Abstract
Purpose
It is unknown whether there are survival disparities between men and women with squamous cell carcinoma of the head and neck (SCCHN), though some data suggest that men have worse outcomes. We conducted a matched-pair study that controlled for several potentially confounding prognostic variables to assess whether a survival advantage exists for female compared with male SCCHN patients receiving similar care.
Experimental Design
We selected 286 female patients and 286 matched male patients from within a prospective epidemiologic study of 1654 patients with incident SCCHN evaluated and treated at a single large multidisciplinary cancer center. Matching variables included age (± 10 years), race/ethnicity, smoking status (never versus ever), tumor site (oral cavity versus oropharynx versus larynx versus hypopharynx), tumor classification (T1–2 versus T3–4), nodal status (negative versus positive), and treatment (surgery, radiation therapy, surgery and radiation therapy, surgery and chemotherapy, chemoradiotherapy, or surgery and chemoradiotherapy).
Results
Matched-pair and log-rank analyses showed no significant differences between women and men in recurrence-free, disease-specific, or overall survival. When the analysis was restricted to individual sites (oral cavity, oropharynx, or larynx/hypopharynx), there was also no evidence of a disparity in survival associated with sex.
Conclusions
We conclude that there is no evidence to suggest that a survival advantage exists for women as compared to men with SCCHN receiving similar multidisciplinary directed care at a tertiary cancer center.
Keywords: Survival disparity, Head and neck cancer, Gender-related disparity, Matched pair study, Gender-related prognosis
Introduction
The incidence, diagnosis, treatment, and outcomes of many diseases are influenced by patient sex, and cancer is no exception. For example, smoking cessation rates are lower among female smokers than male smokers, and the risks of lung cancer associated with smoking appears to be three times greater for women than for men (1). Additionally, there may be more barriers to receiving cancer screening for women than for men (2). At the same time, women are more likely than men to have a specific source of ongoing medical care, potentially enhancing their preventive care4. Among patients with non-small cell lung cancer, response rates to neoadjuvant chemotherapy and survival rates may be greater in women than men (3). On the other hand, rates of post-operative complications from gastric cancer surgery have been reported to be higher among women than among men (4). Differences in social support between men and women may influence cancer-related outcomes. For example, in a group of patients with serious medical conditions such as cancer, the overwhelming majority of divorces occurred when the female spouse rather than the male spouse was suffering from the disease (5). These varied examples illustrate the potential for sex-based disparities in all aspects of the cancer experience.
Only a few studies have been published on the impact of sex on head and neck cancer risk and outcomes. The risk of head and neck cancer associated with smoking appears to be higher in women than in men (6, 7), but the impact of patient sex on head and neck cancer outcomes remains unclear. In a recent analysis of over 20,000 head and neck cancer patients in Florida, the median survival time was 41 months for women and 36 months for men; however, after multivariate adjustment, patient sex was no longer associated with poor outcome (8). Other population-based studies have similarly reported higher mortality rates for men than for women with head and neck cancer (9, 10). In a recent study, Garavello et al matched 71 women with oral tongue cancer to 142 men with oral tongue cancer on age, year of diagnosis, and TNM stage, and while the women had lower rates of recurrence (46% women, 55% men) and cancer-related death (32% women, 39% men), these differences were not statistically significant (11). Franco et al reported a survival advantage for women compared with men with oral cavity cancer (excluding tongue cancers) at a single Brazilian cancer center, with women having a 17% lower risk of recurrence and a 29% lower risk of cancer death (12). Kokoska et al, in contrast, showed no significant influence of patient sex on laryngeal cancer outcome (13). Ildstad et al found improved overall survival for women compared to men with cancers of the oral cavity, oropharynx, and hypopharynx at a single U.S. center; however, when the two groups were matched by stage, the survival advantage did not remain (14). Thus, although women appear to have a higher risk for head and neck cancer from smoking, extant data support that men with head and neck cancer have higher recurrence and mortality rates. However, prior studies have been limited by the use of population-based databases with significant inherent confounding or the use of locally available samples of very limited size.
We performed a matched-pair analysis among patients with squamous cell carcinoma of the head and neck (SCCHN) evaluated and treated at a large multidisciplinary cancer center to determine if there was a survival advantage associated with female sex. Our study differed from previous studies addressing this question in that we employed much more stringent matching criteria, ensured a large sample size, and limited the study to patients treated at a single facility. Thus, this study has attempted to address the shortcomings of the existing literature with respect to confounding and study power.
Materials and Methods
Patient Population
Patients with incident (newly diagnosed, previously untreated) pathologically confirmed SCCHN were entered into a prospective epidemiologic study at The University of Texas M. D. Anderson Cancer Center between May 1995 and June 2008. The study was approved by the Institutional Review Board and patients gave informed consent. Patients with cancers of the salivary glands, nasopharynx, lip, or unknown primary site, and patients initially treated elsewhere were excluded. All subjects were U. S. residents.
