Abstract
Objective
Biological evidence suggests that inflammation might induce type 2 diabetes (T2D), and epidemiological studies have shown an association between higher white blood cell count (WBC) and T2D. However, the association has not been systematically investigated.
Research Design and Methods
Studies were identified through computer-based and manual searches. Previously unreported studies were sought through correspondence. 20 studies were identified (8,647 T2D cases and 85,040 non-cases). Estimates of the association of WBC with T2D were combined using random effects meta-analysis; sources of heterogeneity as well as presence of publication bias were explored.
Results
The combined relative risk (RR) comparing the top to bottom tertile of the WBC count was 1.61 (95% CI: 1.45; 1.79, p = 1.5*10−18). Substantial heterogeneity was present (I2 = 83%). For granulocytes the RR was 1.38 (95% CI: 1.17; 1.64, p = 1.5*10−4), for lymphocytes 1.26 (95% CI: 1.02; 1.56, p = 0.029), and for monocytes 0.93 (95% CI: 0.68; 1.28, p = 0.67) comparing top to bottom tertile. In cross-sectional studies, RR was 1.74 (95% CI: 1.49; 2.02, p = 7.7*10−13), while in cohort studies it was 1.48 (95% CI: 1.22; 1.79, p = 7.7*10−5). We assessed the impact of confounding in EPIC-Norfolk study and found that the age and sex adjusted HR of 2.19 (95% CI: 1.74; 2.75) was attenuated to 1.82 (95% CI: 1.45; 2.29) after further accounting for smoking, T2D family history, physical activity, education, BMI and waist circumference.
Conclusions
A raised WBC is associated with higher risk of T2D. The presence of publication bias and failure to control for all potential confounders in all studies means the observed association is likely an overestimate.
Introduction
Chronic inflammation, characterized by the increased production of cytokines and acute-phase reactants and activation of inflammatory signalling networks [1]–[5], may be involved in the pathogenesis of type 2 diabetes (T2D).Various markers of inflammation have been shown to predict the future diabetes risk, including Interleukin-6 (IL-6) and C-reactive protein (CRP) [1], [5].Obesity, a strong risk factor for T2D is also associated with inflammation as fat tissue releases inflammatory cytokines[6], [7]. Inflammation on its own can affect insulin signalling [3], indirectly increasing the risk of T2D, without the presence of obesity. Inflammation is also thought to promote beta-cell death [8]. However, there is considerable uncertainty about the direction of causality of the relationship between inflammation and T2D.
Evidence from epidemiological studies suggests an association between total peripheral white blood cell (WBC) or leukocyte count, a non-specific marker of inflammation, and diabetes risk[9], [10]. Although a number of studies have been published, they have not been systematically reviewed or meta-analysed. Granulocytes themselves are comprised of neutrophils, basophils and eosinophils[9]. Little is known about the association of each of the subfractions with T2D.
In the present study we systematically review and meta-analyse existing studies of the association between differential WBC count and T2D, including previously unpublished data from 5,021 cases and 43,508 non-cases (with 499 cases and 15,051 non-cases from EPIC-Norfolk study) obtained through correspondence with investigators. We also explore the potential roles of reverse causality, publication bias and confounding.
Methods
A. Systematic review and meta-analysis
Bibliographic search, literature review and data extraction
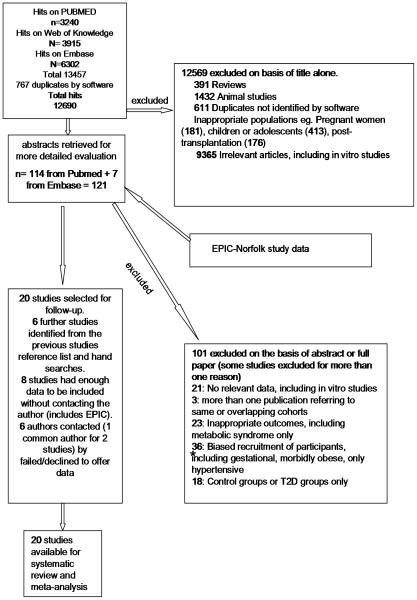
A bibliographic search was conducted by the first author to identify all published evidence on the association between WBC or leukocyte (from now and on, WBC) count and T2D. The search terms included (“leukocyte” OR “leucocyte “OR “white blood”) combined (AND) with diabetes (diabetes” OR “glucose” OR “metabolic syndrome” OR “hyperglycaemia” OR “hyperglycemia”). We searched Pubmed 2.0 (National Library of Medicine) entering each search term as a MeSH, ISI Web of KnowledgeSM version 4.7 (©Thomson Reuters 2009) and Embase (© 2009 Elsevier B.V.), initially without limits with regard to publication date or language. Last searches were conducted in April 2010. Two authors (EGK, ZY) independently reviewed all identified titles (n = 12,705), and subsequently abstracts (n = 136) and full articles (Figure 1). We included evidence from cross-sectional and prospective cohort studies of adults that used standard definitions of T2D [11], adjusted for at least age, sex and BMI (excluded studies n = 1). No case-control studies were identified. For results from the same cohort published more than once (n = 3), we included the study with the largest sample reported (n = 1). We excluded studies of children and adolescents or with participants who had undergone solid organ or bone marrow transplantation (Figure 1). Discrepancies in articles selected for inclusion were addressed by consensus (n = 1). We additionally hand searched reference lists of all articles selected for inclusion. Two authors (EGK, ZY) extracted information from each article selected for inclusion including the number of cases and non-cases, study design and population, measurement of WBC and diagnosis of T2D, effect estimate and 95% confidence intervals for associations between WBC, neutrophil/granulocyte, lymphocyte and monocyte count and T2D risk. Where the risk ratio (odds ratio, relative risk or hazard ratio) was presented using other than three groups of WBC, these were converted to compare the top to bottom tertile of WBC [12].
Figure 1. Information Flow Diagram.
