Abstract
Background
Approximately 3.2 million people in the United States have chronic hepatitis C virus (HCV) infection; the primary cause for adult liver transplantation and a significant burden on healthcare resources. The role of HCV and other risk factors in development of HCC in patients with chronic kidney disease is not well defined.
Aim
To identify specific predictors of hepatocellular carcinoma (HCC) in dialysis patients with chronic HCV.
Methods
This study assessed factors associated with the development of HCC in dialysis patients with chronic HCV. Data were extracted from the United States Renal Database System (USRDS) using ICD-9 codes. Variables included were gender, race, duration on dialysis, and comorbidities (alcohol abuse, drug abuse, HIV, hepatitis B, diabetes and/or presence of cirrhosis).
Results
Among the 32,806 HCV infected subjects, 262 cases had HCC. HCC was 12 times more likely in subjects with cirrhosis (p<.001), 3 times more likely in subjects with alcohol abuse (p<.001), and 1.3 times more likely in subjects with diabetes (p=.04). Asians were 3 times more likely (p<.001) to have HCC. Females were less likely to have HCC compared to males (p=.002). The likelihood of having HCC increased with age (p=.001).
Implications
This population-based study demonstrates that among subjects with HCV on dialysis, those with cirrhosis, Asian race and history of alcohol abuse are at highest risk for development of HCC. Furthermore, these findings indicate links between HCV and HCC which are valuable in case management for identifying; monitoring, and managing dialysis patients with HCC.
Keywords: hepatitis C, hepatocellular carcinoma, dialysis, co-morbidity, end stage renal disease
Introduction
Approximately 1.3 percent of the United States (U.S.) population is estimated to be chronically infected by the hepatitis C virus (HCV) (1). HCV accounts for 60–70 percent of the chronic liver disease and about 1–5 percent of deaths related to chronic liver disease (2). In addition, chronic HCV infection is a major cause of hepatocellular carcinoma (HCC), and accounts for approximately one-third of the cases in the U.S. (3).
The prevalence of HCV is higher in dialysis patients compared to both patients with renal disease not requiring dialysis and the general population (4–6). The rate ranges from 2.4% to 22.9% with a mean of 13.5% (7). Compared to the HCV negative dialysis population, hemodialysis patients with chronic HCV have an increased risk of death from liver disease due to the development of cirrhosis and complications of portal hypertension (8–10). Whether hemodialysis patients are at increased risk for development of HCC is unclear. It is common for HCV infected dialysis patients to have multiple co-morbidities (11) which may be associated with the development of HCC, such as coinfection with hepatitis B virus, diabetes, and cirrhosis.
The purpose of this study was to determine the prevalence and predictive clinical and demographic factors related to HCC in a cohort of End Stage Renal Disease (ESRD) with HCV. The identified predictive factors may provide a guide for clinical practice in identifying patients who are at higher risk of developing HCC. Thereby, appropriate screening and evaluation may be instituted earlier and lead to a reduction in morbidity and mortality associated with HCV-related HCC.
Materials and Methods
The study used data from the United States Renal Data System (USRDS) in conjunction with International Classification of Disease version 9 (ICD-9) codes from Medicare/Medicaid billing data from 1997 to 2003. The USRDS description and 2008 Annual Data report are available at http://www.usrds.org (12). ICD-9 codes have been used in a number of clinical studies (13, 14) including studies using the USRDS data (11, 15). The ICD-9 codes in HCV infected patients has been previously validated in a number of studies (13, 16–19). Additionally, ICD-9 codes for presence of HCV have been shown to have positive and negative predictive values of greater than 90% (19). The Veterans Aging Cohort Study indicated a 78% (kappa 0.42) agreement between the ICD-9 codes and diagnosis of HCV(13). Inclusion criteria for HCV infection were based on presence of at least one inpatient or two outpatient codes for HCV. HCC and other co-morbidities were identified similarly using at least one inpatient or two outpatient ICD-9 codes. Two groups with HCV were then analyzed, those with and without HCC. We analyzed factors predicting HCC, including demographic variables (race, sex, age, and duration on dialysis) and associated co-morbidities, including HBV, diabetes, cirrhosis, alcohol abuse, drug abuse, and co-infection with human immunodeficiency virus (HIV).