All participants completed a standardized epidemiologic questionnaire prospectively at enrollment. Data collected from this questionnaire for the current analysis included date of birth, race/ethnicity, smoking status, alcohol drinking status, marital status, education level, and household income. Patients classified their racial/ethnic background as “White, Anglo, Caucasian”; “Spanish origin (Hispanic)”; “Black (African American)”; “American Indian”; “Asian”; or “other”. Former smokers were defined as smokers who had quit smoking at least 1 year before presentation, and former smokers were grouped with current smokers as “ever smokers”. “Never smokers” were defined as individuals who had smoked fewer than 100 cigarettes in their lifetime. “Current Drinkers” were defined as individuals who had at least one alcoholic drink per week for at least 1 year and who were still drinking in this manner at the time of presentation, while “former drinkers” were defined as those who drunk alcoholic beverages in this manner in the past, but had begun drinking less or stopped drinking at least 1 year before presentation. “Never drinkers” were defined as individuals who never drank or whose drinking had never reached the level of one drink per week for at least 1 year.
Medical records were reviewed for primary tumor site and subsite; clinical stage; treatment; grade; recurrence-free, disease-specific and overall survival as assessed between the initial and final patient contact recorded; and medical comorbidites. Medical record review for follow-up status was performed by otolaryngology-head and neck surgery resident, after training by the senior author, staff head and neck surgeon. Any and all questions which arose in review of the medical record were reviewed personally by the senior author and the treating clinicians were contacted for clarification whenever necessary. Medical record reviews could not be blinded to the sex of the patient, but the matching process was performed blinded to outcomes to avoid any selection bias for the male control subjects included in the study. Grade was classified according to the original histologic description at our institution, and if there was more than 1 tissue specimen at the beginning of treatment and they were of different grades, the more advanced grade was chosen. Tumors were considered moderately differentiated if they were classified as “moderately differentiated”, “moderately well differentiated”, or “moderately poorly differentiated”. Medical comorbidities were classified according to the Adult Comorbidity Evaluation-27, which rates the presence of related comorbidities as engendering mild, moderate, or severe decompensation. This comorbidity index is well established as a prognostic indicator for patients with SCCHN (15, 16).
Within the parent prospective epidemiologic study (N = 1902), there were 392 female and 1262 male patients who received definitive treatment at our institution available for matching. An attempt was made to match each female patient was with one male patient, and matching was performed by individuals blinded to patient outcomes. Matching variables were age (± 10 years), race/ethnicity, smoking status (never versus ever), site of primary tumor (oral cavity, oropharynx, larynx, or hypopharynx), tumor stage (T1–2 versus T3–4), pathological nodal status (negative versus positive), and treatment received (surgery, radiation therapy, surgery and radiation therapy, surgery and chemotherapy, chemoradiotherapy, or surgery and chemoradiotherapy). Furthermore, patients were matched for oral cavity tumor site, subsite was also matched (oral tongue or floor of mouth versus gingivobuccal sites). All patients were treated for curative intent and were free of disease at the end of treatment. Patients were considered disease-free if documented as such at the date of the last visit with the head and neck surgeon, radiation oncologist, or medical oncologist. While this assessment included the relavent imaging studies at that time, this is a retrospective review of a relatively diverse cohort and no universal standards existed for imaging. Many patients had routine serial imaging, while others had symptom/exam directed follow-up imaging. Recurrent disease was documented by biopsy either incisional, excisional, or needle biopsy. However, there were 15 occasions of unequivocal evidence of distant metastases on chest imaging for which patients refused or treating clinicians did not recommend transthoracic or brochoscopic biopsy. Patients were classified as dead with disease only if they had documented recurrent cancer and subsequently having undergone only palliative or no therapy.
Statistical Methods
To detect any significant differences between female and male patients, the frequencies of factors not included in the matching criteria were compared using the Pearson chi-square test. For comparisons in which one or more cells of the contingency table had fewer than 10 observations, Fischer's exact test was used. Student's t test (with adjustment for unequal variances where necessary) was used to compare ages and follow-up times between females and males. All tests were two-sided and P < 0.05 was preset as the cutoff for significance. Recurrence-free, disease-specific, and overall survival, were compared between females and males with Kaplan-Meier estimates and the log-rank test for equality of survival curves. Calculations were completed using Statistical Analysis System software (version 9.1; SAS Institute Inc., Cary, NC). Analysis was completed for time from first appointment, using recurrence and death as censoring variables. Death was categorized as death due to SCCHN or overall death (any cause). In matched-pair analysis, the crude risk for recurrence and death with 95% confidence intervals associated with the sex group was calculated with exact McNemar's chi-square test using STATA software (version 7.0; STATA Corporation, College Station, TX.). Additionally, the risk of recurrence/death was estimated using a multivariable Cox proportional hazards model with adjustment the matching variables and any additional confounders identified by P < 0.1 in frequency tabulations between men and women.
