Abstract
Background and Purpose
In acute stroke, mismatch between lesions seen on diffusion-(DWI) and perfusion-weighted (PWI) MRI has been used to identify ischemic tissue prior to irreversible damage. Nevertheless, the concept of PWI/DWI mismatch is oversimplified and the ischemic tissue metabolic status and outcome are often heterogeneous. Tissue pH, a well-regulated physiological index that alters upon disrupted tissue metabolism, may provide a surrogate metabolic imaging marker that augments the DWI and PWI for penumbra imaging.
Methods
pH-weighted MRI was obtained by probing the pH-dependent amide proton transfer between endogenous mobile proteins/peptides and tissue water. The technique was validated using animal stroke models, optimized for human use, and preliminarily tested for imaging healthy volunteers.
Results
pH-weighted MRI is sensitive and specific to ischemic tissue acidosis. pH MRI can be optimized for clinical use, and a pilot human study showed it is feasible at a standard 3 Tesla MRI scanner.
Conclusions
Ischemic acidosis can be imaged via an endogenous pH-weighted MRI technique, which complements conventional PWI and DWI for penumbra imaging. pH-weighted MRI has been optimized and appears feasible and practical in imaging human subjects. Additional study is necessary to elucidate the diagnostic use of pH MRI in stroke patients.
Keywords: Acidosis, Acute Stroke, DWI, PWI, pH
Introduction
The development of diffusion and perfusion MRI has improved our understanding of acute ischemic tissue damage, and is increasingly utilized to help guide stroke treatment 1–3. Specifically, while thrombolytic therapy can restore blood flow and improve patient outcome, the clinic usage of tissue plasminogen activator (tPA) is still limited due to its narrow three-hour (or, in some locations, 4.5 hour) treatment window 4–8. One approach to overcoming this problem is to develop non-invasive penumbra imaging that might identify patients who may potentially benefit from late tPA treatment with minimal adverse effects 9–14. Because ischemic tissue damage is heterogeneous, salvageable ischemic penumbral tissue may be present well beyond the conventional tPA treatment window 15. Perfusion-weighted (PWI) and diffusion-weighted (DWI) MRI are the most well-established imaging techniques being used to detect regions of reduced blood flow and cytotoxic edema, respectively 16, 17. As such, the PWI/DWI mismatch is postulated to represent ischemic tissue that has not yet undergone severe tissue damage, and sometimes used operationally to define the ischemic penumbra 1, 18, 19.
Whereas the PWI/DWI mismatch provides important pathophysiological insight and can be readily identified, ischemic tissue damage is complex and multifactorial, and the PWI/DWI mismatch provides only an approximation of the penumbra 20, 21. It has been observed that the final infarct volume is generally smaller than the initial PWI lesion, yet larger than the acute DWI lesion. In addition, the DWI abnormality is energetically heterogeneous and portions of some DWI lesions may reverse if treated promptly 22–24. Hence, despite being the most practical method at the present, the concept of PWI/DWI mismatch may be somewhat oversimplified. New imaging markers could augment existing penumbral imaging and provide greater insight into disease pathophysiology and perhaps, if validated, serve to help guide treatment decisions such as late thrombolysis.
As energy production is vital for cell viability, monitoring tissue metabolism may offer additional insights about ischemic tissue damage and outcome 10. It has been shown that cerebral oxygen and glucose metabolism are disrupted at blood flow levels higher than those that cause infarction, and therefore, measurement of oxygen and/or glucose metabolism may provide a sensitive index of early ischemia prior to irreversible damage. Specifically, in ischemic tissue lactic acid is produced due to anaerobic glycolysis, causing tissue acidosis (decreased pH) 25. The cellular energy imbalance is exacerbated due to the reduced buffering capacity of bicarbonate at acidic pH, hypoperfusion, and disrupted oxygen and glucose metabolism, hence, tissue pH may fall even further. As a result, essential ATP-dependent functions such as the critical enzyme Na/K-ATPase are compromised, and without prompt treatment, ischemia will eventually lead to cell death and irreversible tissue damage. Therefore, tissue pH imaging may serve as an important physiological biomarker for tissue viability and dysfunction, complementing the conventional hemodynamic and structurally-based MRI. However, historically, non-invasive in vivo pH imaging has been quite challenging. While 31P and lactate magnetic resonance spectroscopy (MRS) may reflect tissue metabolic state and therefore have been actively investigated, their sensitivity and spatiotemporal resolution are not yet adequate for imaging acute stroke 26.