We identified studies that appeared during the literature review and did not report on the association between WBC and T2D but had potentially collected pertinent data, as evident from their study description. Corresponding authors of these studies (n = 19) were contacted by electronic and regular mail (two electronic reminders) and invited to submit data using a standardized data extraction sheet and uniform analysis plan. We requested odds ratios, relative risks or hazard ratios for the association between WBC and its sub-fractions where available, comparing top to bottom tertile of each measure, adjusting for age, sex, smoking, BMI and waist circumference and using the WHO definition of diabetes[11]. Data on the number of cases and non-cases and 95% confidence intervals for the estimated effects were also requested.
We additionally included unpublished results from the EPIC Norfolk study, described in more detail below, according to the protocol used for obtaining unpublished evidence from other investigators.
Meta-analyses
For the purposes of the meta-analysis, we considered all of odds ratios, risk ratios and hazard ratios as estimates of the relative risk. These relative risks were combined across studies using random effects meta-analysis. Heterogeneity was assessed using the I2 statistic, which represents the proportion of variation in the effect sizes that is attributable to genuine differences across studies rather than to random error [13]. To identify potential sources of heterogeneity between studies and to assess the effect of study characteristics on the results, we repeated the meta-analysis in strata defined by study size, design and method of data collection and ethnicity of participants. The between-study variance was used to quantify the degree of heterogeneity among studies [14]. We also used meta-regression to estimate the effect of each of the covariates on the relative risk. Publication bias was assessed using a funnel plot and Begg and Egger tests [15], [16].Statistical analyses were performed using Stata (version 10.0) statistical software (Stata Corporation, College Station, Texas, USA). Results were presented in forest plots, where the sizes of the boxes for individual studies are inversely proportional to the variances of the log relative risks, and the horizontal lines represent 95% CIs.
B. EPIC-Norfolk cohort
In addition to including results from the European Prospective Investigation of Cancer in Norfolk, UK (EPIC-Norfolk) as part of the overall meta-analysis according to the standard protocol described above, we used prospective data from this cohort to investigate the effect of adjusting for a range of potential confounding factors not consistently available across other studies.
EPIC-Norfolk participants
EPIC-Norfolk is a population-based cohort study, which has previously been described in detail [17]. In brief, men and women aged 40 to 79 years were eligible for participation. In total, 77,630 invitations were sent, 30,447 (39%) individuals consented to take part, with 25,639 (33%) attending the baseline health check between 1993 and 1997. The study was approved by the Norfolk Local Research Ethics Committee and all participants gave written informed consent.
We excluded participants with a history of stroke, myocardial infarction, or cancer (n = 2,460) or prevalent or unconfirmed diabetes at baseline (n = 688).
Measurements
A detailed self-completed health and lifestyle questionnaire was completed at baseline, including questions on family history of diabetes, prescribed medications, occupational social class, smoking status, educational level, and physical activity assessed by a four point index[17]. Participants were invited to attend a baseline health check-up at the study clinic where health checks were carried out by trained research nurses. Anthropometric measurements were taken according to standard protocol[17]. Two further health check-ups were performed, after an average follow-up time of three and thirteen years respectively. Since the baseline health-check visit, there were three follow-up assessments: a postal questionnaire at 18 months, a second health-check visit (1998–2000), and a further postal questionnaire (2002–2004).
Biochemical analyses
Biochemical assays were carried out on samples drawn with participants in the non-fasted state. Blood samples for WBC measurement were stored overnight at room temperature and were collected each morning and transported to the EPIC-Norfolk laboratory in Attleborough (UK). The samples were analysed in a random order using impedance counting technique with an MD18 haematology analyser (Coulter Corporation, Miami, FL, USA). Quality controls were carried out daily. In addition, the Haematology Department of Addenbrooke's Hospital included the EPIC Laboratory in a monthly quality control scheme. The WBC count coefficient of variation for the period of study was ≤3.0%. The standard deviation values for the differential granulocyte, lymphocyte and monocyte percentages were less than or equal to 1.5, 1.5, and 3.0, respectively [18]. Researchers and laboratory personnel did not have access to identifiable information, and could only identify samples by number.
Incident type 2 diabetes
Ascertainment of incident cases of type 2 diabetes involved review of multiple sources of evidence including self-report (self reported doctor diagnosed diabetes, anti-diabetic drug use) during follow-up, linkage to primary and secondary care registers, hospital admissions and mortality data[17]. Criteria for qualification as a confirmed diabetes case were: confirmation of self-report by another data source or diagnosis captured by an external source alone, independently of participation in study follow-up questionnaires or visit. Possible cases based solely on self-report and not confirmed by another data source did not qualify as a confirmed case of diabetes. Cases not meeting the above criteria were excluded (n = 5).
Statistical analysis
Cox proportional hazards regression was used to estimate hazard ratios for the incidence of diabetes by tertiles of WBC, granulocyte, lymphocyte, and monocyte count, using the lowest tertile as the reference category. Results from age and sex adjusted models were compared to those additionally adjusting for smoking status (never, former or current), waist circumference (continuous), BMI (continuous), educational level (below ‘A’ level vs. ‘A’ level and above where ‘A’ level is school education to age 18 years), a positive family history of diabetes, and physical activity level (4 categories ranging from sedentary to active).
Analyses were restricted to participants with full information on total WBC, granulocyte, lymphocyte or monocyte count (n = 15,708). We further excluded 158 participants without complete information on covariates or exclusion criteria.
15,550 participants (499 incident diabetes cases) remained in the analyses. We calculated a Health Behaviours Score (HBS), including information on physical activity, alcohol intake, plasma vitamin C (a biomarker of fruit and vegetable intake) and smoking, as proposed by Khaw et al [19] and compared models with and without an interaction term for HBS, using a likelihood ratio test.
All statistical analyses were performed using Stata/SE 10.0 (Stata-Corp, College Station, Texas, USA). All p values were based on 2-sided tests.