Results
The characteristics of the matched female and male patients are presented in Table 1. As expected, there were no significant differences in the distribution of the patient-specific matching variables (age, race/ethnicity, and smoking status), including when age and smoking status were trichotomized. The median age was 57 years (range 29–84) for the women and 57 years (range 26–87) for the men. While a higher proportion of men than women had moderate to severe medical comorbidities, this difference was not significant (P = 0.078, Table 1). However, significantly more men were current drinkers and significantly more women were never drinkers (P < 0.001). Of note, there was no significant difference in recurrence-free, disease-specific, or overall survival between current drinkers and never or former drinkers (log-rank P = 0.231, 0.279, and 0.693, respectively). A higher proportion of women were widowed, divorced, or separated (P = 0.002), though there were similar distributions in education and income levels for the women and men (Table 1). Of note, there was no significant difference in recurrence-free, disease-specific, or overall survival between married patients and patients who were never married, widowed, divorced, or separated (log-rank P = 0.875, 0.396, and 0.747, respectively).
Table 1.
Characteristics of Females and Matched Males with SCCHN.
| Females (n = 286) |
Males (n = 286) |
||||
|---|---|---|---|---|---|
| Variable | N | (%) | N | (%) | P Value |
| Age* category | .788 | ||||
| < 50 years | 76 | (26.6) | 69 | (24.1) | |
| 50–69 years | 172 | (60.1) | 179 | (62.6) | |
| ≥ 70 years | 38 | (13.3) | 38 | (13.3) | |
| Race/ethnicity* | 1.0 | ||||
| White, Anglo, Caucasian | 264 | (92.3) | 264 | (92.3) | |
| Spanish origin | 10 | (3.5) | 10 | (3.5) | |
| African American | 12 | (7.7) | 12 | (7.7) | |
| Adult comorbidity classification | .078 | ||||
| None or mild | 257 | (89.9) | 243 | (85.0) | |
| Moderate or severe | 29 | (10.1) | 43 | (15.0) | |
| Smoking status* | .980 | ||||
| Current smoker | 140 | (49.0) | 142 | (49.7) | |
| Former smoker | 75 | (26.2) | 73 | (25.5) | |
| Never smoker | 71 | (24.8) | 71 | (24.8) | |
| Alcohol drinking status | <.001 | ||||
| Current drinker | 110 | (38.5) | 162 | (56.6) | |
| Former drinker | 50 | (17.5) | 71 | (24.8) | |
| Never drinker | 126 | (44.1) | 53 | (18.5) | |
| Marital status† | .002 | ||||
| Married | 185 | (66.6) | 215 | (77.9) | |
| Widowed, divorced, or separated | 81 | (29.1) | 46 | (16.7) | |
| Never married | 12 | (4.3) | 15 | (5.4) | |
| Educational level attained† | .840 | ||||
| < High school | 37 | (13.3) | 34 | (12.4) | |
| High School, technichal or vocational school, or assoceate degree or some college | 161 | (57.9) | 166 | (60.3) | |
| Bachelor's degree or advanced degree | 80 | (28.8) | 75 | (27.3) | |
| Income level† | .281 | ||||
| < $25,000 | 75 | (27.8) | 59 | (22.0) | |
| $25,000–74,999 | 112 | (41.5) | 116 | (43.3) | |
| ≥ $75,000 | 83 | (30.7) | 93 | (34.7) | |
| Tumor site* | 1.0 | ||||
| Oral cavity | 132 | (46.2) | 132 | (46.2) | |
| Oral tongue or floor of mouth | 88 | 88 | |||
| Gingivobuccal^ | 44 | 44 | |||
| Oropharynx | 92 | (32.2) | 92 | (32.2) | |
| Tonsil | 48 | 43 | |||
| Other oropharynx | 44 | 49 | |||
| Larynx | 55 | (19.2) | 55 | (19.2) | |
| Supraglottic | 43 | 34 | |||
| Glottic | 12 | 21 | |||
| Hypopharynx | 7 | (2.4) | 7 | (2.4) | |
| Tumor classification* | .992 | ||||
| T1 | 77 | (26.9) | 74 | (25.9) | |
| T2 | 104 | (36.4) | 107 | (37.4) | |
| T3 | 55 | (19.2) | 55 | (19.2) | |
| T4 | 50 | (17.5) | 50 | (17.5) | |
| Nodal classification* | .247 | ||||
| N0 | 140 | (49.0) | 140 | (49.0) | |
| N1 | 49 | (17.1) | 34 | (11.9) | |
| N2 | 89 | (31.1) | 105 | (36.7) | |
| N3 | 8 | (2.8) | 7 | (2.4) | |
| Stage | .621 | ||||
| I | 56 | (19.6) | 54 | (18.9) | |
| II | 54 | (18.9) | 56 | (19.6) | |
| III | 55 | (19.2) | 44 | (15.4) | |
| IV | 121 | (42.3) | 132 | (46.2) | |
| Grade# | .995 | ||||
| Well differentiated | 34 | (11.9) | 34 | (11.9) | |
| Moderately differentiated | 140 | (49.0) | 141 | (49.3) | |
| Poorly differentiated | 76 | (26.6) | 75 | (26.2) | |
| Treatment* | 1.0 | ||||
| Surgery alone | 85 | (29.7) | 85 | (29.7) | |
| Surgery + radiotherapy | 45 | (15.7) | 45 | (15.7) | |
| Surgery + chemotherapy | 1 | (0.3) | 1 | (0.3) | |
| Surgery + chemoradiotherapy | 21 | (7.3) | 21 | (7.3) | |
| Radiotherapy alone | 64 | (22.4) | 64 | (22.4) | |
| Chemoradiotherapy | 70 | (24.5) | 70 | (24.5) | |
Matched variables (age, ± 10 years; race/ethnicity, smoking status, ever versus never; tumor site, oral cavity versus oropharynx versus larynx versus hypopharynx; tumor classification, T1–2 versus T3–4; nodal classification, N0 versus N1–3; and treatment).