To address this unmet biomedical need, chemical exchange saturation transfer (CEST)-based pH MRI has been recently developed 27, 28. As CEST MRI probes pH via the abundant tissue water signal, its pH sensitivity is significantly higher than that of the conventional MRS-based methods, and remains promising for in vivo use. Amide proton transfer imaging, a specific form of CEST MRI that utilizes the composite amide protons from endogenous mobile proteins and peptides, is particular suitable for in vivo pH imaging 29, 30. Specifically, endogenous amide proton exchange is dominantly base-catalyzed, and its exchange rate and hence, pH MRI signal, decreases at the acidic pH present during ischemia. Our preclinical animal stroke studies have demonstrated that pH MRI deficit detects not only the same tissue as evident on DWI, but also additional hypoperfused tissue with altered oxygen metabolism, strongly suggesting that it may serve as a novel metabolic imaging marker for the ischemic penumbra that does not require injection of an exogenous contrast agent. 31. These observations highlight the potential value of performing pH imaging in acute stroke patients. Toward this goal, we have optimized an in vivo pH-weighted MRI protocol and developed necessary image processing tools, and preliminarily tested the pH-weighted MRI at 3 Tesla 32. Here, we describe this methodology and present pilot human pH imaging.
Materials and Methods
Animal Model
Animal studies were conducted following an institutionally approved protocol, Partners. Adult male Wister rats were anesthetized using the isoflurance regimen (1–1.5% during study, 70% N2O/30% O2), and had standard physiological monitoring throughout the study. Global ischemia was induced in three animals via KCl injection through the femoral artery, and additional four animals were subjected to filament middle cerebral artery occlusion (MCAO).
Healthy Volunteer
All studies were approved by institutional review board, Partners (IRB). A healthy male volunteer 34 year old was scanned at 3 Tesla, and consent forms were obtained prior to study.
MRI
Animals were imaged at Bruker 4.7T. The pH-weighted MRI includes continuous wave (CW) saturation prior to single slice spin echo echo planner imaging (EPI) readout (slice thickness of 2.5 mm). The image matrix was 64 by 64, with an isotropic in-plane resolution of 0.5 mm. The repetition time and echo time were TR/TE=6500ms/32 ms, and 16 signal averages (NA) were obtained. The irradiation RF amplitude was 0.75 μT (~30 Hz) with offset varied serially from −6 to 6 ppm per 0.5 ppm. In addition, standard T1, T2 and diffusion images were acquired. For the global ischemia model, MRI was acquired both before and immediately after cardiac arrest. For the focal ischemia model, point resolved solvent suppressed spectroscopy (PRESS) magnetic resonance spectroscopy (MRS) was also obtained, with two regions of interest (ROIs) of 4 mm3 each positioned in the ipsilateral ischemic lesion and contralateral normal areas (TR/TE=1000ms/144 ms, and NA=1024).
Clinical implementation was done on a 3T Siemens Tim Trio scanner (Siemens, Erlangen Germany), using a 32-channel RF receiver head coil. RF irradiation was composed of a train of π pulses, each with a duration of 20 ms with an interval of 20 ms delay (50% duty cycle), interleaved between single shot EPI readout. We used TR/TE=5000ms/12 ms, NA=4 and the total scan time was 2.5 min. The RF offsets were −3.5, 2, 2.75, 3.5, 4.25 and 5 ppm. In addition, standard field mapping was acquired (TR=100ms, ΔTE=2.46 ms), with a scan time of 12 s. Eight slices were acquired and each slice thickness was 6 mm, distance factor of 25%. The FOV was 192 × 192 mm with image matrix being 64 by 64, for both pH MRI and field map to facilitate co-registration, and zero-filled to 128 by 128. Motion artifact was corrected using the standard motion correction FMRIB’s linear image registration tool (MCFLIRT) 33, 34.