The study was a collaborative project with investigators from the University of Pittsburgh, the National Institute of Nursing Research, and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the National Institutes of Health. The study was approved by the Institutional Review Board (IRB) at the University of Pittsburgh, the USRDS sponsors from NIDDK, and the Office of Human Subjects Research at the NIH.
De-identified data were collected, extracted, and organized in SPSS version 15.0 (SPSS Inc, Chicago, Illinois). Descriptive statistical analyses included means, standard deviations, frequencies, and ranges of data. Race, sex and presence of co-morbidity were treated as categorical variables. Age and duration on dialysis were continuous variables. Independent sample t test was used to compare demographic variables. Binary logistic regression was used to analyze the co-morbid factors in subjects with HCV that were associated with HCC. Comorbidities were analyzed after statistically adjusting for the demographic variables (sex, race, age, and duration on dialysis). A value of p≤.05 was set a priori for level of statistical significance.
Results
We identified 32,808 subjects with a diagnosis of HCV on dialysis from 1997 to 2003. The cohort was predominantly male, 58.6%, middle aged, (mean age 56.6 years SD=15.3, range 4.0 – 95.4 years) and African American, 47.1%, (Table 1). Thirty-seven percent were co-infected with HBV, 8.7% were co-infected with HIV, 33.0% had diabetes, 11.5% cirrhosis, 9.5% alcohol abuse, and 8.9% a history of drug abuse. The mean duration on dialysis was 6.1 years (SD=3.8, range 1.3 – 33.3 years). Overall, males were significantly more likely to be co-infected with HBV, have alcohol abuse, or drug abuse (all with p<.001).
Table 1.
Sample characteristics for HCV and HCV with HCC groups
| Variable | Group (All subjects were HCV positive) | |||
|---|---|---|---|---|
| Overall (N=32,806) | No HCC (n=32,544) | HCC (n=262) | Statistic | |
| n (%) | n (%) | n (%) | p value (χ2/t-test) | |
| Sex | ||||
| Male | 19,212 (58.6) | 19,038(58.5) | 174(66.4) | 0.01 |
| Female | 13,594 (41.4) | 13,506(41.5) | 88(33.6) | |
| Race | ||||
| Caucasian | 15,368 (46.8) | 15,255(46.9) | 113(43.1) | |
| Asian | 939 (2.9) | 919(2.8) | 20(7.6) | |
| African American | 15,449 (47.1) | 15,330(47.1) | 119(45.4) | <0.001 |
| Native American | 516 (1.6) | 511(1.6) | 5(1.9) | |
| Other | 534 (1.6) | 529(1.6) | 5(1.9) | |
| Age (M±SD) (yrs) | 56.6 ± 15.3 | 56.6 ± 15.3 | 59.6 ± 12.4 | 0.001 |
| Range (4.0–95.4) | (4.0–95.4) | (17.4–84.7) | ||
| Duration on | 6.1 ± 3.8 | 6.1 ± 3.8 | 5.6 ± 3.5 | 0.04 |
| dialysis (M±SD) (yrs) | Range (1.3–33.3) | (1.3–31.6) | (1.3–33.3) | |
| Co-morbidity | ||||
| Hepatitis B | 12,142 (37.0) | 12,077 (37.1) | 65 (24.8) | <0.001 |
| Diabetes | 10,816 (33.0) | 10,714 (32.9) | 102 (38.9) | 0.04 |
| Cirrhosis | 3,765 (11.5) | 3,609 (11.1) | 156 (59.5) | <0.001 |
| Alcohol Abuse | 3,105 (9.5) | 3,039 (9.3) | 66 (25.2) | <0.001 |
| Drug Abuse | 2,919 (8.9) | 2,910 (8.9) | 9 (3.4) | 0.003 |
| HIV | 2,846 (8.7) | 2,843 (8.7) | 12 (4.6) | 0.02 |
Two hundred and sixty two subjects met the study definition for HCC. The incidence of HCC in the sample on dialysis during the study period was 0.8%. Among subjects with HCC, 66.4% were male, 45.4 % were African American, 43.1% Caucasian, 7.6% Asian, 1.9 % Native American and 1.9 % other race. The mean age in the group with HCC was 59.6 years (SD=12.4, range 17.4–84.7 years). The mean duration on dialysis was 5.6 years (SD=3.5, range 1.3–33.3 years). Compared to those without HCC, subjects with HCC were more likely to be male, Asian, and older (59.6± 12.4 years vs. 56.6±15.3 years) (Table 1). Subjects with HCC were more likely to have diabetes, cirrhosis and alcohol abuse (all with p<.05, Table 1).