Results
A. Systematic review and meta-analysis
Initial searches identified 12,705 articles and abstracts (Figure 1). After exclusions, a total of 27 publications were included, 7 of which reported results on the association between WBC counts and T2D diabetes risk in a format that could be used[9], [10], [20]–[24]. We contacted 19 corresponding authors of the other 20 studies [25]–[44] and received data for 13[25], [27]–[29], [33], [34], [36], [37], [39], [40], [42]–[44]. A total of 6 authors (7 studies) did not respond (n = 6) or declined participation (n = 1). Tabular data from one study could not be used because it was not available in full [27].Our meta-analysis was therefore based on data from 20 independent studies (see Figure 1, Table S1 and PRISMA checklist S1), including EPIC-Norfolk results.
Combined results
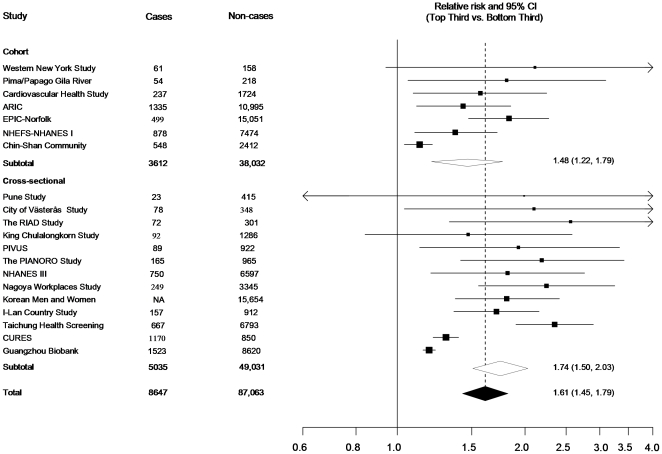
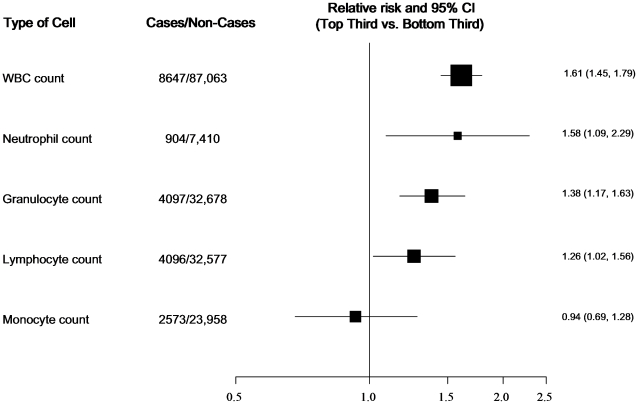
The combined relative risk (RR) comparing the top to bottom tertile of the total WBC count distribution was 1.61 (95% CI: 1.45; 1.79, p = 1.5*10−18). (Figure 2). The combined relative risk was 1.38 (1.17; 1.64, p = 1.5*10−4) for granulocytes, 1.26 (1.02; 1.56, p = 0.029) for lymphocytes, and 0.93 (0.68; 1.28, p = 0.67) for monocytes, comparing the top to bottom tertile of the distribution of each measure (Figure 3).
Figure 2. Forest plot showing study-specific and combined effect estimates comparing the top to bottom tertile of the WBC count distribution.
Figure 3. Forest plot showing combined effect estimates for T2D comparing the top to bottom tertile of the distribution of WBC sub-fractions (Granulocytes include Neutrophils plus Eosinophils plus Basophils).
Heterogeneity
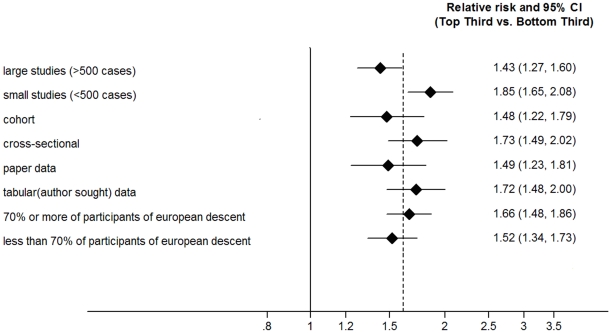
For total WBC, there was a high degree of heterogeneity between the 20 studies (I2 = 83%, p<0.001). I2 was slightly smaller but still statistically significant among prospective cohort studies (I2 = 74%, p<0.001). Sensitivity analyses were used to identify potential sources of heterogeneity between studies. Stratification by study design showed a combined RR for WBC count of 1.73 (95% CI: 1.49; 2.02, p = 7.7*10−13) for prevalent (5,035 cases; 47,008 non-cases) and 1.48 (95% CI: 1.22; 1.79, p = 7.7*10−5).for incident (3,612 cases; 38,032 non-cases) T2D. Subgroup analyses (Figure 4) revealed a lower RR in larger (≥500 cases) compared to smaller studies (RR 1.43, 95% CI: 1.27; 1.60,p = 3.4*10e-9) versus RR 1.85, 95% CI: 1.64; 2.08, p = 3.6*10−25). and no significant difference comparing published to unpublished evidence included in this report (RR 1.49, 95% CI: 1.23; 1.81, p = 5.9*10−5) versus RR 1.72, 95% CI: 1.48; 2.00, p = 1.1*10−12). The combined RR was 1.66 (95% CI 1.48–1.86, p = 2.8*10−18) versus 1.52 (95% CI 1.34–1.73, p = 2.02*10−10) when comparing studies including more than 70% versus less than 70% European descent participants respectively.
Figure 4. Forest plot showing combined effect estimates for T2D comparing the top to bottom tertile of the WBC count distribution.
* dotted line representing combined effect estimate for meta-analysis. Size of rhomboids not informative of weight.
Including ethnicity (percentage of participants of European descent), number of cases, number of participants, source of data (investigator provided versus published data), and type of study (prospective cohort versus cross-sectional study or cross sectional data from a cohort study) in a meta-regression model resulted in a decrease in the value of I2 from 83% to 36.0%. The beta-coefficients and corresponding p values from the meta-regression models using each of the above parameters in turn are presented in table 1.
