Abstract
Allogeneic blood transfusion has an immunomodulatory capacity on its recipients through accumulation of immunologically active substances with blood storage, and prestorage leukoreduction reduces many of these mediators. We investigated lipopolysaccharide (LPS)-induced cytokine response of peripheral blood mononuclear cells (PBMCs) exposed to packed red blood cell (PRBC) supernatants from leukoreduced (LR) or non-leukoreduced (NLR) units with variable duration of storage. PRBC units were collected with or without leukoreduction on Day 0 before routine storage. The plasma fraction (supernatant) was isolated from LR and NLR units after 1 day (D1) or 42 days (D42) of storage and exposed to PBMCs versus control media for 24 h, then with LPS for an additional 24 h. Cell supernatants were analyzed for IL-1β, IL-6, IL-8, IL-10, and TNF-α by cytokine bead array. IL-1β, TNF-α, and IL-6 were significantly elevated in PRBC groups versus control. D42 NLR PRBC supernatant significantly increased secretion of IL-1β and IL-6 compared to D1 NLR PRBC supernatant. LR significantly attenuated the cytokine response of IL-1β. Thus, PRBC supernatant potentiates proinflammatory LPS-induced cytokine secretion from PBMCs. This response is accentuated with storage duration and partially attenuated with leukoreduction. These findings may partially explain the immune activation seen clinically after blood transfusion.
Introduction
Allogeneic blood transfusion (ABT) is recognized to have immunomodulatory effects on its recipient. Clinicians initially had observed the immunologic effects of blood transfusion with respect to improved graft survival in transplant recipients who received pretransplant transfusions, and with respect to the increased recurrence of cancer in patients receiving ABTs (Opelz and others 1973; Gantt 1981; Amato and Pescatori 2006). In the setting of trauma, clinical studies have shown that blood transfusion is an independent risk factor for the development of postinjury multiple organ failure (MOF) and acute lung injury (Moore and others 1997; Silliman and McLaughlin 2006).
Both clinical studies and related in vitro studies of stored packed red blood cells (PRBCs) provide insights into the mechanistic links of transfusion-related immunomodulation. Many immunologically active substances, including cytokines and inflammatory lipids, have been identified in PRBCs, and these potential mediators have been shown to accumulate with blood storage (Silliman and others 1994; Nielsen and others 1996). However, as many of these factors are leukocyte- or platelet-derived, prestorage leukoreduction reduces many of these immunologic mediators (Kristiansson and others 1996; Shanwell and others 1997; Wadhwa and others 2000). The importance of understanding how these bioactive mediators affect the recipient's immune system is apparent, particularly in surgical patients who may require large volumes of blood transfusions. A number of in vitro studies have examined the potential role of stored PRBCs to accentuate the innate immune response of neutrophils (Chin-Yee and others 1998; Zallen and others 2000; Biffl and others 2001; Silliman and others 2003), and neutrophil-mediated injury has been proposed to be central to the two-hit model of MOF. While these studies have emphasized the effect of PRBCs on neutrophil function, peripheral blood mononuclear cells (PBMCs) are also capable of secreting large amounts of circulating cytokines and have been shown to be the predominant source of circulating cytokines among leukocytes (Xing and Remick 2003). In addition, Hensler et al. found that postinjury cytokine levels in severely injured patients who developed MOF correlated with the extent of blood transfusion (Hensler and others 2003). However, the effects of PRBC exposure to PBMCs have not been well-described. In this in vitro study, we investigate the effect PRBC exposure on cytokine production by PBMCs and the effects of storage duration and prestorage leukoreduction.
Materials and Methods
Blood donation and leukoreduction
After informed consent according to guidelines set forth by the Colorado Multiple Institutional Review Board, healthy adult volunteers donated one unit of whole blood, which was separated into components and stored according to the American Association of Blood Banks criteria. Fifty percent, by weight, of the PRBCs were leukoreduced (LR) on Day 0 using Pall BPF4 (Pall Corporate, East Hills, NY, USA) and Fenwall-Sepacell R500-ii (Baxter-Fenwall, Deerfield, IL, USA) third-generation filters before routine storage. Samples from the LR and non-leukoreduced (NLR) PRBCs were drawn via sterile couples on days 1 and 42 of routine storage. The supernatant was isolated from each of these PRBC samples by centrifugation at 5,000g for 7 min followed by an additional spin of 12,500g for 5 min to remove acellular debris. Each supernatant was aliquoted and stored at −70°C until further use. PRBC supernatants from each individual donor were tested separately in each experiment and were not pooled. Up to 16 donors were used to control for interdonor variability, each isolated and processed with and without leukoreduction for 1 to 42 days.