Results
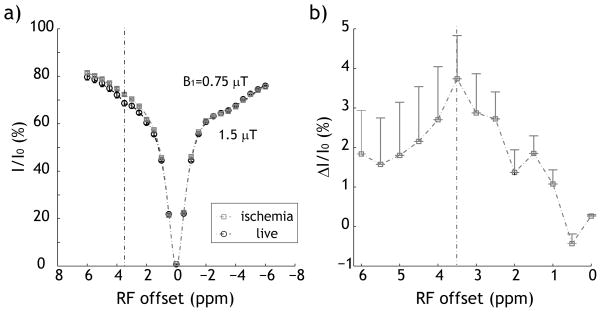
Fig. 1 shows that the pH-weighted MRI measurements changed noticeably upon global ischemia. Specifically, Z-spectrum was obtained by monitoring tissue water signal while the RF irradiation was swept around water resonance. While this technique is similar to magnetization transfer (MT) MRI, it is important to note that its RF irradiation has been optimized for sensitizing proton exchange of endogenous proteins/peptides 30. Notably, the magnetization transfer ratio (MTR) was 68.6 ± 1.1% under normal condition, and increased to 72.4 ± 0.4% postmortem, consistent with the notion that endogenous amide proton exchange is dominantly base-catalyzed around physiological pH (Fig. 1a). The maximal change in Z-spectral intensity was 3.8% peaked at 3.5 ppm, representing the composite amide proton chemical shift (Fig. 1b). This suggested that tissue pH can be assessed via the pH-weighted CEST/APT MRI. In addition, the apparent diffusion coefficient (ADC) decreased from 0.84±0.01 to 0.61±0.02 μm2/ms. In addition, T1 and T2 changed from 1.41±0.03 s and 58.8±0.7 ms to 1.36±0.13 s and 56.3 ± 0.9 ms, respectively, likely attributable to the slightly decreased body temperature and edema during global ischemia.
Fig. 1.
Evaluation of in vivo pH-weighted MRI intensity. Z-spectra were acquired shortly before and immediately after postmortem, which showed signal increase around 3.5 ppm, the composite amide proton chemical shift (Fig. 1a). The difference of Z-spectra clearly showed pH-induced decrease of exchange rate, consistent with the fact the amide proton exchange is dominantly based catalyzed.
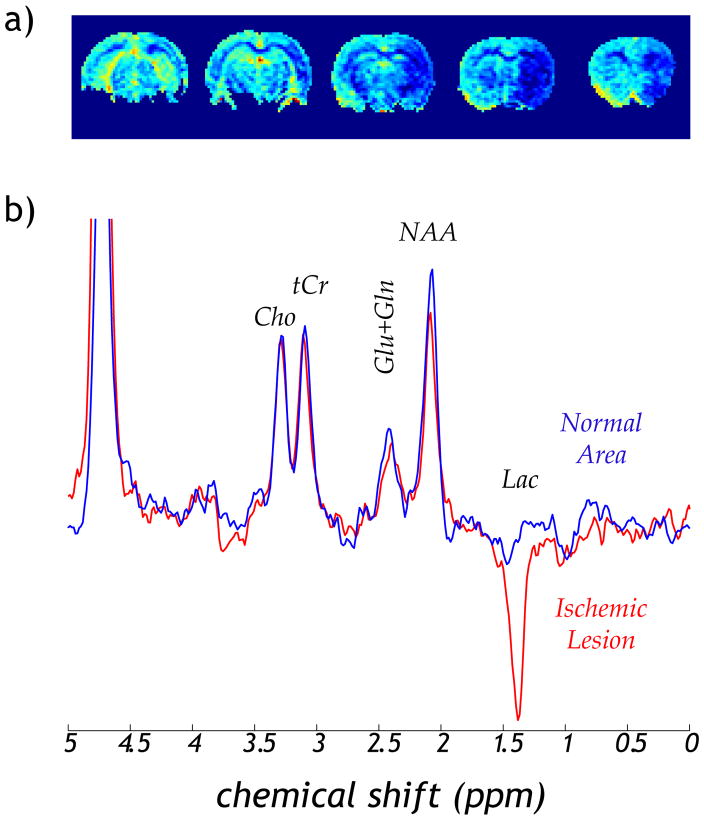
