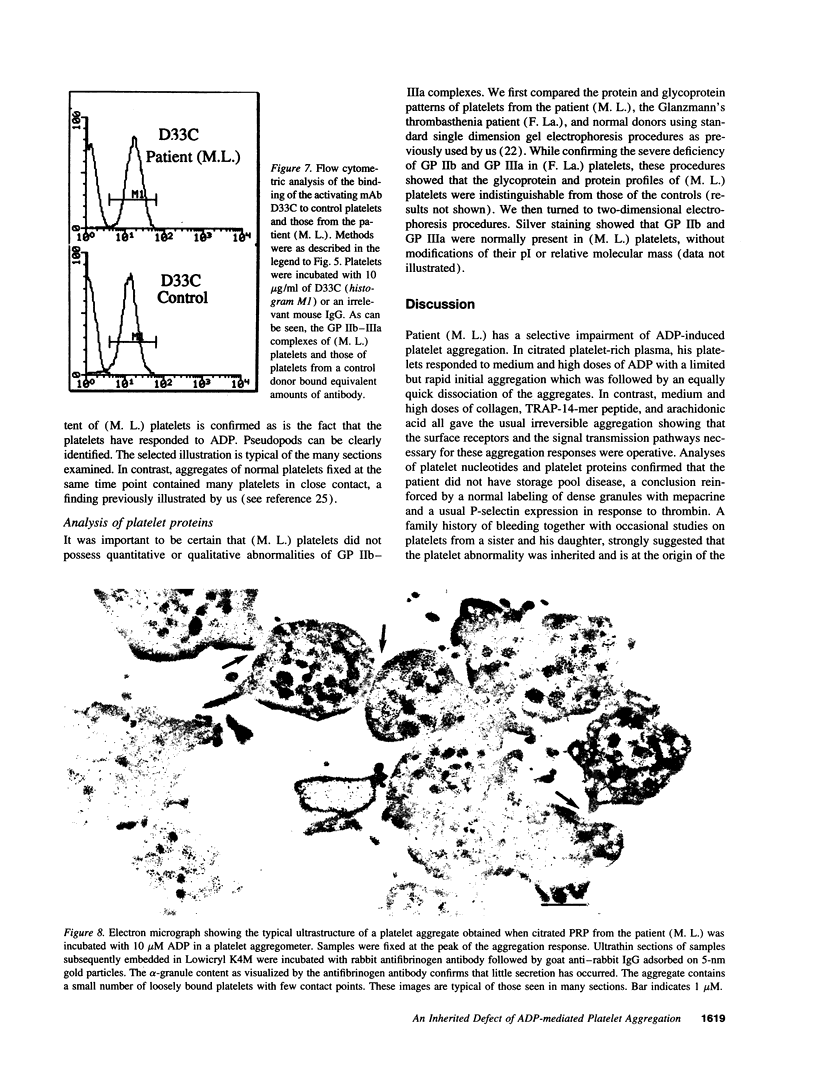
Much discussion has concerned the central role of ADP in platelet aggregation. We now describe a patient (M.L.) with an inherited bleeding disorder whose specific feature is that ADP induces a limited and rapidly reversible platelet aggregation even at high doses. Platelet shape change and other hemostatic parameters were unmodified. A receptor defect was indicated, for, while epinephrine normally lowered cAMP levels of PGE1-treated (M.L.) platelets, ADP was without effect. The binding of [3H]2-methylthio-ADP decreased from 836 +/- 126 molecules/platelet for normals to 30 +/- 17 molecules/platelet for the patient. Flow cytometry confirmed that ADP induced a much lower fibrinogen binding to (M.L.) platelets. Nonetheless, the binding in whole blood of activation-dependent monoclonal antibodies showed that some activation of GP IIb-IIIa complexes by ADP was occurring. Platelets of a patient with type I Glanzmann's thrombasthenia bound [3H]2-methylthio-ADP and responded normally to ADP in the presence of PGE1. Electron microscopy showed that ADP-induced aggregates of (M. L.) platelets were composed of loosely bound shape-changed platelets with few contact points. Thus this receptor defect has a direct influence on the capacity of platelets to bind to each other in response to ADP.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams C., Shattil S. J. Immunological detection of activated platelets in clinical disorders. Thromb Haemost. 1991 May 6;65(5):467–473. [PubMed] [Google Scholar]
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Brass L. F., Hoxie J. A., Manning D. R. Signaling through G proteins and G protein-coupled receptors during platelet activation. Thromb Haemost. 1993 Jul 1;70(1):217–223. [PubMed] [Google Scholar]
- Cattaneo M., Lecchi A., Randi A. M., McGregor J. L., Mannucci P. M. Identification of a new congenital defect of platelet function characterized by severe impairment of platelet responses to adenosine diphosphate. Blood. 1992 Dec 1;80(11):2787–2796. [PubMed] [Google Scholar]
- Chediak J., Telfer M. C., Vander Laan B., Maxey B., Cohen I. Cycles of agglutination-disagglutination induced by ristocetin in thrombasthenic platelets. Br J Haematol. 1979 Sep;43(1):113–126. doi: 10.1111/j.1365-2141.1979.tb03726.x. [DOI] [PubMed] [Google Scholar]
- Colman R. W. Aggregin: a platelet ADP receptor that mediates activation. FASEB J. 1990 Mar;4(5):1425–1435. doi: 10.1096/fasebj.4.5.2407587. [DOI] [PubMed] [Google Scholar]
- Cristalli G., Mills D. C. Identification of a receptor for ADP on blood platelets by photoaffinity labelling. Biochem J. 1993 May 1;291(Pt 3):875–881. doi: 10.1042/bj2910875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack N. J., Hourani S. M. Competitive inhibition by adenosine 5'-triphosphate of the actions on human platelets of 2-chloroadenosine 5'-diphosphate, 2-azidoadenosine 5'-diphosphate and 2-methylthioadenosine 5'-diphosphate. Br J Pharmacol. 1982 Oct;77(2):329–333. doi: 10.1111/j.1476-5381.1982.tb09302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defreyn G., Gachet C., Savi P., Driot F., Cazenave J. P., Maffrand J. P. Ticlopidine and clopidogrel (SR 25990C) selectively neutralize ADP inhibition of PGE1-activated platelet adenylate cyclase in rats and rabbits. Thromb Haemost. 1991 Feb 12;65(2):186–190. [PubMed] [Google Scholar]