Table 1. β coefficients and corresponding p values from the meta-regression models.
| Covariate | β coefficient | P value | N of studies |
| Source of data (tabular vs published paper) | 0.144 | 0.28 | 20 |
| Type of study (cross-sectional vs longitudinal) | 0.164 | 0.213 | 20 |
| Number of cases | −0.0003 | 0.018 | 19* |
| Number of participants | −0.000002 | 0.89 | 20 |
| Percentage of Caucasian participants | 0.142 | 0.316 | 20 |
*Number of cases not available for one study [60]. β –coefficient represents the change in log relative risk per unit increase in the relevant covariate. Each model includes each covariate as an explanatory variable and the log relative risk as the outcome variable.
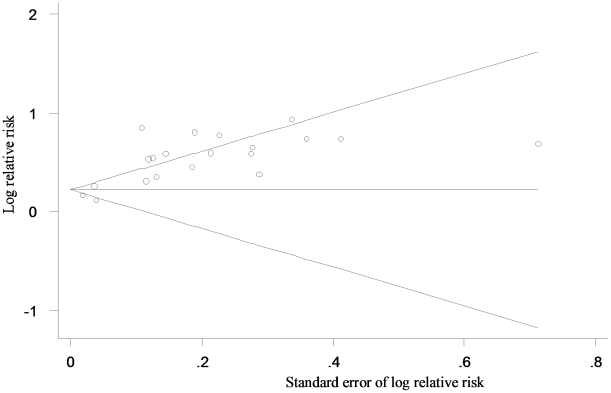
Publication bias
A funnel plot indicated the presence of publication bias in these studies (Figure 5). Significant publication bias was also observed using Egger's bias test (p = 0.011 for prospective cohort and p<0.0001 for cross-sectional studies).
Figure 5. Begg's Funnel Plot* for visual assessment of the presence of publication bias for all studies included in the meta-analysis (each study is represented by an open circle).
*Tests for Publication Bias. For Prospective Cohort Studies (n = 9), Egger's bias 2.50 (p 0.011). For Cross-Sectional Studies (n = 13), Egger's bias 2.64 (p<0.001). Overall Egger's bias p<0.001.
Confounding
Since limited information was available from the published studies, we were unable to assess the impact of confounding based on results from published studies. Instead, we assessed the impact of confounding in the EPIC-Norfolk cohort as described below.
B. EPIC-Norfolk cohort
We assessed the impact of confounding in EPIC-Norfolk study participants with detailed covariate information (499 incident cases, 15,051 non-cases). The following were associated with lower WBC counts: female sex, lower BMI, lower waist circumference, lower age, never smoking (Table 2).
Table 2. Distribution of T2D risk factors according to tertiles of total WBC count at baseline, EPIC-Norfolk Study.
| Total WBC tertiles | 1 (n = 5,477) | 2 (n = 5,120) | 3 (n = 4,953) | P for trend |
| Tertile range, * 10(9)/L | 1–5.8 | 5.8–7.0 | 7.1–40.5 | |
| Sociodemographic variables | ||||
| Age, y | 57.6±9.3 | 58.1±9.3 | 58.0±9.5 | 0.01 |
| Sex, n (% female) | 3,137 (57.3) | 2,778 (54.3) | 2,677 (54.1) | <0.001 |
| Education level, n (%) | 0.004* | |||
| ‘A’ level† and above | 3,045 (55.6) | 2,867 (56.0) | 2,622 (52.9) | |
| Below ‘A’ level† | 2,432 (44.4) | 2,253 (44.0) | 2,331 (47.06) | |
| Anthropometric measures | ||||
| BMI, Kg/m2 | 25.8±3.6 | 26.3±3.8 | 26.5±3.8 | <0.001* |
| Waist circumference, cm | 86.3±11.9 | 88.2±12.2 | 89.2±12.8 | <0.001* |
| Health related behaviours | ||||
| Physical activity level, n (%) | <0.001* | |||
| Active | 1,107 (20.2) | 953(18.6)) | 856 (17.3) | |
| Moderately active | 1,294 (23.6) | 1,157(22.6) | 1,084(21.9) | |
| Moderately inactive | 1,566(28.6) | 1,501 (29.3) | 1,346 (27.2) | |
| Inactive | 1,510 (27.6) | 1,509 (29.5) | 1,667 (33.7) | |
| Smoking status, n(%) | <0.001* | |||
| Never | 2,961 (54.1) | 2,417 (47.2) | 1,933(39.0) | |
| Former | 2,244 (41.0) | 2,186 (42.7) | 1,939 (39.2) | |
| Current | 272 (5.0) | 517 (10.1) | 1081 (21.8) | |
| Medical history | ||||
| Family history of diabetes present, n (%) | 682(12.5) | 676 (13.2) | 644 (13.0) | 0.32* |
Data are means ± standard deviation. P values are derived using the Kruskal-Wallis test for continuous variables and the chi-squared test for categorical variables.
‘A’ level = Advanced Level General Certificate of Education, ‘O’ level = Ordinary Level General Certificate of Education.
*age and sex adjusted.
Table 3 shows hazard ratios for incident T2D by tertiles of total WBC and sub-fractions distribution. We decided a priori to include T2D family history, physical activity and educational level in our models since they have been previously associated with peripheral WBC [45]–[48]. The age and sex adjusted HR for WBC count of 2.19 (95% CI: 1.74; 2.75) was reduced to 1.82 (95% CI: 1.45; 2.29) after further accounting for smoking status, T2D family history, physical activity, education, BMI and waist circumference, comparing the top and bottom tertiles of the total WBC distribution. Adjusting only for age, sex, smoking, BMI, waist circumference the HR for WBC count was 1.82 (95% CI 1.44, 2.29), comparing the top and bottom tertiles of the total WBC distribution.