We also preliminarily compared pH-weighted MRI with lactate MRS using the focal ischemia animal model. The pH-weighted MRI was calculated as MTR asymmetry, (i.e., Iref − Ilabel/I0), where Iref and Tlabel are reference and label with RF applied at −3.5 and 3.5 ppm, respectively, and I0 is the scan without RF irradiation. The pH MRI showed a large pH deficit across the hypoperfused right MCA territory (Fig. 2a). In addition, the MRS showed that the lactate level in the ipsilateral stroke lesion was greatly elevated, but lactate was nearly absent in the contralateral normal region, similar as the findings of Jokivarsi et al. 35 (Fig. 2b). Moreover, a subtle NAA decrease was observed, consistent with early neuronal damage during acute ischemia.
Fig. 2.
MRI and MRS characterization of acute focal ischemia. a) pH-weighted MRI showed acute pH deficit in the hypoperfused brain region. b) MRS detected significantly elevated lactate, suggesting ischemic acidosis. In addition, subtle decrease of NAA was observed, an early maker of neuronal damage.
Fig. 3 shows a pilot pH-weighted MRI of a normal volunteer. It is important to point out that the field inhomogeneity was compensated based on the co-registered field map. In addition, pH-weighted image was calculated by taking the difference between the label scan (3.5 ppm) and mean of two reference scans (2 and 5 ppm) instead of the commonly used MTR asymmetry analysis. This alternative method was significantly less susceptible to the intrinsic MTR asymmetry shift. The pH-weighted MRI appeared reasonably homogeneous within the brain, which should facilitate detection of subtle pH lesion in acute stroke patients.
Fig. 3.
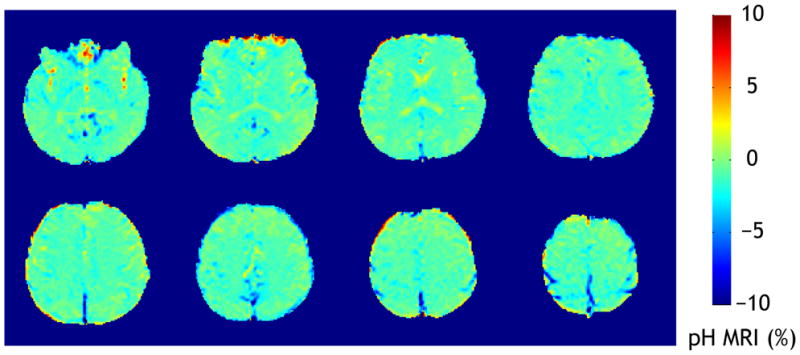
pH-weighted MRI of a normal subject at 3 Tesla. The B0-inhomogeneity compensated pH-weighted MRI appeared reasonably homogeneous within the brain, which should facilitate detection of pH lesion in stroke patients.