Subjects with cirrhosis were almost 12 times more likely to have HCC (p<.001) [Table 2]. Similarly, subjects with alcohol abuse were 3 times (p<.001) more likely and those with diabetes were 1.3 times (p=.04) more likely to have HCC. Univariate analysis revealed that HCV-infected patients on dialysis with HCC were less likely to abuse drugs (p=.003), or be co-infected with HIV (p=.02) or HBV (p=.05) (Table 2).
Table 2.
Demographic and co-morbid predictors of HCC in HCV infected subjects on dialysis using univariate analysis
| p value | Odds Ratio | 95% C.I. for Exp(B) | ||
|---|---|---|---|---|
| Lower | Upper | |||
| ¥Race (overall) | <0.001 | |||
| Asian | <0.001 | 3.1 | 1.92 | 5.01 |
| African American | 0.14 | 1.23 | 0.94 | 1.61 |
| Native American | 0.36 | 1.53 | 0.62 | 3.78 |
| Other | 0.47 | 1.39 | 0.57 | 3.43 |
| ΦSex | 0.002 | 0.66 | 0.51 | 0.86 |
| Age | 0.001 | 1.02 | 1.01 | 1.02 |
| Duration on dialysis | 0.2 | 0.98 | 0.94 | 1.01 |
| Co-morbidities | ||||
| Hepatitis B | <0.001 | 0.56 | 0.42 | 0.74 |
| Diabetes | 0.04 | 1.3 | 1.01 | 1.67 |
| Cirrhosis | <0.001 | 11.8 | 9.2 | 15.14 |
| Alcohol Abuse | <0.001 | 3.27 | 2.47 | 4.33 |
| Drug Abuse | 0.003 | 0.36 | 0.19 | 0.71 |
| HIV | 0.02 | 0.5 | 0.28 | 0.9 |
Caucasians were the reference group.
Males were the reference group.
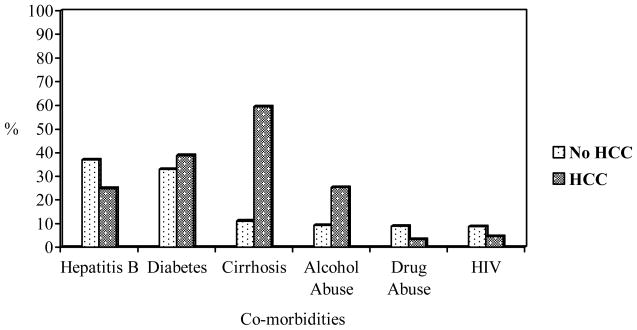
Approximately 1.3 percent of the variability in HCC was explained by the demographic variables (Nagelkerke R2 =.013). Asians were 3 times more likely (p<.001) to have HCC as compared to Caucasians. Among persons with HCC, co-infection with HBV was three times more prevalent in Asians than in other races (χ2(1) = 1.22, p =0.01). Females were less likely to have HCC compared to males (p=.002). The likelihood of HCC increased by 1.25 times for each year increase in age (p=.001). There was a significant increase in prediction of HCC by certain co-morbidities after adjusting for the demographic variables (sex, race, age, and duration on dialysis) using bivariate logistic regression model (χ2(6) = 387.0, p <0.001). Specifically, cirrhosis (p<.001), HBV (p=.05) and drug abuse (p=.002) were found to be significant after adjusting for demographic variables in the statistical model used (Table 3). Almost fifteen percent of the variability in HCC was explained by the co-morbidities in this model (Nagelkerke R2 =.145). The correlational matrix revealed a significant correlation between cirrhosis and alcohol abuse (r=.35, p<.001) [Table 4]. A comparison of percentages of co-morbid conditions between those with and without HCC is shown in Figure 1. Twenty-three of the 262 patients with HCC (9%) died during the study period. The median duration on dialysis of those who died was 9.5 years (range- 2–33 years).