Table 3. Hazard Ratio (95% CI) of incident T2D by tertiles of total WBC and sub-fractions.
| Lowest tertile | Middle tertile | Highest tertile | P for trend | |
| WBC (n) | 5,477 | 5,120 | 4,953 | |
| Mean 103 cells L−1 | 4.9 | 6.4 | 8.5 | |
| Diabetes (n) | 111 | 160 | 228 | |
| Range | ≤5.7 | 5.8–7.0 | ≥7.1 | |
| Model 1 | 1 | 1.47 (1.17–1.90) | 2.19 (1.74–2.75) | <0.001 |
| Model 2 | 1 | 1.33 (1.04–1.70) | 1.82 (1.45–2.29) | <0.001 |
| Granulocytes (n) | 5,499 | 4,940 | 5,111 | |
| Mean 103 cells L−1 | 2.7 | 3.8 | 5.5 | |
| Diabetes (n) | 131 | 155 | 213 | |
| Range | ≤3.3 | 3.4–4.3 | ≥4.4 | |
| Model 1 | 1 | 1.28 (1.01–1.61) | 1.68 (1.35–2.08) | <0.001 |
| Model 2 | 1 | 1.14 (0.90–1.43) | 1.45 (1.17–1.81) | 0.001 |
| Lymphocytes (n) | 5,646 | 5,319 | 4,585 | |
| Mean 103 cells L−1 | 1.44 | 1.97 | 2.78 | |
| Diabetes (n) | 134 | 151 | 214 | |
| Range | ≤1.7 | 1.8–2.2 | ≥2.3 | |
| Model 1 | 1 | 1.21 (0.96–1.53) | 2.02 (1.63–2.51) | <0.001 |
| Model 2 | 1 | 1.09 (0.86–1.37) | 1.66 (1.33–2.06) | <0.001 |
| Monocytes (n) | 5,754 | 5,899 | 3,897 | |
| Mean 103 cells L−1 | 0.23 | 0.48 | 1.00 | |
| Diabetes (n) | 156 | 199 | 144 | |
| Range | ≤0.3 | 0.4–0.6 | ≥0.7 | |
| Model 1 | 1 | 1.17 (0.94–1.44) | 1.22 (0.97–1.54) | 0.07 |
| Model 2 | 1 | 1.10 (0.90–1.36) | 1.14(0.90–1.42) | 0.268 |
Model 1: adjusted for age and sex (n = 15,550).
Model 2: as model 1 plus smoking status, family history of diabetes, physical activity level, education level, BMI and waist circumference (n = 15,550).
Analysis within participants with an HbA1c of less than 6.5% at baseline
Baseline HbA1c data were available for only a subset of EPIC-Norfolk (n = 9558). 9392 individuals had a baseline HbA1c of less than 6.5%, 166 of whom developed incident diabetes. The age and sex adjusted HR for WBC count of 2.07 (95% CI: 1.41 3.04) was reduced to 1.87 (95% CI: 1.27; 2.76) after further accounting for smoking status, T2D family history, physical activity, education, BMI and waist circumference, comparing the top and bottom tertiles of the total WBC distribution.
Analysis within normal total WBC count limits
When restricting analyses to individuals with WBC counts within the normal range (4 to 11*109/L) results were similar to when including participants irrespective of the normal range (as in Table 3). Comparing the top to bottom tertile of the relevant distribution, the HR was 1.87 (1.47; 2.39) for total WBC count, 1.45 (1.15–1.83) for granulocytes, 1.73 (1.37–2.17) for lymphocytes and 1.11 (0.88–1.41) for monocytes.
Analysis with Health Behaviours score (HBS)
Given the associations between WBC and the range of heath behaviours, we investigated whether associations between WBC count and T2D differed according to groups defined by adverse versus healthier lifestyle choices, assuming that unmeasured confounders clustered according to groups defined by measured behaviours. When comparing models including HBS, family history of diabetes, education level, BMI and waist circumference with and without an interaction term between WBC and HBS, there was no significant difference between the models, so no evidence of an interaction was detected (p for interaction 0.21).
Discussion
Summary of Findings
The present meta-analysis includes evidence about the association between WBC count and T2D from 20 cross-sectional and prospective cohort observational studies, comprising a total of 8,647 T2D cases and 85,040 non cases. Total WBC count was significantly associated with T2D, after adjustment for age, sex, smoking, BMI, waist circumference. Total granulocyte (and subset neutrophil) as well as lymphocyte but not monocyte count were also significantly associated with T2D, after adjustment for age, sex, smoking, BMI, waist circumference The findings were similar for incident T2D in the EPIC-Norfolk cohort analysis, where further adjustment for measured confounders showed the potential for residual confounding.
The available biological data have strongly suggested that T2D is an inflammatory disease [1]–[5].Various markers of inflammation predict the future diabetes risk, including IL-6, CRP[1], sialic acid, and orosomucoid[5]. An inflammation score that included the above four parameters at baseline increased the future T2D risk almost four fold, when comparing the extreme quintiles in non-smoking individuals[49]. A recent analysis from the ARIC cohort showed that WBC, a marker for inflammation, contributed to the short term increased risk of T2D among participants recently quitting smoking[50].
We did not find an increased risk of incident T2D for participants belonging to the higher monocyte tertile. Our findings agree with the previously reported results of the ARIC study [9]. Several stimuli, including pro-inflammatory as well as metabolic stimuli increase the recruitment of monocytes to peripheral tissues, where they differentiate to macrophages and dendritic cells [51]. The destination of monocytes is therefore not the bloodstream and hence peripheral enumeration is not representative of monocyte tissue presence or a possible local monocyte-mediated tissue effect.
Possible mechanisms that could link inflammation and diabetes include interruptions of the insulin signalling in the liver by inflammatory molecules like IL-6 [52]or a pro-inflammatory effect on insulin[53], or insulin resistance[54], [55]. Obesity, a major risk factor for diabetes, is a state of chronic inflammation and is associated with elevated levels of CRP[56], IL-6 [6] and plasminogen activator inhibitor-1 (PAI-1) [7]. Thus, it is possible that the association between inflammation and T2D is mediated by obesity. Studies involving tight matching for obesity suggest that there is an association between WBC count and insulin resistance but may be subject to residual confounding[47].In the current analyses, we adjusted for both waist circumference as well as BMI to control for confounding associated with obesity. Also, we investigated possible sources of confounding in this report using the EPIC-Norfolk study. The observation that the effect size, adjusted for a wide range of possible confounding factors, was lower that that adjusted for age and sex alone does suggest the potential for confounding. Of course, residual confounding by factors not considered at all or by factors we have considered but measured imprecisely can not be excluded.