Discussion
Our work confirms reports of pre-clinical pH-weighted MRI 31. We extend earlier work by translating this method to the clinical setting, and obtained promising pilot data from a healthy volunteer. Given that pH is well regulated under normal physiological conditions, it may serve as a specific surrogate biomarker for altered tissue metabolism, particularly useful for characterizing heterogeneous ischemic tissue damage. Our preclinical study showed that abnormalities detected on pH-MRI correlate with much of the PWI/DWI mismatch 31. While these pre-clinical studies have been crucial to verify the methodology, considerable effort has been required for clinical translation. Specifically, we have optimized the image acquisition and processing, including optimizing the pulsed-RF irradiation, correcting motion and field inhomogeneity artifacts, and compensating the concomitant intrinsic MTR asymmetry shift 36. The scan time has been significantly reduced so that patients may be imaged with only minimal or no interference with their clinical care. It is important to point out that additional fast multi-slice pH MRI pulse sequence and image processing algorithms are currently being evaluated, which may further facilitate routine clinic use of pH MRI. For instance, tissue pH MRI may serve as an imaging metabolic biomarker for guiding late thrombolytic treatment and evaluating novel therapeutics that aims to improve tissue metabolism 37.
Our study utilized the endogenous amide proton signal and provided only pH-weighted information. However, pH-weighted MRI contrast could change slightly in the presence of changes in relaxation times, such as in the setting of vasogenic edema. While such issues are presumably not a major problem for hyperacute stroke patients, these confounds might be present in the setting of subacute or chronic ischemia. In addition, our current study utilized reference images around the composite amide proton offset instead of the conventional reference scan at −3.5 ppm to minimize concomitant MT asymmetry effect. In summary, our goal is to fully develop pH MRI and evaluate its diagnostic utility in clinic, and ultimately to augment our diagnostic capability of stroke and other debilitating diseases.
Summary
Our study demonstrated a non-invasive endogenous pH-weighted MRI technique in an animal model of cerebral ischemia, and translated it to initial human use. This work confirms that pH-weighted MRI is feasible in the clinic, which may ultimately provide complementary information to the routine PWI and DWI scans. Additional study is needed to test whether pH MRI may augment routine clinical MRI and ultimately help guide stroke treatment.
Acknowledgments
The authors are grateful to Dr. Enfeng Wang for surgical assistance. This study was supported in part by grants from AHA/SDG 0835384N, NIH/NIBIB 1K01EB009771 and NIH/NINDS 1R21NS061119.
Footnotes
Conflicts of Interest/Disclosures
The authors have no conflict of interest.
References
- 1.Warach S. Thrombolysis in stroke beyond three hours: Targeting patients with diffusion and perfusion mri. Ann Neurol. 2002;51:11–13. doi: 10.1002/ana.10109. [DOI] [PubMed] [Google Scholar]
