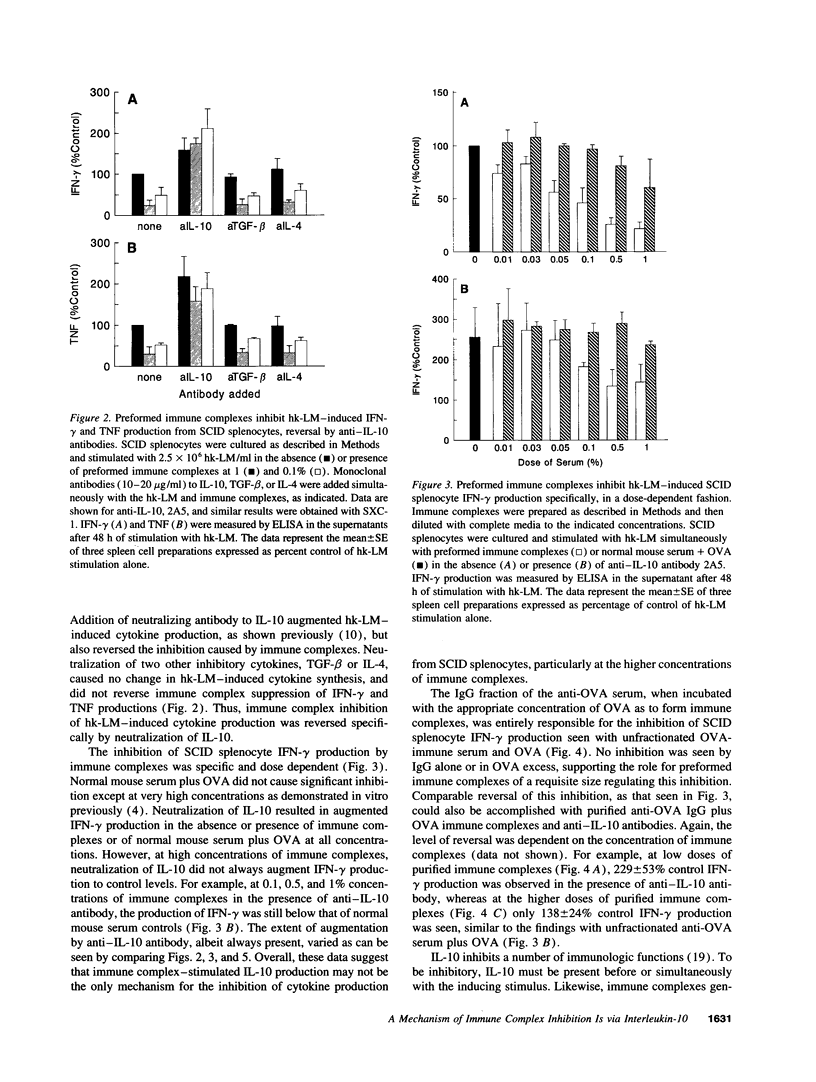
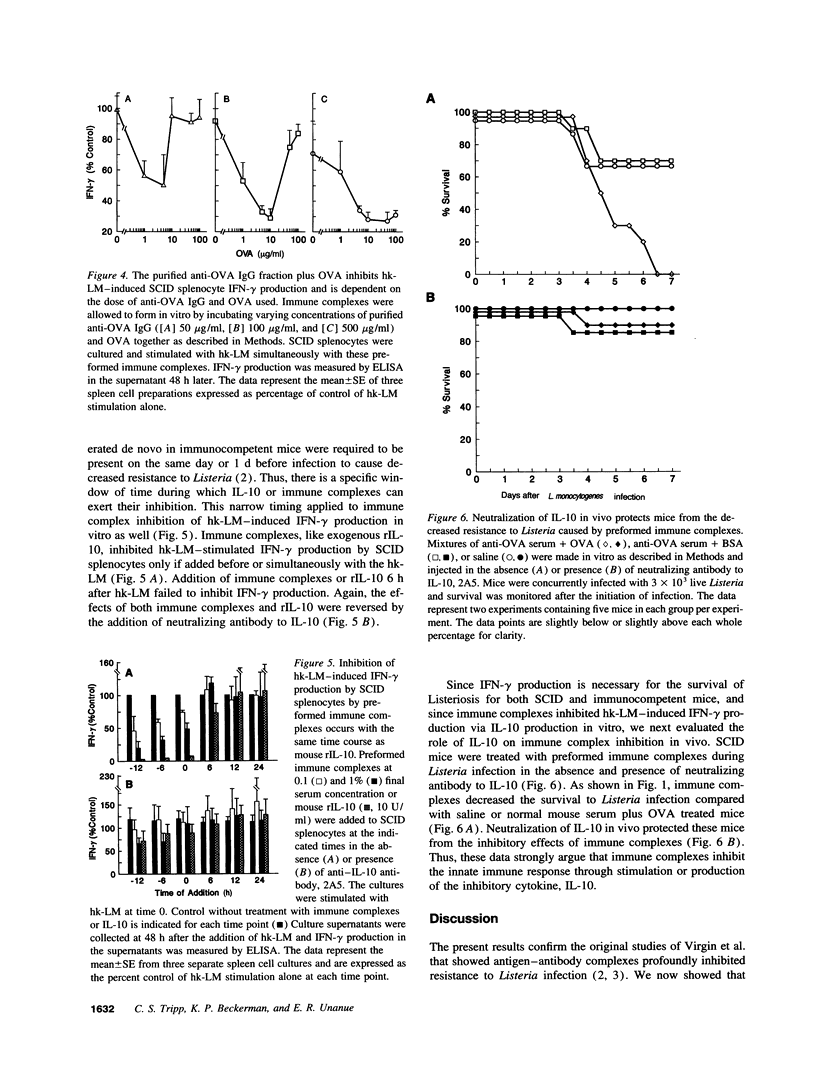
Abstract
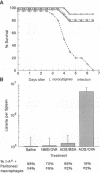
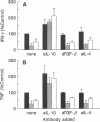
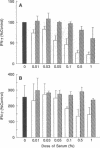
The presence of soluble antigen-antibody complexes renders mice highly susceptible to infection with the intracellular pathogen Listeria monocytogenes. In this report we show that this inhibition is manifest at the level of the innate immune response and is mediated by IL-10. Like immuno-competent mice, mice with the severe combined immunodeficient mutation (SCID) injected with immune complexes died from a sublethal dose of L. monocytogenes. These mice were protected if pretreated with neutralizing antibodies to IL-10. In vitro, immune complexes stimulated IL-10 production by SCID splenocytes and splenic macrophages. Likewise, immune complexes inhibited TNF and IFN-gamma production by SCID splenocytes cultured with heat-killed-L. monocytogenes. This inhibition was reversed by neutralization of IL-10 but not IL-4 or TGF-beta. Immune complexes and rIL-10 inhibited cytokine production by SCID splenocytes if added before or simultaneously with heat-killed-L. monocytogenes. These data support a model in which immune complexes modulate host defense and the immune response by stimulating the production of IL-10 from macrophages.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Bosma M. J., Bosma G. C., Unanue E. R. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell-independent mechanism. J Immunol. 1986 Jul 1;137(1):4–9. [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Unanue E. R. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991 Dec;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Sheehan K. C., Schreiber R. D., Unanue E. R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989 Jul 1;143(1):127–130. [PubMed] [Google Scholar]
- Boonpucknavig S., Wongsawang S., Boonpucknavig V., Bhamarapravati N. Serum-soluble malarial antigens and immune complex nephritis in Plasmodium berghei berghei infected mice. J Trop Med Hyg. 1976 Jun;79(6):116–119. [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A., Aste-Amezaga M., Valiante N. M., Ma X., Kubin M., Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993 Sep 1;178(3):1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drutz D. J., Gutman R. A. Renal manifestations of leprosy: glomerulonephritis, a complication of erythema nodosum leprosum. Am J Trop Med Hyg. 1973 Jul;22(4):496–502. doi: 10.4269/ajtmh.1973.22.496. [DOI] [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991 Sep;59(9):2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza I., Green R., Schreiber R. D. Inhibition of macrophage tumoricidal activity by immune complexes and altered erythrocytes. J Immunol. 1983 Nov;131(5):2117–2121. [PubMed] [Google Scholar]