Table 3.
Co-morbid predictors of HCC in HCV infected subjects on dialysis using bivariate logistic model adjusted for race, sex, age, and duration on dialysis
| Co-morbidities | p value | Odds Ratio | 95% C.I. for Exp(B) | |
|---|---|---|---|---|
| Lower | Upper | |||
| Hepatitis B | 0.05 | 0.74 | 0.56 | 0.99 |
| Diabetes | 0.24 | 1.17 | 0.9 | 1.52 |
| Cirrhosis | <0.001 | 11.74 | 8.89 | 15.5 |
| Alcohol Abuse | 0.25 | 1.21 | 0.88 | 1.66 |
| Drug Abuse | 0.002 | 0.34 | 0.17 | 0.67 |
| HIV | 0.34 | 0.75 | 0.41 | 1.36 |
Table 4.
Correlations among co-morbidities in the group with HCC (n=262)
| Correlation (p value) | Hepatitis B | Diabetes | Cirrhosis | Alcohol Abuse | Drug Abuse | HIV |
|---|---|---|---|---|---|---|
| Hepatitis B | - | |||||
| Diabetes | −0.04 (0.5) | - | ||||
| Cirrhosis | −0.07 (0.28) | 0.18 (0.004) ** | - | |||
| Alcohol Abuse | −0.05 (0.44) | 0.13 (0.03) * | 0.35 (<0.001) ** | - | ||
| Drug Abuse | −0.06 (0.34) | −0.02 (0.73) | −0.06 (0.35) | 0.13 (0.03) * | - | |
| HIV | 0.001 (0.99) | −0.14 (0.03) * | −0.04 (0.49) | −0.04 (0.49) | 0.16 (0.01) ** | - |
p≤0.05;
p≤0.01
Figure 1.
Bar graph showing comparison of percentages of individual co-morbidities among groups with and without HCC
Discussion
In our national cohort of 32,808 HCV infected patients on dialysis, 262 were diagnosed with HCC. We found that Asians were three times more likely to have HCC compared to Caucasians. These findings concur with the those of Cheng and colleagues, stressing the growing importance of chronic HCV infection in the Asian American population (20). Studies have reported that Asian Americans have a 4-fold higher risk of liver cancer (21, 22). As the prevalence of HCV in Asian populations increases (23) special attention needs to be provided to this group.
The relationship between cirrhosis and HCC, as identified from previous studies, was reconfirmed (24). This study demonstrated that patients with cirrhosis were twelve times more likely to have HCC. Cirrhosis increases the risk for development of HCC likely due to increased hepatocyte turnover and increased risk for mutations. The issue remains as to whether earlier monitoring or treatment in this specific cohort may prevent complications of the cirrhosis. This study like other studies found that males are at a higher risk of having HCC (24). Higher rates of HCV/HBV co-infection, alcohol abuse, and drug abuse in males, may be co-factors that put them at higher risk.
Our findings are important in the context of increasing numbers of patients who are on dialysis with HCV and potentially multiple co-morbidities. All co-morbidities in the analysis had significant prediction to presence of HCC. Diabetes and alcohol abuse were factors that increased the risk of having HCC. The presence of cirrhosis, alcohol abuse and diabetes may have a negative impact on HCC related patient morbidity and mortality. Hence, strategies such as cessation of alcohol, consideration of anti-viral therapy, and diabetes prevention measures may reduce the additive risk toward developing HCC.