Human PBMC isolation
Venous blood was collected from healthy volunteers in sterile pyrogen-free syringes containing heparin sodium as an anticoagulant. PBMCs were isolated using a 3% dextran sedimentation followed by layering the leukocyte-rich upper layer over Ficoll-Hypaque (Amersham Biosciences, Uppsala, Sweden). The gradient was centrifuged at 400g for 30 min and the PBMC layer was collected. The PBMCs were washed once and resuspended at 1 ×107/mL in RPMI 1640 culture medium (Mediatech, Herndon, VA, USA) supplemented with 10% fetal calf serum, 1:100 glutamine, and 1:100 penicillin/streptomycin. PBMCs from each individual volunteer were tested separately in each experiment.
Measurement of PBMC cytokine production
The PBMCs were incubated for 24 h in cell culture media, which consisted of RPMI 1640 culture medium supplemented with fetal calf serum, glutamine, penicillin/streptomycin (control media), with or without 5% plasma supernatant by volume from Day 1 (D1) NLR PRBCs, Day 42 (D42) NLR PRBCs, or Day 42 (D42) LR PRBCs. Following this 24 h incubation period, the PBMC cultures were stimulated 100 ng/mL lipopolysaccharide (LPS; Escherichia coli serotype 055:B5, Sigma Aldrich, St. Louis, MO, USA) for an additional 24 h. After the 24 h LPS stimulation period, cell culture supernatants were collected. Measurements of the cytokines IL-1β, IL-6, IL-8, IL-10, and TNF-α in the supernatants were measured by using a Becton Dickinson Human Inflammation cytometric bead quantitative array according to manufacturer's instructions. Briefly, the culture supernatants were incubated with a mixture of six distinct fluorescent bead populations, each of which had been labeled with antibodies specific for one of the cytokines of interest. A second cocktail of labeled antibodies were then added to the bead–supernatant mixture to label the captured cytokines. Each sample was run on a BD FACSCaliber (Beckton Dickinson, Franklin Lakes, NJ, USA) and the individual cytokines identified by the fluorescence intensity of the bead. Cytokine levels were quantified by the fluorescence intensity of the associated labeled antibody as compared to a standard curve. The results were then analyzed using the BD CBA analysis software.
Statistical analysis
Analysis of variance using the Fisher's exact test for post hoc comparisons was used to determine differences among the groups. Statistical significance was considered at the P < 0.05 level. All data are reported as mean ± SEM.