Missing data: 8 women and 10 men for marital status; 8 women and 11 men for education level attained; 16 women and 18 men for income level.
Grade was not recorded in 36 females and 36 males.
One female and the corresponding matched male had a primary tumor of the mandibular gingiva and a simultaneous second primary tumor of the oropharynx.
The tumor-specific characteristics for the matched pairs are also listed in Table 1. Again, there were no significant differences in the distribution of matching variables (tumor classification, pathological nodal status, and treatment), including when tumor classification and nodal classification were further segregated. In addition, stage and grade were not distributed differently between males and females. The most common tumor sites were the oral cavity (46%) and oropharynx (32%).
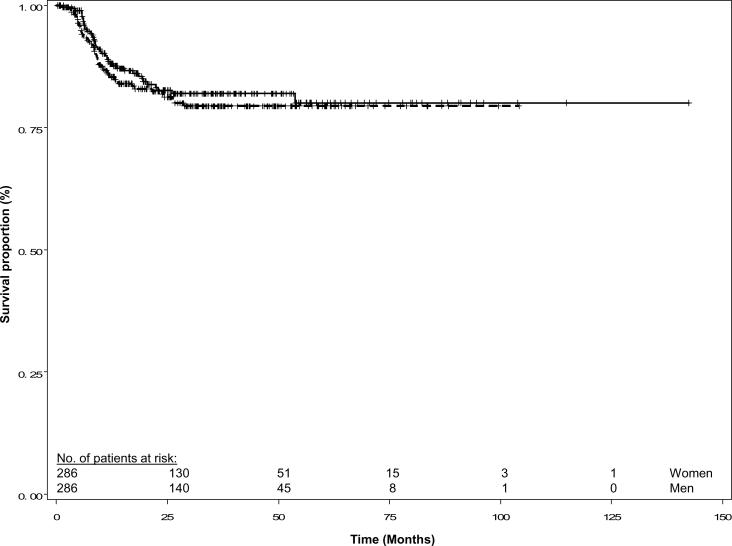
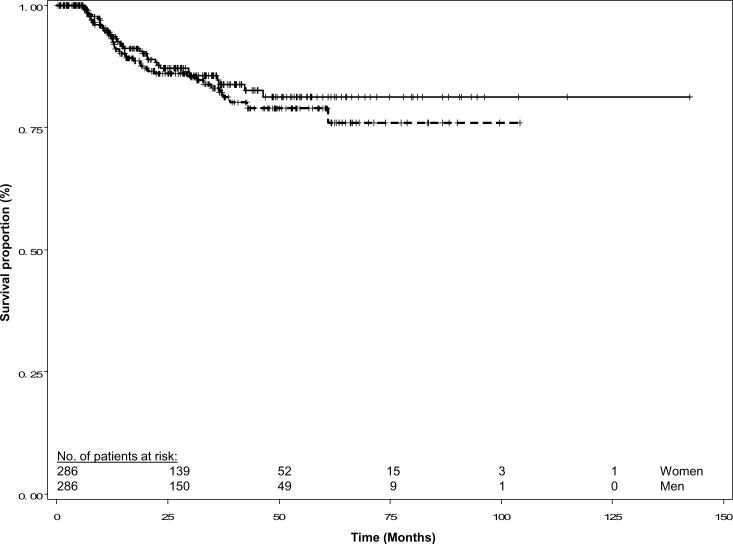
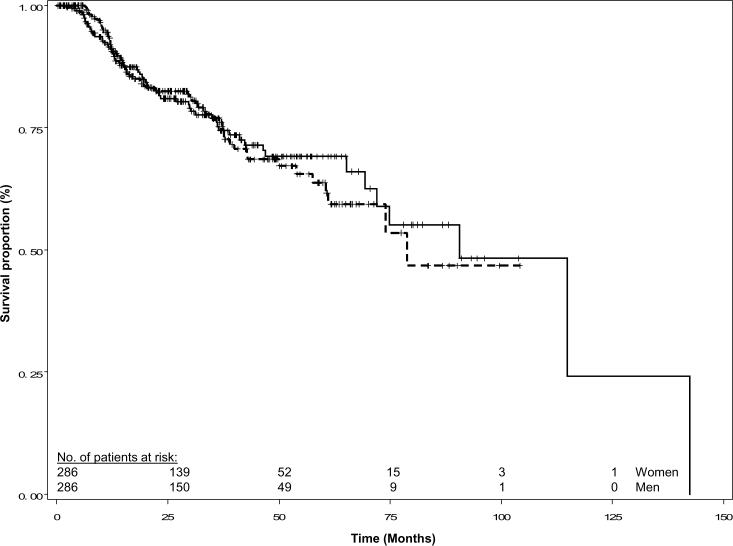
The median follow up time for patients alive at last contact was 27.9 months for the women and 30.7 months for the matched men. Women suffered 43 recurrences while men suffered 48 recurrences. The Kaplan-Meier recurrence-free survival curves were similar for the two groups (log-rank P = 0.473, Figure 1). In addition, the disease-specific and overall survival curves were similar for women and men (log-rank P = 0.495 and 0.765, respectively; Figures 2 and 3). Overall, 34 women and 40 men died of SCCHN, and 65 women and 63 men died of any cause.
Fig. 1.
Recurrence-free survival of female and matched male patients with squamous cell carcinoma of the head and neck. Females are represented by the solid line and males by the dashed line; (log rank P = 0.473).
Fig. 2.
Disease-specific survival of female and matched male patients with squamous cell carcinoma of the head and neck. Females are represented by the solid line and males by the dashed line; (log rank P = 0.495).
Fig. 3.
Overall survival of female and matched male patients with squamous cell carcinoma of the head and neck. Females are represented by the solid line and males by the dashed line; (log rank P = 0.765).