- Ezekowitz R. A., Austyn J., Stahl P. D., Gordon S. Surface properties of bacillus Calmette-Guérin-activated mouse macrophages. Reduced expression of mannose-specific endocytosis, Fc receptors, and antigen F4/80 accompanies induction of Ia. J Exp Med. 1981 Jul 1;154(1):60–76. doi: 10.1084/jem.154.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Flores Villanueva P. O., Chikunguwo S. M., Harris T. S., Stadecker M. J. Role of IL-10 on antigen-presenting cell function for schistosomal egg-specific monoclonal T helper cell responses in vitro and in vivo. J Immunol. 1993 Sep 15;151(6):3192–3198. [PubMed] [Google Scholar]
- Garb K. S., Stavitsky A. B., Olds G. R., Tracy J. W., Mahmoud A. A. Immune regulation in murine schistosomiasis japonica: inhibition of in vitro antigen- and mitogen-induced cellular responses by splenocyte culture supernatants and by purified fractions from serum of chronically infected mice. J Immunol. 1982 Dec;129(6):2752–2758. [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., O'Garra A., Murphy K. M. Pathogen-induced Th1 phenotype development in CD4+ alpha beta-TCR transgenic T cells is macrophage dependent. Int Immunol. 1993 Apr;5(4):371–382. doi: 10.1093/intimm/5.4.371. [DOI] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- June C. H., Contreras C. E., Perrin L. H., Lambert P. H., Miescher P. A. Circulating and tissue-bound immune complex formation in murine malaria. J Immunol. 1979 Jun;122(6):2154–2161. [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- Kullberg M. C., Pearce E. J., Hieny S. E., Sher A., Berzofsky J. A. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J Immunol. 1992 May 15;148(10):3264–3270. [PubMed] [Google Scholar]
- Moore K. W., O'Garra A., de Waal Malefyt R., Vieira P., Mosmann T. R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Moran C. J., Ryder G., Turk J. L., Waters M. F. Evidence for circulating immune complexes in lepromatous leprosy. Lancet. 1972 Sep 16;2(7777):572–573. doi: 10.1016/s0140-6736(72)91962-9. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Fiorentino D. F., Leverah J., Moore K. W., Bond M. W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990 Nov 1;145(9):2938–2945. [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Street N. F., Budd R., O'Garra A., Fong T. A., Bond M. W., Moore K. W., Sher A., Fiorentino D. F. Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol Rev. 1991 Oct;123:209–229. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., Plotz P. H. The role of autoantibodies in autoimmune disease. Annu Rev Immunol. 1993;11:79–104. doi: 10.1146/annurev.iy.11.040193.000455. [DOI] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Olveda R., Tracy J. W., Mahmoud A. A. Adoptive transfer of modulation of granuloma formation and hepatosplenic disease in murine schistosomiasis japonica by serum from chronically infected animals. J Immunol. 1982 Mar;128(3):1391–1393. [PubMed] [Google Scholar]
- Pedersen B. K., Thomsen B. S., Nielsen H. Inhibition of natural killer cell activity by antigen-antibody complexes. Allergy. 1986 Nov;41(8):568–574. doi: 10.1111/j.1398-9995.1986.tb00348.x. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Kinet J. P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- Ridley M. J., Ridley D. S. The immunopathology of erythema nodosum leprosum: the role of extravascular complexes. Lepr Rev. 1983 Jun;54(2):95–107. doi: 10.5935/0305-7518.19830015. [DOI] [PubMed] [Google Scholar]
- Rogers H. W., Sheehan K. C., Brunt L. M., Dower S. K., Unanue E. R., Schreiber R. D. Interleukin 1 participates in the development of anti-Listeria responses in normal and SCID mice. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1011–1015. doi: 10.1073/pnas.89.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Espinosa O., Mendez-Navarrete I., Estrada-Parra S. Presence of C1q-reactive immune complexes in patients with leprosy. Clin Exp Immunol. 1972 Oct;12(2):215–223. [PMC free article] [PubMed] [Google Scholar]
- Santoro F., Capron M., Joseph M., Rousseaux-Prevost R., Capron A. Circulating antigens and immune complexes in Schistosoma mansoni-infected rats. Characterization by radioimmunoprecipitation-PEG assay (RIPEGA). Clin Exp Immunol. 1978 Jun;32(3):435–442. [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Hicks L. J., Celada A., Buchmeier N. A., Gray P. W. Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity. J Immunol. 1985 Mar;134(3):1609–1618. [PubMed] [Google Scholar]
- Sheehan K. C., Ruddle N. H., Schreiber R. D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989 Jun 1;142(11):3884–3893. [PubMed] [Google Scholar]