- Di Minno G., Cerbone A. M., Mattioli P. L., Turco S., Iovine C., Mancini M. Functionally thrombasthenic state in normal platelets following the administration of ticlopidine. J Clin Invest. 1985 Feb;75(2):328–338. doi: 10.1172/JCI111705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet C., Cazenave J. P. ADP induced blood platelet activation: a review. Nouv Rev Fr Hematol. 1991;33(5):347–358. [PubMed] [Google Scholar]
- Gachet C., Cazenave J. P., Ohlmann P., Hilf G., Wieland T., Jakobs K. H. ADP receptor-induced activation of guanine-nucleotide-binding proteins in human platelet membranes. Eur J Biochem. 1992 Jul 1;207(1):259–263. doi: 10.1111/j.1432-1033.1992.tb17046.x. [DOI] [PubMed] [Google Scholar]
- George J. N., Caen J. P., Nurden A. T. Glanzmann's thrombasthenia: the spectrum of clinical disease. Blood. 1990 Apr 1;75(7):1383–1395. [PubMed] [Google Scholar]
- George J. N., Nurden A. T., Phillips D. R. Molecular defects in interactions of platelets with the vessel wall. N Engl J Med. 1984 Oct 25;311(17):1084–1098. doi: 10.1056/NEJM198410253111705. [DOI] [PubMed] [Google Scholar]
- Gralnick H. R., Williams S. B., McKeown L., Shafer B., Connaghan G. D., Hansmann K., Vail M., Magruder L. Endogenous platelet fibrinogen: its modulation after surface expression is related to size-selective access to and conformational changes in the bound fibrinogen. Br J Haematol. 1992 Mar;80(3):347–357. doi: 10.1111/j.1365-2141.1992.tb08144.x. [DOI] [PubMed] [Google Scholar]
- Greco N. J., Tandon N. N., Jackson B. W., Jamieson G. A. Low structural specificity for nucleoside triphosphates as antagonists of ADP-induced platelet activation. J Biol Chem. 1992 Feb 15;267(5):2966–2970. [PubMed] [Google Scholar]
- Gulino D., Ryckewaert J. J., Andrieux A., Rabiet M. J., Marguerie G. Identification of a monoclonal antibody against platelet GPIIb that interacts with a calcium-binding site and induces aggregation. J Biol Chem. 1990 Jun 5;265(16):9575–9581. [PubMed] [Google Scholar]
- Hanash S. M., Neel J. V., Baier L. J., Rosenblum B. B., Niezgoda W., Markel D. Genetic analysis of thirty-three platelet polypeptides detected in two-dimensional polyacrylamide gels. Am J Hum Genet. 1986 Mar;38(3):352–360. [PMC free article] [PubMed] [Google Scholar]
- Hardisty R., Pidard D., Cox A., Nokes T., Legrand C., Bouillot C., Pannocchia A., Heilmann E., Hourdillé P., Bellucci S. A defect of platelet aggregation associated with an abnormal distribution of glycoprotein IIb-IIIa complexes within the platelet: the cause of a lifelong bleeding disorder. Blood. 1992 Aug 1;80(3):696–708. [PubMed] [Google Scholar]
- Haslam R. J. Interactions of the pharmacological receptors of blood platelets with adenylate cyclase. Ser Haematol. 1973;6(3):333–350. [PubMed] [Google Scholar]
- Heilmann E., Hourdillé P., Pruvost A., Paponneau A., Nurden A. T. Thrombin-induced platelet aggregates have a dynamic structure. Time-dependent redistribution of glycoprotein IIb-IIIa complexes and secreted adhesive proteins. Arterioscler Thromb. 1991 May-Jun;11(3):704–718. doi: 10.1161/01.atv.11.3.704. [DOI] [PubMed] [Google Scholar]