Results
Effects of PRBC supernatants on LPS-induced cytokine production
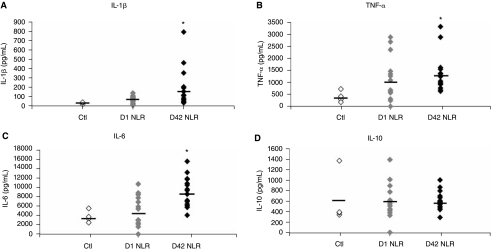
To determine the cytokine response from PRBC exposure, IL-1β, IL-6, IL-8, IL-10, and TNF-α levels were measured from LPS-stimulated PBMCs. Day 1 (D1) and Day 42 (D42) units were tested to evaluate the effect of storage on cytokine production. IL-1β increased 2.5-fold in the Day 1 NLR PRBC group versus control and the Day 42 NLR PRBC group increased >7-fold over control, being significantly higher than either the Day 1 NLR PRBC group or control (182 ± 50.4 and 64.8 ± 10.1 vs. 25.4 ± 6.5 pg/mL IL-1β in D42 NLR PRBC, D1 NLR PRBC, and control groups, respectively, P < 0.05 for D42 NLR PRBC group vs. D1 and control groups; Fig. 1A; n = 16 for D42, 15 for D1, and 4 for Ctl). Comparing levels of secreted TNF-α, PRBC supernatant also attenuated its release: TNF-α levels in the D1 NLR PRBC group increased nearly 3-fold over control (1155 ± 246 vs. 403 ± 118 pg/mL, respectively; n = 14 for D1 and 4 for Ctl); whereas more than a 3-fold increase was observed with D42 NLR PRBC group versus control (1365 ± 186 vs. 403 ± 118 pg/mL, respectively, P < 0.05 vs. control; Fig. 1B; n = 16 for D42). More modest yet significant increases over control were noted with IL-6 production: D1 NLR PRBC group with a 1.4-fold increase and D42 NLR PRBC with a 2.4-fold increase over control, respectively (5231 ± 827 and 8931 ± 761 vs. 3720 ± 642 pg/mL for the D1, D42, and control groups, respectively, P < 0.05 for D42 vs. D1 and control; Fig. 1C; n = 16 for D42, 14 for D1 and 4 for Ctl). Measurements of IL-10 production, however, were not significantly different in either NLR PRBC groups compared to control (Fig. 1D). In addition, IL-8 levels were also not significantly different between groups (31 ± 4.2 vs. 41 ± 5.0 vs. 47 ± 3.1 pg/mL for Ctl, D1 and D42, respectively). Taken together, both D1 and D42 NLR PRBCs potentiated the production of the proinflammatory cytokines IL-1β, TNF-α, and IL-6 in response to subsequent LPS stimulation compared to the control-LPS group, and prolonged storage to 42 days significantly potentiated IL-β and IL-6 secretion even higher than fresh units.
FIG. 1.
PRBC supernatant potentiates LPS-induced proinflammatory cytokine release by PBMCs. (A) The amount of IL-1β secreted from PBMCs exposed to control media, Day 1 non-leukoreduced PRBC supernatant (D1 NLR), or Day 42 non-leukoreduced PRBC supernatant (D42 NLR) is shown as a scatter plot with the mean represented by a bar. D42 NLR PRBC supernatant significantly increased IL-1β secretion versus control and D1 NLR supernatant (*P < 0.05 vs. Ctl and D1 NLR; n = 4 for control, n = 15 for D1 NLR and n = 16 for D42 NLR). (B) D42 NLR supernatant exposure resulted in increased TNF-α versus control and significantly increased IL-6 (C) versus control and D1 NLR PRBC supernatant (*P < 0.05 vs. Ctl and D1 NLR groups). (D) IL-10 levels were not different between groups.
Effects of leukoreduction of PRBCs on LPS-induced cytokine production
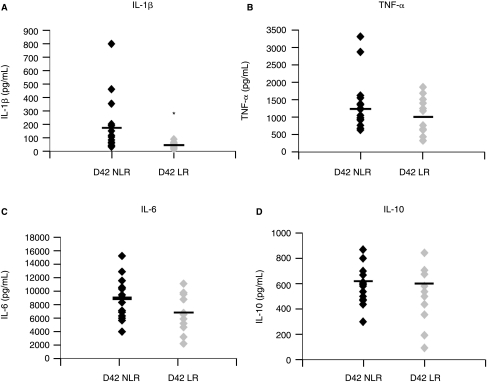
Given that D42 PRBC samples potentiated proinflammatory cytokine production, we next sought to determine if prestorage leukoreduction of D42 units would attenuate the production of cytokines stimulated by LPS. Exposure to D42 LR PRBC supernatant did not significantly alter secretion of TNF-α (1084 ± 147 pg/mL) and IL-6 (6739 ± 784 pg/mL) versus the non-leukoreduced units (1365 ± 186 and 8931 ± 761 pg/mL for TNF-α and IL-6, respectively; Fig. 2B and C; n = 12 for LR 16 and NLR). The D42 LR PRBC group significantly decreased the amount of IL-1β produced, a >4-fold reduction versus the NLR group (42 ± 6.5 vs. 182 ± 50.4 pg/mL for LR vs. NLR groups, respectively, P < 0.05; Fig. 2A; n = 12 for LR 16 and NLR). IL-10 levels were not significantly altered by leukoreduction (Fig. 2D) nor were IL-8 levels (47 ± 5.0 vs. 53 ± 5.4 pg/mL for NLR and LR, respectively). Thus, LR appears to attenuate the proinflammatory cytokine production of LPS-stimulated cells.