Subjects with HBV, HIV, and drug abuse despite significant univariate predictions were less likely to have HCC in the logistic model. Previous studies demonstrated that these three factors had an additive independent effect toward the development of HCC. After adjustment for covariates (sex, race, age, and duration on dialysis), HBV, cirrhosis, and drug abuse were factors that predicted the presence of HCC. However, the lower association between HBV and HCC remains unclear. Possible explanations for the lower association in HBV patients may be due to attrition for the period of time in which the data were collected, in that patients with these conditions die of liver failure prior to development of HCC. Another possible explanation may include inaccurate ICD-9 coding of active versus resolved chronic HBV infection by health care practitioners. In our cohort, patients with HBV were significantly less likely to have alcohol abuse. Therefore, abstinence from alcohol may be protective in this cohort (25). The role of HBV and development of HCC in HCV positive patients on dialysis should be examined in future studies.
Strengths of this study included a large and diverse sample that was representative of the national population on dialysis with HCV with respect to gender, ethnicity, and geographic location. The study involved a collaborative team that economically utilized a large public domain data set for hypothesis testing. The USRDS is a uniform source of clinical data that may lay the foundation for future studies.
There are limitations to this study. The study design model used was secondary in nature and therefore, to properly assess causal relationships a prospective longitudinal study would be optimal. The use of retrospective ICD-9 codes for diagnosis of HCV, HCC, and other comorbidities is a drawback as coding is dictated by the health care provider. Therefore, the absence of a particular ICD-9 coding does not necessarily exclude the presence of HCV, HCC, or other co-morbidities. The analysis of additional variables such as geographic location, treatment history, HCV genotype or RNA, viral load and further co-morbidities are considerations to be made in future studies. The findings from this study are specific to the HCV infected hemodialysis subgroup of patients and may not necessarily be generalized to the overall population of patients with HCV. It may be difficult to generalize these findings to other non-U.S. patient populations as U.S. patients on dialysis are eligible for disability and healthcare resources that may be unavailable elsewhere.
In the last twenty years there has been a significant increase in the incidence of HCC related to HCV in the general population (26). The disease has a long natural history, which may be significantly modified by co-morbidities (27) as seen in this study. The course of disease in dialysis patients versus those with normal kidney functions remains controversial as certain studies suggest even though incidence of HCV in patients on chronic hemodialysis is higher than the rest of the population, HCC is less frequently reported in the hemodialysis setting (24). Conversely, other studies suggest that HCV infected individuals have a lower survival rate which may be due to HCC and cirrhosis related mortality (9, 28). These contrasting results may be due to differences in demographic and clinical factors among different populations with HCV and may be important in predicting progression to HCC in patients with chronic HCV infection. Factors associated with HCC in HCV infected persons on dialysis are not well described. Therefore, these findings are important as they describe demographic and co-morbidity differences that exist in this large population-based study. Hemodialysis with multiple comorbidities such as diabetes, alcoholism, and cirrhosis along with being of Asian or African American descent may be factors that yield negative outcomes. This also suggests that persons of Asian race on dialysis should be screened earlier and/or more frequently for HCC. Additionally, patients should be counseled about additive risk of alcohol abuse toward the progression of HCC. The role of HBV, drug abuse, and HIV in this subgroup of patients still remains unclear. Thus clinicians should carefully monitor for the presence of the aforementioned risk factors in HCV patients to recognize paramount risk and provide early and preventive interventions.