- Hourdille P., Bernard P., Belloc F., Pradet A., Sanchez R., Boisseau M. R. Platelet abnormalities in thermal injury. Study of platelet-dense bodies stained with mepacrine. Haemostasis. 1981;10(3):141–152. doi: 10.1159/000214398. [DOI] [PubMed] [Google Scholar]
- Hourdillé P., Heilmann E., Combrié R., Winckler J., Clemetson K. J., Nurden A. T. Thrombin induces a rapid redistribution of glycoprotein Ib-IX complexes within the membrane systems of activated human platelets. Blood. 1990 Oct 15;76(8):1503–1513. [PubMed] [Google Scholar]
- Janes S. L., Wilson D. J., Chronos N., Goodall A. H. Evaluation of whole blood flow cytometric detection of platelet bound fibrinogen on normal subjects and patients with activated platelets. Thromb Haemost. 1993 Oct 18;70(4):659–666. [PubMed] [Google Scholar]
- Kieffer N., Phillips D. R. Platelet membrane glycoproteins: functions in cellular interactions. Annu Rev Cell Biol. 1990;6:329–357. doi: 10.1146/annurev.cb.06.110190.001553. [DOI] [PubMed] [Google Scholar]
- Macfarlane D. E. 2-Methylthioadenosine [beta-32P]diphosphate: synthesis and use as probe of platelet ADP receptors. Methods Enzymol. 1992;215:137–142. doi: 10.1016/0076-6879(92)15059-l. [DOI] [PubMed] [Google Scholar]
- Macfarlane D. E., Mills D. C., Srivastava P. C. Binding of 2-azidoadenosine [beta-32P]diphosphate to the receptor on intact human blood platelets which inhibits adenylate cyclase. Biochemistry. 1982 Feb 2;21(3):544–549. doi: 10.1021/bi00532a020. [DOI] [PubMed] [Google Scholar]
- Macfarlane D. E., Mills D. C. The effects of ATP on platelets: evidence against the central role of released ADP in primary aggregation. Blood. 1975 Sep;46(3):309–320. [PubMed] [Google Scholar]
- Macfarlane D. E., Srivastava P. C., Mills D. C. 2-Methylthioadenosine[beta-32P]diphosphate. An agonist and radioligand for the receptor that inhibits the accumulation of cyclic AMP in intact blood platelets. J Clin Invest. 1983 Mar;71(3):420–428. doi: 10.1172/JCI110786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure M. O., Kakkar A., Cusack N. J., Born G. V. Evidence for the dependence of arterial haemostasis on ADP. Proc R Soc Lond B Biol Sci. 1988 Aug 23;234(1276):255–262. doi: 10.1098/rspb.1988.0047. [DOI] [PubMed] [Google Scholar]
- McGuire T. F., Corey S. J., Sebti S. M. Lovastatin inhibits platelet-derived growth factor (PDGF) stimulation of phosphatidylinositol 3-kinase activity as well as association of p85 subunit to tyrosine-phosphorylated PDGF receptor. J Biol Chem. 1993 Oct 25;268(30):22227–22230. [PubMed] [Google Scholar]
- Meurs H., Kauffman H. F., Koeter G., De Vries K. Extraction of cyclic AMP for the determination in the competitive protein binding assay. Clin Chim Acta. 1980 Sep 8;106(1):91–97. doi: 10.1016/0009-8981(80)90378-2. [DOI] [PubMed] [Google Scholar]
- Mills D. C., Figures W. R., Scearce L. M., Stewart G. J., Colman R. F., Colman R. W. Two mechanisms for inhibition of ADP-induced platelet shape change by 5'-p-fluorosulfonylbenzoyladenosine. Conversion to adenosine, and covalent modification at an ADP binding site distinct from that which inhibits adenylate cyclase. J Biol Chem. 1985 Jul 5;260(13):8078–8083. [PubMed] [Google Scholar]