Limitations
Measurement error could affect our assessment of exposures, outcomes and confounding factors. Differences in the methods used to measure WBC counts as well as different performance of the same assays at different time periods might have contributed to error in longitudinal studies. In general, such measurement error would attenuate the measure of association if non-differential with regard to the case status. Some cases of type 1 diabetes (T1D) might have been inadvertently included; however, such misclassification is likely to have a minimal impact on effect sizes, given that T1D constitutes only 5–10% of all diabetes cases. Also, given the ascertainment methods used, misclassification is unlikely in prospective cohorts; all except three (NHEFS-NHANES I, NHANES III, Pima Papago Gila River) included incident cases occurring after age 35 years. The majority of cross-sectional studies asked about the type of diabetes when assessing prevalent diabetes or excluded participants using insulin within the first year of diagnosis.
It is possible that hyperglycaemia itself has an impact on WBC levels. In people with diabetes, WBC levels are lowered by treatment with rosiglitazone[57], [58] which may be due to the lowering of glucose levels or an immunomodulatory effect of this class of drugs. However, similar reductions in WBC have been observed with other types of glucose lowering drugs including acarbose[59]. To investigate the possibility of reverse causality, we compared the difference in strength of the association with T2D between cross-sectional and prospective cohort studies. The higher combined RR of WBC in cross-sectional compared to prospective cohort studies could be suggestive of reverse causality. However, since the difference between combined RR between cross-sectional and cohort studies was not statistically significant and because of evidence of presence of publication bias, reverse causality is less likely. Moreover, limiting analyses to healthier people at baseline by excluding participants with a possible or confirmed history of chronic diseases including T2D at baseline, and limiting the analysis to individuals with a baseline HbA1c of less than 6.5%, the risk estimate was almost identical to that in the entire dataset further decreasing the attractiveness of a reverse causality hypothesis, at least in the EPIC-Norfolk cohort.
Results of this study are based on a systematic and comprehensive literature review, including data from both prospective cohorts and cross-sectional studies, and previously unpublished data for a large number of participants. Incomplete retrieval of available results and studies is possible, since the studies included were all published in the English language. However, additional searches showed that no article in a language other that English that fitted the inclusion criteria could be identified. To address variations in design and diabetes ascertainment we asked corresponding authors to report their cases following the same diabetes definition (WHO 1999) and standardize their effect estimates for the same set of covariates (age, sex, smoking, BMI and waist circumference). Despite these attempts of standardisation, heterogeneity between investigator sought studies was not totally eliminated. The funnel plot does indicate the potential for publication bias despite our efforts to obtain data that were not published. This could have been due to the fact that our search strategy, although exhaustive, was more likely to identify studies with reported results on WBC and T2D associations. Sensitivity analyses revealed a significantly lower RR in larger (≥500 cases) compared to smaller studies but no significant difference comparing published to unpublished evidence.
Summary of conclusions
In summary, these results suggest that WBC is positively associated with the risk of T2D. However, the presence of publication bias and failure to control for all potential confounders in all studies suggests that the observed association may be an overestimate of the truth. We cannot exclude the possibility of reverse causality or residual confounding. Approaches such as the use of genetic determinants of WBC as instrumental variables may be useful to deal with these as yet unresolved issues.
Supporting Information
Summary of the studies of association between WBC and T2D included in the meta-analysis
(0.09 MB RTF)
PRISMA Checklist of items to include when reporting a systematic review or meta-analysis (diagnostic review consisting of cohort studies)
(0.07 MB DOC)
Acknowledgments
We thank the volunteers, general practitioners and staff of the EPIC-Norfolk study.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: EGK is supported by the Medical Research Council through a Clinical Research Training Fellowship and the Cambridge BRC-NIHR. EPIC-Norfolk is supported by grant funding from the Medical Research Council and Cancer Research United Kingdom with additional support from the Stroke Association, British Heart Foundation, Research Into Ageing, and the Academy of Medical Science. The sponsors had no role in the design and conduct of the study, collection, management, analysis and interpretation of the data, and preparation, review, or approval of the manuscript. VL is supported by the Rachadapiseksompoj Faculty of Medicine Research Fund Chulalongkorn University, Thailand, and by the National Institutes of Health (grants T37-TW00049 and T37-MD-100449). PIVUS work was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, the Royal Swedish Academy of Science, Uppsala University Hospital and AstraZeneca R&D, Moelndal. The sponsors had no role in the PIVUS study design, analyses, writing or decision to publish the manuscript. The Cardiovascular Health Study research was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant numbers U01 HL080295, R01 HL-075366 and R01 HL-094555 from the National Heart, Lung, and Blood Institute; R01 AG-023629, R01 AG-15928, R01 AG-20098, and AG-027058 from the National Institute on Aging; the University of Pittsburgh Claude. D. Pepper Older Americans Independence Center P30-AG-024827, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. JO and GN's work was supported by grants from Vastmanland's research foundation against cardiovascular disease and SparbanksstiftelsenNya. GN had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Pradhan, AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 2.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–92. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 3.Hotamisligil, GS Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 4.Hak AE, Stehouwer CD, Bots ML, Polderman KH, Schalkwijk CG, et al. Associations of C-reactive protein with measures of obesity, insulin resistance, and subclinical atherosclerosis in healthy, middle-aged women. Arterioscler Thromb Vasc Biol. 1999;19:1986–91. doi: 10.1161/01.atv.19.8.1986. [DOI] [PubMed] [Google Scholar]