- 2.Davis S, Donnan G, Butcher K, Parsons M. Selection of thrombolytic therapy beyond 3 h using magnetic resonance imaging. Curr Opin Neurol. 2005;18:47–52. doi: 10.1097/00019052-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Parsons MW, Christensen S, McElduff P, Levi CR, Butcher KS, De Silva DA, Ebinger M, Barber PA, Bladin C, Donnan GA, Davis SM. Pretreatment diffusion- and perfusion-mr lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab. 2010 doi: 10.1038/jcbfm.2010.3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-pa stroke study group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 5.Smith MA, Doliszny KM, Shahar E, McGovern PG, Arnett DK, Luepker RV. Delayed hospital arrival for acute stroke: The minnesota stroke survey. Annals of Internal Medicine. 1998;129:190–196. doi: 10.7326/0003-4819-129-3-199808010-00005. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor RE, McGraw P, Edelsohn L. Thrombolytic therapy for acute ischemic stroke: Why the majority of patients remain ineligible for treatment. Annals of Emergency Medicine. 1999;33:9–14. doi: 10.1016/s0196-0644(99)70411-7. [DOI] [PubMed] [Google Scholar]
- 7.Katzan IL, Furlan AJ, Lloyd LE, Frank JI, Harper DL, Hinchey JA, Hammel JP, Qu A, Sila CA. Use of tissue-type plasminogen activator for acute ischemic stroke: The cleveland area experience. JAMA. 2000;283:1151–1158. doi: 10.1001/jama.283.9.1151. [DOI] [PubMed] [Google Scholar]
- 8.Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from tpa therapy?: An analysis of patient eligibility. Neurology. 2001;56:1015–1020. doi: 10.1212/wnl.56.8.1015. [DOI] [PubMed] [Google Scholar]
- 9.Astrup J, Siesjo B, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 10.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 11.Kidwell C, Alger J, Saver J. Evolving paradigms in neuroimaging of the ischemic penumbra. Stroke. 2004;35:2662–2665. doi: 10.1161/01.STR.0000143222.13069.70. [DOI] [PubMed] [Google Scholar]
- 12.Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35:265926–265961. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- 13.Kang D, Chalela J, Dunn W, Warach S. Mri screening before standard tissue plasminogen activator therapy is feasible and safe. Stroke. 2005;36:1939–1943. doi: 10.1161/01.STR.0000177539.72071.f0. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez RG. Imaging-guided acute ischemic stroke therapy: From “Time is brain” To “Physiology is brain”. Am J Neuroradiol. 2006;27:728–735. [PMC free article] [PubMed] [Google Scholar]
- 15.Darby D, Barber P, Gerraty R, Desmond P, Yang Q, Parsons M, Li T, Tress B, Davis S. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion mri. Stroke. 1999;30:2043–2052. doi: 10.1161/01.str.30.10.2043. [DOI] [PubMed] [Google Scholar]
- 16.Hjort N, Butcher K, Davis SM, Kidwell CS, Koroshetz WJ, Rother J, Schellinger PD, Warach S, Ostergaard L on behalf of the UTI. Magnetic resonance imaging criteria for thrombolysis in acute cerebral infarct. Stroke. 2005;36:388–397. doi: 10.1161/01.STR.0000152268.47919.be. [DOI] [PubMed] [Google Scholar]
- 17.Wu O, Christensen S, Hjort N, Dijkhuizen RM, Kucinski T, Fiehler J, Thomalla G, Rother J, Ostergaard L. Characterizing physiological heterogeneity of infarction risk in acute human ischaemic stroke using mri. Brain. 2006;129:2384–2393. doi: 10.1093/brain/awl183. [DOI] [PubMed] [Google Scholar]
- 18.Schlaug G, Benfield A, Baird AE, Siewert B, Lovblad KO, Parker RA, Edelman RR, Warach S. The ischemic penumbra: Operationally defined by diffusion and perfusion mri. Neurology. 1999;53:1528–1537. doi: 10.1212/wnl.53.7.1528. [DOI] [PubMed] [Google Scholar]
- 19.Schaefer PW, Ozsunar Y, He J, Hamberg LM, Hunter GJ, Sorensen AG, Koroshetz WJ, Gonzalez RG. Assessing tissue viability with mr diffusion and perfusion imaging. Am J Neuroradiol. 2003;24:436–443. [PMC free article] [PubMed] [Google Scholar]
- 20.Kidwell C, Alger J, Saver J. Beyond mismatch: Evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- 21.Sobesky J, Weber OZ, Lehnhardt F-G, Hesselmann V, Neveling M, Jacobs A, Heiss W-D. Does the mismatch match the penumbra?: Magnetic resonance imaging and positron emission tomography in early ischemic stroke. Stroke. 2005;36:980–985. doi: 10.1161/01.STR.0000160751.79241.a3. [DOI] [PubMed] [Google Scholar]