There were 71 discordant pairs in which one patient of the matched pair had a recurrence, and the other did not. In 33 of the pairs, the woman had a recurrence, while in 38 of the pairs, the man had a recurrence (Table 2). The concordant pairs included 205 pairs in which neither the man nor the woman had a recurrence and 10 pairs in which both the man and the woman had a recurrence. In crude matched-pair analysis, there was no significant increased risk of disease recurrence associated with male sex (Table 2). Similar analyses with regard to disease-specific and overall survival also demostrated no significant increased risk associated with male sex (Table 2). Additionally, no evidence of a recurrence or survival disparity associated with patient sex after adjustment for the potential confounders of comorbidity classification, alcohol drinking status, and marital status (Table 2). When the same crude matched-pair analysis was segregated by tumor site (oral cavity, oropharyngeal, or larynx/hypopharynx) (Table 3), no evidence of a recurrence or survival difference between women and men was revealed. Finally, in Kaplan-Meier survival analysis segregated by tumor site, we found no difference between women and men in recurrence-free, disease-specific, or overall survival (Table 3).
Table 2.
Analysis of Risk for Death or Recurrence Associated with Female Sex.
| Matched Pairs |
|||||
|---|---|---|---|---|---|
| Males |
Risk of Recurrence or Death Associated with Female Sex (95% confidence interval); P Value* | P-Value from Log-rank Test of Equality of Survival Curves | Adjusted Risk of Recurrence or Death Associated with Female Sex (95% confidence interval); P Value† | ||
| Females | Recurrence or Death | No Event | |||
| No recurrence | 38 | 205 | |||
| Recurrence | 10 | 33 | 0.9 (0.5–1.4); P = 0.635 | 0.473 | 0.9 (0.6–1.4); P = 0.619 |
| Alive | 32 | 220 | |||
| Death from cancer | 8 | 26 | 0.8 (0.5–1.4); P = 0.512 | 0.495 | 0.9 (0.5–1.4); P = 0.487 |
| Alive | 36 | 185 | |||
| Death from any cause | 27 | 38 | 1.1 (0.7–1.7); P = 0.908 | 0.765 | 1.0 (0.7–1.5); P = 0.868 |
McNemar's chi-squared test.
Multivariable Cox proportional hazards models adjusted for age, race/ethnicity, adult comorbidity classification, smoking status, alcohol drinking status, marital status, site, T-stage, N-stage, Stage, and Treatment.
Table 3.