Acknowledgments
The data reported here have been supplied by the United States Renal Data System (USRDS) http://www.usrds.org. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
Support was provided by the Symptoms Management Branch, National Institute of Nursing Research, Intramural Research Program, National Institutes of Health, Department of Health and Human Services. Additional support was provided by the following: Clinical & Translational Science Institute Fellowship (Henderson): NIH, 1TL1 RR024155-01(Reis), NIH/NIDA, DA016175-01A1, (Butt); University of Pittsburgh School of Nursing, Educational Innovation Fund; Duquesne University School of Nursing, Dean’s Fund (Henderson). Special thanks to Drs. Thelma Patrick and Mary Ann Thurkettle for their assistance with the pilot phase.
Abbreviations
- HCV
hepatitis C virus
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
- ESRD
end-stage renal disease
- USRDS
United States Renal Database
- ICD-9
International Classification of Disease version 9
- NINR
National Institute of Nursing Research
- IRB
Institutional Review Board
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- HIV
human immunodeficiency virus
- SD
standard deviation
- NIAID
National Institute of Allergy and Infectious Diseases
Footnotes
Approvals
The protocol was approved by the Office of Human Subjects at the National Institutes of Health, Department of Health and Human Services #3917. The protocol was also approved by the University of Pittsburgh Institutional Review Board approval #0403077.
References
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006 May 16;144(10):705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Infectious Diseases, Centers for Disease Control and Prevention. Hepatitis C virus infection in the United States. Available at: http://www.cdc.gov/ncidod/diseases/hepatitis/c/plan/HCV_infection.htm.
- 3.El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36(5B):s74–s83. doi: 10.1053/jhep.2002.36807. [DOI] [PubMed] [Google Scholar]
- 4.US Renal Data System. USRDS 2002 Annual Data Report. The National Institutes of Health, National Institute of Diabetes and Digestive Diseases; Bethesda, MD: 2002. p. 564. [Google Scholar]
- 5.Fabrizi F, Martin P, Dixit V, Brezina M, Cole MJ, Gerosa S, et al. Quantitative assessment of HCV load in chronic hemodialysis patients: A cross-sectional survey. Nephron. 1998 Dec;80(4):428–33. doi: 10.1159/000045215. [DOI] [PubMed] [Google Scholar]
- 6.Niu MT, Coleman PJ, Alter MJ. Multicenter study of hepatitis C virus infection in chronic hemodialysis patients and hemodialysis center staff members. Am J Kidney Dis. 1993 Oct;22(4):568–73. doi: 10.1016/s0272-6386(12)80930-9. [DOI] [PubMed] [Google Scholar]
- 7.Tokars JI, Alter MJ, Miller E, Moyer LA, Favero MS. National surveillance of dialysis associated diseases in the United States--1994. ASAIO J. 1997 Jan–Feb;43(1):108–19. [PubMed] [Google Scholar]
- 8.Espinosa M, Martin-Malo A, Alvarez de Lara MA, Aljama P. Risk of death and liver cirrhosis in anti-HCV-positive long-term haemodialysis patients. Nephrol Dial Transplant. 2001 Aug;16(8):1669–74. doi: 10.1093/ndt/16.8.1669. [DOI] [PubMed] [Google Scholar]
- 9.Fabrizi F, Takkouche B, Lunghi G, Dixit V, Messa P, Martin P. The impact of hepatitis C virus infection on survival in dialysis patients: Meta-analysis of observational studies. J Viral Hepat. 2007 Oct;14(10):697–703. doi: 10.1111/j.1365-2893.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- 10.Stehman-Breen CO, Emerson S, Gretch D, Johnson RJ. Risk of death among chronic dialysis patients infected with hepatitis C virus. Am J Kidney Dis. 1998 Oct;32(4):629–34. doi: 10.1016/s0272-6386(98)70027-7. [DOI] [PubMed] [Google Scholar]
- 11.Butt AA, Evans R, Skanderson M, Shakil AO. Comorbid medical and psychiatric conditions and substance abuse in HCV infected persons on dialysis. J Hepatology. 2006;44:864–8. doi: 10.1016/j.jhep.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 12.The United States Renal Database System. [Accessed September 4, 2008.];USRDS 2008 Annual Data Report. Available at: http://www.usrds.org.