- 5.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799–805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 7.Lundgren CH, Brown SL, Nordt TK, Sobel BE, Fujii Setal. Elaboration of type-1 plasminogen activator inhibitor from adipocytes. A potential pathogenetic link between obesity and cardiovascular disease. Circulation. 1996;93:106–10. doi: 10.1161/01.cir.93.1.106. [DOI] [PubMed] [Google Scholar]
- 8.Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, et al. Mechanisms of Beta-Cell Death in Type 2 Diabetes. Diabetes. 2005;54:S108–S113. doi: 10.2337/diabetes.54.suppl_2.s108. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. The Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 10.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, et al. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:455–61. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Geneva, Switzerland: 2006. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: A report of the World Health Organization and International Diabetes Federation. pp. 1–46. [Google Scholar]
- 12.Danesh J, Collins R, Appleby P, Peto R, et al. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–82. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10:1665–77. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 15.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day NE, Oakes S, Luben R, Khaw KT, Bingham SA, et al. EPIC-Norfolk: Study design and characteristics of the cohort. British Journal of Cancer. 1999;80:95–103. [PubMed] [Google Scholar]
- 18.Smith MR, Kinmonth AL, Luben RN, Bingham S, Day NE, et al. Smoking status and differential white cell count in men and women in the EPIC-Norfolk population. Atherosclerosis. 2003;169:331–337. doi: 10.1016/s0021-9150(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 19.Khaw KT, Wareham N, Bingham S, Welch A, Luben R, et al. Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Med. 2008;5:e12. doi: 10.1371/journal.pmed.0050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford, ES Leukocyte count, erythrocyte sedimentation rate, and diabetes incidence in a national sample of US adults. Am J. 2002;Epidemiol155:57–64. doi: 10.1093/aje/155.1.57. [DOI] [PubMed] [Google Scholar]
- 21.Chien K, Cai T, Hsu H, Su T, Chang W, et al. A prediction model for type 2 diabetes risk among Chinese people. Diabetologia. 2009;52:443–50. doi: 10.1007/s00125-008-1232-4. [DOI] [PubMed] [Google Scholar]
- 22.Kim DJ, Noh JH, Lee BW, Choi YH, Chung JH, et al. The associations of total and differential white blood cell counts with obesity, hypertension, dyslipidemia and glucose intolerance in a Korean population. J Korean Med Sci. 2008;23:193–8. doi: 10.3346/jkms.2008.23.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muntner P, He J, Chen J, Fonseca V, Whelton PK. Prevalence of non-traditional cardiovascular disease risk factors among persons with impaired fasting glucose, impaired glucose tolerance, diabetes, and the metabolic syndrome: analysis of the Third National Health and Nutrition Examination Survey (NHANES III). Ann Epidemiol. 2004;14:686–95. doi: 10.1016/j.annepidem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Stranges S, Rafalson LB, Dmochowski J, Rejman K, Tracy RP, et al. Additional contribution of emerging risk factors to the prediction of the risk of type 2 diabetes: evidence from the Western New York Study. Obesity (Silver Spring) 2008;16:1370–6. doi: 10.1038/oby.2008.59. [DOI] [PubMed] [Google Scholar]
- 25.Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, et al. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50:2384–9. doi: 10.2337/diabetes.50.10.2384. [DOI] [PubMed] [Google Scholar]
- 26.Chen LK, Lin MH, Chen ZJ, Hwang SJ, Chiou ST. Association of insulin resistance and hematologic parameters: study of a middle-aged and elderly Chinese population in Taiwan. J Chin Med Assoc. 2006;69:248–53. doi: 10.1016/S1726-4901(09)70251-5. [DOI] [PubMed] [Google Scholar]
- 27.Chuo SK, Li JC, Tsai WC, Wu DA, Kuo SW, et al. Correlations between white blood cell count and metabolic syndrome in middle-age Taiwanese. Endocr Res. 2005;31:39–50. doi: 10.1080/07435800500229151. [DOI] [PubMed] [Google Scholar]
- 28.Gokulakrishnan K, Deepa R, Sampathkumar R, Balasubramanyam M, Mohan V. Association of leukocyte count with varying degrees of glucose intolerance in Asian Indians: the Chennai Urban Rural Epidemiology Study (CURES-26). Metab Syndr Relat Disord. 2009;7:205–10. doi: 10.1089/met.2008.0024. [DOI] [PubMed] [Google Scholar]
- 29.Ingelsson E, Hulthe J, Lind L. Inflammatory markers in relation to insulin resistance and the metabolic syndrome. Eur J Clin Invest. 2008;38:502–9. doi: 10.1111/j.1365-2362.2008.01962.x. [DOI] [PubMed] [Google Scholar]
- 30.shizaka N, Ishizaka Y, Toda E, Nagai R, Yamakado M. Association between smoking, hematological parameters, and metabolic syndrome in Japanese men. Diabetes Care. 2006;29:741. doi: 10.2337/diacare.29.03.06.dc05-2245. [DOI] [PubMed] [Google Scholar]
- 31.Ishizaka N, Ishizaka Y, Toda E, Nagai R, Yamakado M. Association between cigarette smoking, white blood cell count, and metabolic syndrome as defined by the Japanese criteria. Intern Med. 2007;46:1167–70. doi: 10.2169/internalmedicine.46.0136. [DOI] [PubMed] [Google Scholar]
- 32.Kim JA, Choi YS, Hong JI, Kim SH, Jung HH, et al. Association of metabolic syndrome with white blood cell subtype and red blood cells. Endocr J. 2006;53:133–9. doi: 10.1507/endocrj.53.133. [DOI] [PubMed] [Google Scholar]
- 33.Lao XQ, Neil Thomas G, Jiang C, Zhang W, Adab P, et al. White blood cell count and the metabolic syndrome in older Chinese: the Guangzhou Biobank Cohort Study. Atherosclerosis. 2008;201:418–24. doi: 10.1016/j.atherosclerosis.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 34.Lohsoonthorn V, Dhanamun B, Williams MA. Prevalence of metabolic syndrome and its relationship to white blood cell count in a population of Thai men and women receiving routine health examinations. Am J Hypertens. 2006;19:339–45. doi: 10.1016/j.amjhyper.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Marques-Vidal P, Mazoyer E, Bongard V, Gourdy P, Ruidavets JB, et al. Prevalence of insulin resistance syndrome in southwestern France and its relationship with inflammatory and hemostatic markers. Diabetes Care. 2002;25:1371–7. doi: 10.2337/diacare.25.8.1371. [DOI] [PubMed] [Google Scholar]