- Mills D. C., Puri R., Hu C. J., Minniti C., Grana G., Freedman M. D., Colman R. F., Colman R. W. Clopidogrel inhibits the binding of ADP analogues to the receptor mediating inhibition of platelet adenylate cyclase. Arterioscler Thromb. 1992 Apr;12(4):430–436. doi: 10.1161/01.atv.12.4.430. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Dupuis D., Kunicki T. J., Caen J. P. Analysis of the glycoprotein and protein composition of Bernard-Soulier platelets by single and two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Clin Invest. 1981 May;67(5):1431–1440. doi: 10.1172/JCI110172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden A. T., Nurden P. A review of the role of platelet membrane glycoproteins in the platelet-vessel wall interaction. Baillieres Clin Haematol. 1993 Sep;6(3):653–690. doi: 10.1016/s0950-3536(05)80193-3. [DOI] [PubMed] [Google Scholar]
- Peerschke E. I. The platelet fibrinogen receptor. Semin Hematol. 1985 Oct;22(4):241–259. [PubMed] [Google Scholar]
- Pidard D., Montgomery R. R., Bennett J. S., Kunicki T. J. Interaction of AP-2, a monoclonal antibody specific for the human platelet glycoprotein IIb-IIIa complex, with intact platelets. J Biol Chem. 1983 Oct 25;258(20):12582–12586. [PubMed] [Google Scholar]
- Savi P., Dol F., Herbert J. M. ADP-dependence of platelet activation induced by a thrombin receptor agonist. Nouv Rev Fr Hematol. 1993 Apr;35(2):115–119. [PubMed] [Google Scholar]
- Shattil S. J., Cunningham M., Hoxie J. A. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood. 1987 Jul;70(1):307–315. [PubMed] [Google Scholar]
- Shattil S. J., Hoxie J. A., Cunningham M., Brass L. F. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985 Sep 15;260(20):11107–11114. [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Codina J., Unson C. G., Spiegel A. M. Gi2 mediates alpha 2-adrenergic inhibition of adenylyl cyclase in platelet membranes: in situ identification with G alpha C-terminal antibodies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7809–7813. doi: 10.1073/pnas.86.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugarova T. P., Budzynski A. Z., Shattil S. J., Ruggeri Z. M., Ginsberg M. H., Plow E. F. Conformational changes in fibrinogen elicited by its interaction with platelet membrane glycoprotein GPIIb-IIIa. J Biol Chem. 1993 Oct 5;268(28):21080–21087. [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Yao S. K., Ober J. C., McNatt J., Benedict C. R., Rosolowsky M., Anderson H. V., Cui K., Maffrand J. P., Campbell W. B., Buja L. M. ADP plays an important role in mediating platelet aggregation and cyclic flow variations in vivo in stenosed and endothelium-injured canine coronary arteries. Circ Res. 1992 Jan;70(1):39–48. doi: 10.1161/01.res.70.1.39. [DOI] [PubMed] [Google Scholar]
- Zamarron C., Ginsberg M. H., Plow E. F. A receptor-induced binding site in fibrinogen elicited by its interaction with platelet membrane glycoprotein IIb-IIIa. J Biol Chem. 1991 Aug 25;266(24):16193–16199. [PubMed] [Google Scholar]
- Zawilska K. M., Born G. V., Begent N. A. Effect of ADP-utilizing enzymes on the arterial bleeding time in rats and rabbits. Br J Haematol. 1982 Feb;50(2):317–325. doi: 10.1111/j.1365-2141.1982.tb01922.x. [DOI] [PubMed] [Google Scholar]