- 13.Butt AA, Fultz SL, Kwoh CK, Kelley D, Skanderson M, Justice AC. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology. 2004 Jul;40(1):115–9. doi: 10.1002/hep.20289. [DOI] [PubMed] [Google Scholar]
- 14.Giordano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El-Serag H, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007 May 9;297(18):2010–7. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- 15.Butt AA, Khan UA, Skanderson M. Predictors of mortality in HCV and HCV-HIV co-infected persons on dialysis. Journal of Clinical Gastroenterology. 2008 doi: 10.1097/mcg.0b013e3181574d58. [DOI] [PubMed] [Google Scholar]
- 16.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003 Feb 20;348(8):702–10. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 17.Giordano TP, Kramer JR, Souchek J, Richardson P, El-Serag HB. Cirrhosis and hepatocellular carcinoma in HIV-infected veterans with and without the hepatitis C virus: A cohort study, 1992–2001. Arch Intern Med. 2004 Nov 22;164(21):2349–54. doi: 10.1001/archinte.164.21.2349. [DOI] [PubMed] [Google Scholar]
- 18.Kramer JR, Giordano TP, Souchek J, El-Serag HB. Hepatitis C coinfection increases the risk of fulminant hepatic failure in patients with HIV in the HAART era. J Hepatol. 2005 Mar;42(3):309–14. doi: 10.1016/j.jhep.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005 Jan;100(1):56–63. doi: 10.1111/j.1572-0241.2005.40670.x. [DOI] [PubMed] [Google Scholar]
- 20.Cheng JT, Hsien C, Sun HE, Tong MJ. The emerging importance of chronic hepatitis C infection in Asian Americans. Am J Gastroenterol. 2006 Dec;101(12):2737–43. doi: 10.1111/j.1572-0241.2006.00831.x. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen MH, Keeffe EB. Chronic hepatitis B and hepatitis C in Asian Americans. Rev Gastroenterol Disord. 2003 Summer;3(3):125–34. [PubMed] [Google Scholar]
- 22.Nguyen MH, Whittemore AS, Garcia RT, Tawfeek SA, Ning J, Lam S, et al. Role of ethnicity in risk for hepatocellular carcinoma in patients with chronic hepatitis C and cirrhosis. Clin Gastroenterol Hepatol. 2004 Sep;2(9):820–4. doi: 10.1016/s1542-3565(04)00353-2. [DOI] [PubMed] [Google Scholar]
- 23.Barnes JB, Bennett CE. Census 2000 brief. U.S. Census Bureau; 2002. The Asian population:2000. [Google Scholar]
- 24.Ishida H, Agishi T, Koyama I, Sawada T, Murakami T, Utsumi K, et al. Hemodialysis paradox: Survey on the incidence rate of hepatocellular carcinoma in antihepatitis virus C-antibody-positive chronic hemodialysis patients. Artif Organs. 2001 Jan;25(1):58–60. doi: 10.1046/j.1525-1594.2001.025001058.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang HI, Yeh SH, Chen PJ, Iloeje UH, Jen CL, Su J, et al. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst. 2008 Aug 20;100(16):1134–43. doi: 10.1093/jnci/djn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: An update. Ann Intern Med. 2003 November 18;139(10):817–23. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 27.Kiyosawa K, Sodeyama T, Tanaka E, Gibo Y, Yoshizawa K, Nakano Y, et al. Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: Analysis by detection of antibody to hepatitis C virus. Hepatology. 1990 Oct;12(4 Pt 1):671–5. doi: 10.1002/hep.1840120409. [DOI] [PubMed] [Google Scholar]
- 28.Butt AA, Skanderson M, McGinnis KA, Ahuja T, Bryce CL, Barnato AE, et al. Impact of hepatitis C virus infection and other comorbidities on survival in patients on dialysis. J Viral Hepat. 2007 Oct;14(10):688–96. doi: 10.1111/j.1365-2893.2007.00853.x. [DOI] [PubMed] [Google Scholar]