Risk for Death or Recurrence Associated with Female Sex (Segregated by Cancer Site).
| Matched pairs |
||||
|---|---|---|---|---|
| Males |
Risk of Recurrence or Death Associated with Female Sex (95% confidence interval); P Value* | P Value from Log-rank Test of Equality of Survival Curves | ||
| Females | Recurrence or Death | No Event | ||
| Oral Cavitv (131 pairs†*) | ||||
| No recurrence | 19 | 91 | ||
| Recurrence | 5 | 16 | 0.8 (0.4–1.7); P = 0.736 | 0.575 |
| Alive | 17 | 96 | ||
| Death from cancer | 4 | 14 | 0.8 (0.4–1.8); P = 0.720 | 0.651 |
| Alive | 17 | 82 | ||
| Death from any cause | 12 | 20 | 1.2 (0.6–2.4); P = 0.743 | 0.868 |
| Oropharynx (92 pairs) | ||||
| No recurrence | 9 | 72 | ||
| Recurrence | 3 | 8 | 0.9 (0.3–2.6); P = 1.0 | 0.762 |
| Alive | 8 | 74 | ||
| Death from cancer | 3 | 7 | 0.9 (0.3–2.8); P = 1.0 | 0.773 |
| Alive | 13 | 63 | ||
| Death from any cause | 8 | 8 | 0.6 (0.2–1.6); P = 0.383 | 0.446 |
| Larynx/Hypopharynx (62 pairs) | ||||
| No recurrence | 10 | 41 | ||
| Recurrence | 3 | 8 | 0.8 (0.3–2.3); P = 0.815 | 0.651 |
| Alive | 7 | 49 | ||
| Death from cancer | 1 | 5 | 0.7 (0.2–2.6); P = 0.774 | 0.662 |
| Alive | 6 | 40 | ||
| Death from any cause | 7 | 9 | 1.5 (0.5–5.1); P = 0.607 | 0.537 |
McNemar's chi-squared test.
One pair having simultaneous primaries of the oral cavity and the oropharynx were omitted from this analysis segregated by site.
Discussion
Using a matched-pair design, we found no evidence of a recurrence or survival advantage for women compared to men with SCCHN among patients evaluated and treated at a large multidisciplinary cancer center.
The principal strength of this study was the stringent matching for 6 variables of known prognostic significance: age, race/ethnicity, smoking status, tumor site, tumor classification, nodal status-–as well as type of treatment (17, 18). Additionally, when the smoking, tumor classification and nodal status were further segregated, there remained no significant difference between women and men in these important prognostic variables. Furthermore, all patients were evaluated and treated at a single large multidisciplinary cancer center and had treatment category included in the matching criteria. Finally, degree of decompensation according to the Adult Comorbidity Evaluation-27, an established measurement of patient comorbidity and an independent prognostic factor for SCCHN patients (15, 16), was not distributed differently between female and male patients.
Another major strength of this study was the power associated with the large sample size of 286 matched pairs, which does not include the additional statistical precision provided by our extensive matching process.
Finally, the inclusion of smoking (never versus ever) and tumor site among our matching criteria may in part control for the proportion of human papillomavirus (HPV)-associated cancers in each group (association with this virus is a recently documented predictor of good prognosis), because “never smoker” status is likely a strong surrogate for HPV-associated cancers (19, 20). However, we must consider that inability to classify each cancer in this retrospective cohort as HPV positive or HPV negative as a shortcoming. Clearly, the greatest influence of HPV on prognosis is established for oropharynx cancer, the disease for which HPV prevalence is the highest. In fact, there is not similar high-quality data clearly demonstrating improved prognosis of HPV positive oral cavity cancer patients or laryngeal cancer patients. Certainly the very limited HPV prevalence in these subgroups has hampered such analyses. While we were unable to categorize this entire dataset as HPV positive or HPV negative, it is very unlikely that major confounding of survival due to HPV is present for the oral cavity and larynx/hypopharynx groups. However, site alone is admittedly a crude attempt to control for HPV, but we have also matched on smoking. The most recent and sophisticated (both in the statistical approach and the controlled trial in which it was performed) publication on this topic clearly demonstrates that the clearest prognostic benefit of HPV among oropharyngeal cancer patients is for those without significant smoking history.(21) Thus we would argue that matching for both site and smoking provides substantial control for the effect of HPV on survival. In further support that our matching process was a reasonable surrogate for HPV matching, we found no evidence of a difference in the HPV positive proportion between men and women amongst the 130 oral cavity cancers and 41 oropharyngeal cancers in this matched-pair cohort for which we did have HPV data (P = 0.528 and 0.248, respectively).