FIG. 2.
Leukoreduction attenuates proinflammatory cytokine secretion by LPS-stimulated PBMCs. (A) The amount of IL-1β secreted from PBMCs exposed to control Day 42 non-leukoreduced PRBC supernatant (D42 NLR) and Day 42 leukoreduced PRBC supernatant (D42 LR) is shown as a scatter plot with the mean represented as a bar. D42 LR PRBC supernatant significantly decreased IL-1β secretion versus D42 NLR supernatant (*P < 0.05 vs. D42 NLR group; n = 16 for D42 NLR, n = 12 for D42 LR). D42 LR supernatant exposure resulted in nonsignificant reductions in (B) TNF-α and (C) IL-6 secretion. (D) IL-10 levels were not altered.
Discussion
In this study, we found PRBC supernatant potentiated LPS-induced proinflammatory cytokine secretion by PBMCs. IL-1β, TNF-α, and IL-6 levels were significantly higher in PRBC-exposed groups than control, while IL-8 and IL-10 levels were not altered. Furthermore, prolonged storage of PRBCs significantly increased this potentiation as IL-1β and IL-6 levels were significantly higher from PBMCs exposed to Day 42 supernatants versus Day 1 supernatants. In addition, prestorage leukoredeuction attenuated this proinflammatory cytokine release as IL-1β levels were significantly lower in PBMCs exposed to leukoreduced (LR) supernatants compared to non-leukoreduced (NLR) supernatants. TNF-α and IL-6 levels were also lower after LR PRBC exposure versus NLR exposure, but they did not achieve statistical significance.
Potentiation of proinflammatory cytokines may partially explain clinical immune up-regulation after ABT. IL-1 is a potent proinflammatory cytokine that results in increased expression of other proinflammatory cytokines including IL-6 and IL-8, and elevated levels of IL-1 have been identified in arthritis, inflammatory bowel disease, graft-versus-host disease, and other proinflammatory conditions (Arend 2002). TNF-α also has important immune activation roles, and anti-TNF-α therapies have been developed for treatment of rheumatoid arthritis and inflammatory bowel disease (Locksley and others 2001). IL-6 is a crucial mediator of the acute phase response and inflammation, and elevated levels have been correlated with an increased risk of MOF (Dimopoulou and others 2008; Lausevic and others 2008). Collectively, IL-1, IL-6, and TNF-α act together to active immune up-regulation as they play a major role in initiating the inflammatory response in sepsis (Jean-Baptiste 2007). Thus, elevation of these cytokines may mediate transfusion-related inflammation. Indeed, elevated serum IL-6 and TNF-α levels have been identified after blood transfusion (Hensler and others 2003; Milasiene and others 2007).
However, ABT can also result in immune suppression as is evident by improved graft tolerance, increased cancer recurrence, decreased severity of autoimmune disease, and increased infection risk in transfused patients (Peters and others 1989; Opelz and others 1997; Chang and others 2000; Amato and Pescatori 2006). It is likely that the disparate findings of pro- and anti-inflammation after ABT result from the complex interactions between bioactive substances found in transfused blood and the recipient's immune cells in the context of the underlying illness. In the current study, one arm of the immune response was evaluated as LPS was selected as an investigational tool to induce PBMC-derived secretion of cytokines. More specifically, LPS elicits monocyte and dendritic cell cytokine production as toll-like receptor 4 (TLR4), the LPS receptor, is primarily expressed on these cells (Takeda and others 2003).