- 36.Muscari A, Antonelli S, Bianchi G, Cavrini G, Dapporto S, et al. Serum C3 is a stronger inflammatory marker of insulin resistance than C-reactive protein, leukocyte count, and erythrocyte sedimentation rate: comparison study in an elderly population. Diabetes Care. 2007;30:2362–8. doi: 10.2337/dc07-0637. [DOI] [PubMed] [Google Scholar]
- 37.Nagasawa N, Tamakoshi K, Yatsuya H, Hori Y, Ishikawa M, et al. Association of white blood cell count and clustered components of metabolic syndrome in Japanese men. Circ J. 2004;68:892–7. doi: 10.1253/circj.68.892. [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi N, Sato M, Shirai K, Nakajima K, Murakami S, et al. Associations between white blood cell count and features of the metabolic syndrome in Japanese male office workers. Ind Health. 2002;40:273–7. doi: 10.2486/indhealth.40.273. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson G, Hedberg P, Jonason T, Lonnberg I, Tenerz A, et al. White blood cell counts associate more strongly to the metabolic syndrome in 75-year-old women than in men: a population based study. Metab Syndr Relat Disord. 2007;5:359–64. doi: 10.1089/met.2007.0012. [DOI] [PubMed] [Google Scholar]
- 40.Temelkova-Kurktschiev T, Siegert G, Bergmann S, Henkel E, Koehler C, et al. Subclinical inflammation is strongly related to insulin resistance but not to impaired insulin secretion in a high risk population for diabetes. Metabolism. 2002;51:743–9. doi: 10.1053/meta.2002.32804. [DOI] [PubMed] [Google Scholar]
- 41.Tian JY, Yang Y, Cheng Q, Huang HE, Li R, et al. Association of WBC count and glucose metabolism among Chinese population aged 40 years and over. Diabetes Res Clin Pract. 2008;82:132–8. doi: 10.1016/j.diabres.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Wang YY, Lin SY, Liu PH, Cheung BM, Lai WA. Association between hematological parameters and metabolic syndrome components in a Chinese population. J Diabetes Complications. 2004;18:322–7. doi: 10.1016/S1056-8727(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 43.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, et al. The metabolic syndrome and insulin resistance: relationship to haemostatic and inflammatory markers in older non-diabetic men. Atherosclerosis. 2005;181:101–8. doi: 10.1016/j.atherosclerosis.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 44.Yajnik CS, Joglekar CV, Lubree HG, Rege SS, Naik SS, et al. Adiposity, inflammation and hyperglycaemia in rural and urban Indian men: Coronary Risk of Insulin Sensitivity in Indian Subjects (CRISIS) Study. Diabetologia. 2008;51:39–46. doi: 10.1007/s00125-007-0847-1. [DOI] [PubMed] [Google Scholar]
- 45.McCarthy DA, Dale MM. The leucocytosis of exercise. A review and model. Sports Med. 1988;6:333–63. doi: 10.2165/00007256-198806060-00002. [DOI] [PubMed] [Google Scholar]
- 46.Natale VM, Brenner IK, Moldoveanu AI, Vasiliou P, Shek P, et al. Effects of three different types of exercise on blood leukocyte count during and following exercise. Sao Paulo Med J. 2003;121:9–14. doi: 10.1590/S1516-31802003000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pannacciulli N, Giorgino F, Martina RA, Resta O, Giorgino R, et al. Effect of family history of type 2 diabetes on white blood cell count in adult women. Obes Res. 2003;11:1232–7. doi: 10.1038/oby.2003.169. [DOI] [PubMed] [Google Scholar]
- 48.Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, et al. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. Eur J Epidemiol. 2007;22:55–66. doi: 10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- 49.Duncan BB, Schmidt MI, Chambless LE, Folsom AR, Carpenter M, et al. Fibrinogen, other putative markers of inflammation, and weight gain in middle-aged adults—the ARIC study. Atherosclerosis Risk in Communities. Obes Res. 2000;8:279–86. doi: 10.1038/oby.2000.33. [DOI] [PubMed] [Google Scholar]
- 50.Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, Smoking Cessation, and Risk for Type 2 Diabetes Mellitus. Annals of Internal Medicine. 2010;152:10–17. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Furth R, Diesselhoff-den Dulk MMC, Mattie H. QUANTITATIVE STUDY ON THE PRODUCTION AND KINETICS OF MONONUCLEAR PHAGOCYTES DURING AN ACUTE INFLAMMATORY REACTION. The Journal of Experimental Medicine. 1973;138:1314–1330. doi: 10.1084/jem.138.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–9. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- 53.Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, et al. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86:3257–65. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 54.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Dandona P, Aljada A, Chaudhuri A, Mohanty P. Endothelial dysfunction, inflammation and diabetes. Rev Endocr Metab Disord. 2004;5:189–97. doi: 10.1023/B:REMD.0000032407.88070.0a. [DOI] [PubMed] [Google Scholar]
- 56.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 57.Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, et al. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106:679–84. doi: 10.1161/01.cir.0000025403.20953.23. [DOI] [PubMed] [Google Scholar]
- 58.Van Wijk JP, Cabezas MC, Coll B, Joven J, Rabelink TJ, et al. Effects of rosiglitazone on postprandial leukocytes and cytokines in type 2 diabetes. Atherosclerosis. 2006;186:152–9. doi: 10.1016/j.atherosclerosis.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Hanefeld M, Schaper F, Koehler C, Bergmann S, Ugocsai P, et al. Effect of acarbose on postmeal mononuclear blood cell response in patients with early type 2 diabetes: the AI(I)DA study. Horm Metab Res. 2009;41:132–6. doi: 10.1055/s-0028-1119407. [DOI] [PubMed] [Google Scholar]
- 60.Kim JA, Park HS. White blood cell count and abdominal fat distribution in female obese adolescents. Metabolism. 2008;57:1375–9. doi: 10.1016/j.metabol.2008.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the studies of association between WBC and T2D included in the meta-analysis
(0.09 MB RTF)
PRISMA Checklist of items to include when reporting a systematic review or meta-analysis (diagnostic review consisting of cohort studies)
(0.07 MB DOC)