- Sher A., Fiorentino D., Caspar P., Pearce E., Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991 Oct 15;147(8):2713–2716. [PubMed] [Google Scholar]
- Sher A., Gazzinelli R. T., Oswald I. P., Clerici M., Kullberg M., Pearce E. J., Berzofsky J. A., Mosmann T. R., James S. L., Morse H. C., 3rd Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992 Jun;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Stevens W. J., Feldmeir H., Bridts C. H., Daffalla A. A. IgG and IgE circulating immune complexes, total serum IgE and parasite related IgE in patients with mono- or mixed infection with Schistosoma mansoni and/or S. haematobium. Influence of therapy. Clin Exp Immunol. 1983 Apr;52(1):144–152. [PMC free article] [PubMed] [Google Scholar]
- Tripp C. S., Gately M. K., Hakimi J., Ling P., Unanue E. R. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-gamma. J Immunol. 1994 Feb 15;152(4):1883–1887. [PubMed] [Google Scholar]
- Tripp C. S., Wolf S. F., Unanue E. R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Kurt-Jones E. A., Wittenberg G. F., Unanue E. R. Immune complex effects on murine macrophages. II. Immune complex effects on activated macrophages cytotoxicity, membrane IL 1, and antigen presentation. J Immunol. 1985 Dec;135(6):3744–3749. [PubMed] [Google Scholar]
- Virgin H. W., 4th, Unanue E. R. Suppression of the immune response to Listeria monocytogenes. I. Immune complexes inhibit resistance. J Immunol. 1984 Jul;133(1):104–109. [PubMed] [Google Scholar]
- Virgin H. W., 4th, Wittenberg G. F., Bancroft G. J., Unanue E. R. Suppression of immune response to Listeria monocytogenes: mechanism(s) of immune complex suppression. Infect Immun. 1985 Nov;50(2):343–353. doi: 10.1128/iai.50.2.343-353.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Wittenberg G. F., Unanue E. R. Immune complex effects on murine macrophages. I. Immune complexes suppress interferon-gamma induction of Ia expression. J Immunol. 1985 Dec;135(6):3735–3743. [PubMed] [Google Scholar]
- Wherry J. C., Schreiber R. D., Unanue E. R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991 May;59(5):1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde A. A., de Waal Malefijt R., Huijbens R. J., de Vries J. E., Figdor C. G. IL-10 stimulates monocyte Fc gamma R surface expression and cytotoxic activity. Distinct regulation of antibody-dependent cellular cytotoxicity by IFN-gamma, IL-4, and IL-10. J Immunol. 1992 Dec 15;149(12):4048–4052. [PubMed] [Google Scholar]