Other investigators have examined the cytokine response to in vitro blood product exposure. Biedler and others (2002) and Mynster and others (1998) have investigated the TNF-α response of recipient whole blood to allogeneic whole blood or allogeneic whole blood supernatant, respectively. In contrast to the present study, they found TNF-α levels decreased in cells exposed to stored whole blood, and this reduction in TNF-α secretion was partially ameliorated by leukoreduction. However, exposure of recipient cells to whole blood or whole blood supernatant may elicit a different cytokine response than PRBC supernatant as whole blood contains different amounts and ratios of cells (particularly leukocytes) and plasma than PRBCs. In addition, investigation of whole blood is not as clinically relevant as PRBCs, and the current study investigated supernatant from PRBCs processed in the standard fashion according to the American Association of Blood Banks criteria. Supernatant was used to more precisely determine if the cell-free fraction of PRBCs modulate the immune response. PBMCs were investigated to model the recipient cytokine response. Furthermore, in the prior studies LPS was added concomitantly with the blood products rather than conditioning the cells first with exposure to ABT, then providing the induction agent as in the current study. The ABT exposure prior to LPS induction was performed to model the “two-hit” phenomenon of sequential insults seen clinically.
The current finding that prolonged storage enhances the proinflammatory response to ABT coincides with clinical data, which suggest that a storage lesion in blood products may contribute to worse prognosis in patients receiving older units of blood. Cardiac surgery patients have an increased mortality if transfused blood stored longer than 14 days compared to recipients of blood stored less than 14 days, although the mechanism of this increased mortality remains unclear, and the age of transfused blood correlates with the risk of MOF (Zallen and others 1999; Koch and others 2008). In addition, leukoreduction has been proposed, in part, to reduce the effects of leukocyte-derived products in ABT and has been adopted universally in Europe and Canada. Thus, the present study was undertaken to further investigate the potential attenuation of immune modulation by leukoreduced ABT. However, we found that although leukoreduction attenuates the IL-1β response, it only partially mitigates the TNF-α and IL-6 response. These results concur with some clinical studies as leukoreduction has not been definitively proven to ameliorate the immune activation of ABT. In one double-blind, randomized, controlled, clinical trial, prestorage leukoreduction did not decrease the incidence of acute lung injury in trauma patients (Watkins and others 2008). Leukoreduction has also been shown to have no effect on the neutrophil resilience mediated by ABT (Biffl and others 2001). Thus, given the present findings, it seems leukoreduction only partially ameliorates the immune response to ABT.
The mechanism of the ABT-invoked proinflammatory cytokine response is uncertain. PRBC supernatant was used in the present study to isolate the soluble factors present in a PRBC transfusion. Thus, this study more precisely defines which fraction of a PRBC transfusion modulates the immune response and suggests the effect is due to cell- or plasma-derived factors. The PRBC units underwent high-speed centrifugation to obtain the supernatant and dead or dying cells from units with prolonged storage duration may have thus released more immunologically active mediators such as RNA or DNA than more fresh units. However, these differences would also be present in vivo as more of the transfused cells from older units are dead or dying, and thus the difference seen between fresh and old units is due to the storage effect itself rather than the processing technique. In addition, because leukoreduction partly ameliorated this effect, it seems one of the factors involved originates from the leukocyte. Lipid or cytokine mediators are potential targets as both are important immune activators, and both have been implicated in other aspects of transfusion-related immunomodulation (Silliman and others 1994; Shanwell and others 1997). Other potential mediators that have been suggested include histamine, eosinophil cationic protein, eosinophil protein X, myeloperoxidase, and plasminogen activator inhibitor-1 (Nielsen and others 1996). Furthermore, the assay used to measure cytokine levels primarily detects free cytokines, and thus soluble cytokine antagonists, such as sIL-1RII, may alter the free cytokine levels in the cell supernatants. Additional experiments directly measuring the level of cytokine antagonists in the PRBC supernatant or measuring the total amount of secreted cytokine, bound or free, by the PBMCs would be necessary to determine the effect of soluble cytokine antagonists on the level of secreted free cytokines. Nevertheless, the cytokine antagonists in the PRBC supernatant would also be present clinically in a blood transfusion and thus the cytokine levels seen in vitro would likely be proportional to those seen in vivo.