- 22.Nicoli F, Lefur Y, Denis B, Ranjeva JP, Confort-Gouny S, Cozzone PJ. Metabolic counterpart of decreased apparent diffusion coefficient during hyperacute ischemic stroke: A brain proton magnetic resonance spectroscopic imaging study. Stroke. 2003;34:82–87. doi: 10.1161/01.STR.0000078659.43423.0A. [DOI] [PubMed] [Google Scholar]
- 23.Guadagno JV, Warburton EA, Jones PS, Day DJ, Aigbirhio FI, Fryer TD, Harding S, Price CJ, Green HA, Barret O, Gillard JH, Baron JC. How affected is oxygen metabolism in dwi lesions?: A combined acute stroke pet-mr study. Neurology. 2006;67:824–829. doi: 10.1212/01.wnl.0000233984.66907.db. [DOI] [PubMed] [Google Scholar]
- 24.Fiehler J, Knudsen K, Kucinski T, Kidwell CS, Alger JR, Thomalla G, Eckert B, Wittkugel O, Weiller C, Zeumer H, Rother J. Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke. 2004;35:514–519. doi: 10.1161/01.STR.0000114873.28023.C2. [DOI] [PubMed] [Google Scholar]
- 25.Siesjo BK. Pathophysiology and treatment of focal cerebral ischemia: Part ii: Mechanisms of damage and treatment. J of Neurosurgery. 1992;77:337–354. doi: 10.3171/jns.1992.77.3.0337. [DOI] [PubMed] [Google Scholar]
- 26.Höhn-Berlage M, Okada Y, Kloiber O, Hossmann KA. Imaging of brain tissue ph and metabolites. A new approach for the validation of volume–selective nmr spectroscopy. NMR Biomed. 1989;2:240–245. doi: 10.1002/nbm.1940020512. [DOI] [PubMed] [Google Scholar]
- 27.Ward KM, Balaban RS. Determination of ph using water protons and chemical exchange dependent saturation transfer (cest) Magn Reson Med. 2000;44:799–802. doi: 10.1002/1522-2594(200011)44:5<799::aid-mrm18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Sun PZ, Sorensen AG. Imaging ph using the chemical exchange saturation transfer (cest) mri: Correction of concomitant rf irradiation effects to quantify cest mri for chemical exchange rate and ph. Magn Reson Med. 2008;60:390–397. doi: 10.1002/mrm.21653. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Payen J, Wilson DA, Traystman RJ, van Zijl PCM. Using the amide proton signals of intracellular proteins and peptides to detect ph effects in mri. Nature Med. 2003;9:1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 30.Sun PZ, Zhou J, Huang J, van Zijl P. Simplified quantitative description of amide proton transfer (apt) imaging during acute ischemia. Magn Reson Med. 2007;57:405–410. doi: 10.1002/mrm.21151. [DOI] [PubMed] [Google Scholar]
- 31.Sun PZ, Zhou J, Sun W, Huang J, van Zijl PCM. Detection of the ischemic penumbra using ph-weighted mri. J Cereb Blood Flow Metab. 2007;27:1129–1136. doi: 10.1038/sj.jcbfm.9600424. [DOI] [PubMed] [Google Scholar]
- 32.Sun PZ, Benner T, Kumar A, Sorensen AG. An investigation of optimizing and translating ph-sensitive pulsed-chemical exchange saturation transfer (cest) imaging to a 3 T clinical scanner. Magn Reson Med. 2008;60:834–841. doi: 10.1002/mrm.21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 34.Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in fsl. NeuroImage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 35.Jokivarsi KT, Gröhn HI, Gröhn OH, Kauppinen RA. Proton transfer ratio, lactate, and intracellular ph in acute cerebral ischemia. Magn Reson Med. 2007;57:647–653. doi: 10.1002/mrm.21181. [DOI] [PubMed] [Google Scholar]
- 36.Sun PZ, Farrar CT, Sorensen AG. Correction for artifacts induced by b0 and b1 field inhomogeneities in ph–sensitive chemical exchange saturation transfer (cest) imaging. Magn Reson Med. 2007;58:1207–1215. doi: 10.1002/mrm.21398. [DOI] [PubMed] [Google Scholar]
- 37.Xiong Z, Zhu X, Chu X, Minami M, Hey J, Wei W, MacDonald J, Wemmie J, Price M, Welsh M. Neuroprotection in ischemia: Blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]