Although patient sex has been shown to affect diagnosis, evaluation, treatment, and survivorship for a variety of cancer types, patient sex as an independent prognostic factor for SCCHN has not been adequately explored. While issues such as increased sensitivity to tobacco carcinogens and lower smoking cessation rates among women likely influence SCCHN risk among women who smoke (1, 6), we were interested in a potential survival advantage among women treated for SCCHN. One previous study has hypothesized that estrogen deficiency and elevated glucose in post-menopausal women may be added risk factors in females with oral cancer (22). However, this study only analyzed the etiology of oral cancer and did not determine if these factors affected survival. No study to date has explored the hormonal effect on survival in head and neck cancer.
Our work has several limitations including potential referral and other biases as well as confounders not controlled for. Firstly, our female and male SCCHN patient groups may not represent the broader U.S. population of women and men diagnosed and treated for SCCHN, and this may limit the generalizability of our results. However, our intent was not to explore national differences in survival, but rather to understand whether similar women and men with similar SCCHN extent undergoing similar multidisciplinary treatment have demonstrable differences in survival. Second, while we did control for smoking, our female group had a non-significantly lower comorbidity index and as history of significantly less alcohol use; however, both of these potential confounders would have biased the results towards better survival for women and we found no evidence to support a survival advantage for women. Additionally, there was no apparent alteration in our risk estimates when comorbidity classification, alcohol drinking status, and marital status were included in multivariable models. Thirdly, while misclassification of survival outcomes is certainly possible, this is unlikely. In addition, to confirming survival outcomes documented in the medical record by the treating services, independent survival outcomes are documented in the tumor registry section of our medical records and these dates are confirmed with national and state death indices. Additionally, misclassification of disease-free status is also certainly possible, though we can identify no reason why such potential misclassifications in survival outcomes or disease-free status would be differential between women and men. Finally, while we controlled for race/ethnicity, we did not control for socioeconomic or marital status. There were no differences in socioeconomic status between our female and male SCCHN patient groups, however, the female patients were more likely to be widowed, divorced, or separated. Within this cohort of patients we found no evidence of an influence by marital status on survival, nor did we observe any significant changes in risk estimates when adjusted for comorbidity classification, alcohol drinking status, and marital status.
In conclusion, our findings show no evidence of an association between female sex and lower risk of SCCHN recurrence or improved survival among similar patients receiving similar multidisciplinary evaluation and treatment. It is likely that sex-related mortality disparities seen in previous population-level studies of SCCHN are principally accounted for by differences in incidence (much higher for men)—which are associated with the higher prevalence of exposures to tobacco, alcohol, and likely HPV in men--rather than by disease differences inherent to patient sex. It is also possible that survival advantages observed for women in uncontrolled populations may be accounted for by less smoking, less alcohol use, and hence fewer medical comorbidities among women. Future efforts to reduce the morbidity and mortality associated with SCCHN should concentrate on eliminating the exposures linked to SCCHN and addressing the reasons why men have a higher prevalence of these exposures.
Statement of Translational Relevence.
This work shows that when men and women are matched by prognostic factors and treatment, there is no significant survival difference based on sex in patients with head and neck cancer. This finding is in contrast to the findings of the many population-based studies that have illustrated a survival advantage in women. Possible reasons for the sex-related survival disparities in previous studies are that women and men have different patient and disease characteristics and were treated differently. Future practice should focus on preventing these risk factors in men in an attempt to decrease the incidence of head and neck cancer.
Acknowledgements
The authors wish to thank Liliana Mugartegui, Kathryn Patterson, and Margaret Lung for patient recruiting; Mark Zafereo, Sal Aleem, Mary Borer, Katrina Chaung, and Fei Dong for assistance with medical records review; and Stephanie Deming for manuscript editing.
Grant Support The University of Texas M. D. Anderson Cancer Center Institutional Research Grants (PI: E. M. Sturgis) and start-up funds (to E. M. Sturgis) and a Career Development Award (to E. M. Sturgis) NIH SPORE grant in head and neck cancer CA097007-05 (PI: Waun Ki Hong); NIH K-12 grant 88084 (to E. M. Sturgis; PI: Robert C. Bast); NIH R01 grant ES-11740 (to Q. Wei); Centers for Disease Control and Prevention K01 grant DP001120 (to L. R. Reitzel); and NIH P-30 grant CA 16672 (to M. D. Anderson Cancer Center).