In conclusion, allogeneic blood transfusion potentiates LPS-induced proinflammatory cytokine secretion from normal human PBMCs. This potentiation is enhanced by prolonged storage and partially attenuated by prestorage leukoreduction. These findings may partially explain the immune activation seen clinically after blood transfusion. Further investigation of the in vivo cytokine and immune response in recipients of blood transfusion will advance the understanding of transfusion-related immune activation and may provide therapeutic strategies to abrogate this effect.
Acknowledgment
This work was supported in part by NIH grants (Nos. P50GM49222 and T32GM08315).
References
- Amato A. Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane database of systematic reviews (Online) 2006:CD005033. doi: 10.1002/14651858.CD005033.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Biedler AE. Schneider SO. Seyfert U. Rensing H. Grenner S. Girndt M. Bauer I. Bauer M. Impact of alloantigens and storage-associated factors on stimulated cytokine response in an in vitro model of blood transfusion. Anesthesiology. 2002;97:1102–1109. doi: 10.1097/00000542-200211000-00011. [DOI] [PubMed] [Google Scholar]
- Biffl WL. Moore EE. Offner PJ. Ciesla DJ. Gonzalez RJ. Silliman CC. Plasma from aged stored red blood cells delays neutro-phil apoptosis and primes for cytotoxicity: abrogation by post-storage washing but not prestorage leukoreduction. J Trauma. 2001;50:426–431. doi: 10.1097/00005373-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Chang H. Hall GA. Geerts WH. Greenwood C. McLeod RS. Sher GD. Allogeneic red blood cell transfusion is an independent risk factor for the development of postoperative bacterial infection. Vox Sanguinis. 2000;78:13–18. doi: 10.1159/000031143. [DOI] [PubMed] [Google Scholar]
- Chin-Yee I. Keeney M. Krueger L. Dietz G. Moses G. Supernatant from stored red cells activates neutrophils. Transfusion Med (Oxford, England) 1998;8:49–56. doi: 10.1046/j.1365-3148.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- Dimopoulou I. Orfanos S. Kotanidou A. Livaditi O. Giamarellos-Bourboulis E. Athanasiou C. Korovesi I. Sotiropoulou C. Kopterides P. Ilias I. Kanellakopoulou K. Armaganidis A. Plasma pro- and anti-inflammatory cytokine levels and outcome prediction in unselected critically ill patients. Cytokine. 2008;41:263–267. doi: 10.1016/j.cyto.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Gantt CL. Red blood cells for cancer patients. Lancet. 1981;2:363. doi: 10.1016/s0140-6736(81)90673-5. [DOI] [PubMed] [Google Scholar]
- Hensler T. Heinemann B. Sauerland S. Lefering R. Bouillon B. Andermahr J. Neugebauer EA. Immunologic alterations associated with high blood transfusion volume after multiple injury: effects on plasmatic cytokine and cytokine receptor concentrations. Shock (Augusta, Ga) 2003;20:497–502. doi: 10.1097/01.shk.0000095058.62263.1f. [DOI] [PubMed] [Google Scholar]
- Jean-Baptiste E. Cellular mechanisms in sepsis. J Intensive Care Med. 2007;22:63–72. doi: 10.1177/0885066606297123. [DOI] [PubMed] [Google Scholar]
- Koch CG. Li L. Sessler DI. Figueroa P. Hoeltge GA. Mihaljevic T. Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- Kristiansson M. Soop M. Shanwell A. Sundqvist KG. Prestorage versus bedside white blood cell filtration of red blood cell concentrates: effects on the content of cytokines and soluble tumor necrosis factor receptors. J Trauma. 1996;40:379–383. doi: 10.1097/00005373-199603000-00009. [DOI] [PubMed] [Google Scholar]
- Lausevic Z. Lausevic M. Trbojevic-Stankovic J. Krstic S. Stojimirovic B. Predicting multiple organ failure in patients with severe trauma. Can J Surg. 2008;51:97–102. [PMC free article] [PubMed] [Google Scholar]
- Locksley RM. Killeen N. Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Milasiene V. Stratilatovas E. Characiejus D. Kazbariene B. Norkiene V. TGF-beta1 and TNF-alpha after red blood cell transfusion in colorectal cancer patients. Exp Oncol. 2007;29:67–70. [PubMed] [Google Scholar]
- Moore FA. Moore EE. Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620–624. [PubMed] [Google Scholar]
- Mynster T. Dybkjoer E. Kronborg G. Nielsen HJ. Immunomodulating effect of blood transfusion: is storage time important? Vox Sanguinis. 1998;74:176–181. [PubMed] [Google Scholar]
- Nielsen HJ. Reimert CM. Pedersen AN. Brunner N. Edvardsen L. Dybkjaer E. Kehlet H. Skov PS. Time-dependent, spontaneous release of white cell- and platelet-derived bioactive substances from stored human blood. Transfusion. 1996;36:960–965. doi: 10.1046/j.1537-2995.1996.36111297091738.x. [DOI] [PubMed] [Google Scholar]
- Opelz G. Sengar DP. Mickey MR. Terasaki PI. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc. 1973;5:253–259. [PubMed] [Google Scholar]
- Opelz G. Vanrenterghem Y. Kirste G. Gray DW. Horsburgh T. Lachance JG. Largiader F. Lange H. Vujaklija-Stipanovic K. Alvarez-Grande J. Schott W. Hoyer J. Schnuelle P. Descoeudres C. Ruder H. Wujciak T. Schwarz V. Prospective evaluation of pretransplant blood transfusions in cadaver kidney recipients. Transplantation. 1997;63:964–967. doi: 10.1097/00007890-199704150-00010. [DOI] [PubMed] [Google Scholar]
- Peters WR. Fry RD. Fleshman JW. Kodner IJ. Multiple blood transfusions reduce the recurrence rate of Crohn's disease. Dis Colon Rectum. 1989;32:749–753. doi: 10.1007/BF02562122. [DOI] [PubMed] [Google Scholar]
- Shanwell A. Kristiansson M. Remberger M. Ringden O. Generation of cytokines in red cell concentrates during storage is prevented by prestorage white cell reduction. Transfusion. 1997;37:678–684. doi: 10.1046/j.1537-2995.1997.37797369441.x. [DOI] [PubMed] [Google Scholar]
- Silliman CC. Bjornsen AJ. Wyman TH. Kelher M. Allard J. Bieber S. Voelkel NF. Plasma and lipids from stored platelets cause acute lung injury in an animal model. Transfusion. 2003;43:633–640. doi: 10.1046/j.1537-2995.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- Silliman CC. Clay KL. Thurman GW. Johnson CA. Ambruso DR. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124:684–694. [PMC free article] [PubMed] [Google Scholar]
- Silliman CC. McLaughlin NJ. Transfusion-related acute lung injury. Blood Rev. 2006;20:139–159. doi: 10.1016/j.blre.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Takeda K. Kaisho T. Akira S. Toll-like receptors. Ann Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Wadhwa M. Seghatchian MJ. Dilger P. Contreras M. Thorpe R. Cytokine accumulation in stored red cell concentrates: effect of buffy-coat removal and leucoreduction. Transfusion Sci. 2000;23:7–16. doi: 10.1016/s0955-3886(00)00049-7. [DOI] [PubMed] [Google Scholar]
- Watkins TR. Rubenfeld GD. Martin TR. Nester TA. Caldwell E. Billgren J. Ruzinski J. Nathens AB. Effects of leukoreduced blood on acute lung injury after trauma: a randomized controlled trial. Crit Care Med. 2008;36:1493–1499. doi: 10.1097/CCM.0b013e318170a9ce. [DOI] [PubMed] [Google Scholar]
- Xing L. Remick DG. Relative cytokine and cytokine inhibitor production by mononuclear cells and neutrophils. Shock. 2003;20:10–16. doi: 10.1097/01.shk.0000065704.84144.a4. [DOI] [PubMed] [Google Scholar]
- Zallen G. Moore EE. Ciesla DJ. Brown M. Biffl WL. Silliman CC. Stored red blood cells selectively activate human neutrophils to release IL-8 and secretory PLA2. Shock. 2000;13:29–33. doi: 10.1097/00024382-200013010-00006. [DOI] [PubMed] [Google Scholar]
- Zallen G. Offner PJ. Moore EE. Blackwell J. Ciesla DJ. Gabriel J. Denny C. Silliman CC. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]