Footnotes
References
- 1.Gasperino J, Rom WN. Gender and lung cancer. Clin Lung Cancer. 2004;5:353–9. doi: 10.3816/CLC.2004.n.013. [DOI] [PubMed] [Google Scholar]
- 2.Friedemann-Sanchez G, Griffin JM, Partin MR. Gender differences in colorectal screening barriers and information needs. Health Expect. 2007;10:148–160. doi: 10.1111/j.1369-7625.2006.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerfolio RJ, Bryant AS, Scott E, et al. Women with pathologic stage I, II, and III non-small cell lung cancer have better survival than men. Chest. 2006;130:1796–1802. doi: 10.1378/chest.130.6.1796. [DOI] [PubMed] [Google Scholar]
- 4.Sah BK, Zhu ZG, Wang XY, et al. Post operative complications of gastric cancer surgery: female gender at high risk. Eur J Cancer Care. 2009;18:202–8. doi: 10.1111/j.1365-2354.2008.01036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glantz MF, Chamberlain MC, Liu Q, et al. Gender disparity in the rate of partner abandonment in patients with serious medical illnesses. Cancer. 2009;115:5237–42. doi: 10.1002/cncr.24577. [DOI] [PubMed] [Google Scholar]
- 6.Freedman ND, Abnet CC, Leitzmann MF, Hollenbeck AR, Schatzkin A. Prospective investigation of the cigarette smoking-head and neck cancer association by sex. Cancer. 2007;110:1593–601. doi: 10.1002/cncr.22957. [DOI] [PubMed] [Google Scholar]
- 7.Muscat JE, Richie JP, Thompson S, Wynder EL. Gender differences in smoking and risk for oral cancer. Cancer Res. 1996;56:5192–7. [PubMed] [Google Scholar]
- 8.Molina MA, Cheung MC, Perez EA, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer. Cancer. 2008;113:2797–806. doi: 10.1002/cncr.23889. [DOI] [PubMed] [Google Scholar]
- 9.McLean A, Lemay W, Vila P, Wegner M, Remington P. Disparities in oral and pharyngeal cancer incidence and mortality among Wisconsin residents, 1999–2002. WMJ. 2006;105:32–5. [PubMed] [Google Scholar]
- 10.Goldberh HI, Lockwood SA, Wyatt SW, Crossett LS. Trends and differentials in mortality from cancers of the oral cavity and pharynx in the United States, 1973–1987. Cancer. 1994;74:565–72. doi: 10.1002/1097-0142(19940715)74:2<565::aid-cncr2820740206>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Garavello W, Spreafico R, Somigliana E, Gaini L, Pignataro L, Gaini RM. Prognostic influence of gender in patients with oral tongue cancer. Otolaryngol Head Neck Surg. 2008;138:768–71. doi: 10.1016/j.otohns.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 12.Franco EL, Dib LL, Pinto DS, Lombardo V, Contesini H. Race and gender influences on the survival of patients with mouth cancer. J Clin Epidemiol. 1993;46:37–46. doi: 10.1016/0895-4356(93)90007-n. [DOI] [PubMed] [Google Scholar]
- 13.Kokoska MS, Piccirillo JF, Haughey BH. Gender differences in cancer of the larynx. Ann Otol Rhinol Laryngol. 1995;104:419–24. doi: 10.1177/000348949510400601. [DOI] [PubMed] [Google Scholar]
- 14.Ildstad ST, Tollerud DJ, Bigelow ME, Remensnyder JP. Squamous cell carcinoma of the head and neck and Massachusetts General Hospital: a comparison of biologic characteristics in men and women. Surgery. 1986;99:7–14. [PubMed] [Google Scholar]
- 15.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–7. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 16.Hall SF, Groome PA, Rothwell D. The impact of comorbidity on the survival of patients with squamous cell carcinoma of the head and neck. Head Neck. 2000;22:317–22. doi: 10.1002/1097-0347(200007)22:4<317::aid-hed1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Chen Chen LM, Li G, Reitzel LR, et al. Matched-pair analysis of race or ethnicity in outcomes of head and neck cancer patients receiving similar multidisciplinary care. Cancer Prev Res. 2009;2:782–91. doi: 10.1158/1940-6207.CAPR-09-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pytynia KP, Grant JR, Etzel CJ, Roberts DB, Wei Q, Sturgis EM. Matched-pair analysis of survival of never-smokers and ever-smokers with squamous cell carcinoma of the head and neck. J Clin Oncol. 2004;22:3981–8. doi: 10.1200/JCO.2004.02.133. [DOI] [PubMed] [Google Scholar]
- 19.Dahlstrom KR, Adler-Storthz K, Etzel CJ, et al. Human papillomavirus type 16 infection and squamous cell carcinoma of the head and neck in never smokers: A matched pair analysis. Clin Cancer Res. 2003;9:2620–6. [PubMed] [Google Scholar]
- 20.Dahlstrom KR, Little JA, Zafereo ME, Lung M, Wei Q, Sturgis EM. Squamous cell carcinoma of the head and neck in never smoker - never drinkers: A descriptive epidemiologic study. Head Neck. 2008;30:75–84. doi: 10.1002/hed.20664. [DOI] [PubMed] [Google Scholar]
- 21.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cáncer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SUBA Z. Gender-related hormonal risk factors for oral cancer. Pathol Oncol Res. 13:195–202. doi: 10.1007/BF02893499. [DOI] [PubMed] [Google Scholar]