Abstract
In order to develop the most effective Th1 immunity, naïve CD4+ T cells must acquire the capacity to induce the expression of IFN-γ and to silence Th2 cytokine-producing potential. Although the IFN-γ-STAT1 and the IL-12-STAT4 pathways have been demonstrated to be important in inducing the IFN-γ-producing capacity in Th1 cells, their respective roles in silencing the IL-4-producing potential in Th1 cells remain unclear. In this study, we investigated the role of the IFN-γ and the IL-12 pathways in silencing the IL-4-producing potential in Th1 cells. We found that IFN-γ was essential to silence the IL-4-producing potential in Th1 cells, while IL-12 only partially suppressed the IL-4-producing potential. IFN-γ depended on STAT1 and IL-12 depended on STAT4 to suppress the IL-4-producing potential. We showed that the IL-12-STAT4 pathway and the IFN-γ-STAT1 pathway converge at the point of T-bet. Our study demonstrates that the IFN-γ-STAT1-T-bet signaling pathway is the major pathway that leads to silencing the IL-4-producing potential of Th1 cells.
Introduction
The present paradigm of Th1-cell differentiation emphasizes the significance of IL-12 as a key regulator (Hsieh and others 1993; Neurath and others 2002; Seder and Paul 1994). IL-12 mediates its function by binding to the β1 and β2 chains of the IL-12 receptors (IL-12Rβ2) (Chua and others 1994; Presky and others 1996). The binding of IL-12 to its receptors activates Jak2 and Tyk2, which leads to STAT4 activation (Bacon and others 1995a, 1995b; Jacobson and others 1995). Activated STAT4 can induce IFN-γ expression (Xu and others 1996), the hallmark cytokine of Th1 cells. Recently, a T-box family transcription factor, called T-bet, has emerged as a master transcription factor in Th1-cell development (Szabo and others 2000, 2003). T-bet has been reported to either suppress the induction of GATA3 or to compete with GATA3 for DNA binding (Hwang and others 2005; Usui and others 2006). Furthermore, an Il4 silencer region in the mouse Il4 gene has been uncovered (Agarwal and Rao 1998; Lee and others 2001). It has also been shown that T-bet and Runx3 are recruited into this silencer region to silence the Il4 gene in developing Th1 cells (Djuretic and others 2007).
While the pathways that promote Th1-cell differentiation have been elucidated, the pathways that lead to Th1-cell commitment (i.e., silencing of the Il4 gene in Th1 cells) remain obscure. The pathways that lead to silencing of the Il4 gene are important in developing Th1 immunity because failure to silence the IL-4-producing potential in Th1 cells has been shown to result in ineffective protection against Leishmania major infection (Ansel and others 2004). In the literature, the conclusion that IL-12 is essential for Th1-cell lineage commitment was based on experiments in which the presence of IL-12 and anti-IL-4 antibodies were used in Th1-inducing conditions (Hsieh and others 1993; Seder and Paul 1994). Under traditional Th1-inducing conditions, the differentiating CD4+ T cells, as a result of the IL-12 treatment, could produce a small amount of IFN-γ, which may contribute to the silencing of the IL-4-producing potential. Nonetheless, such a possibility has not yet been formally tested.
In this study, we used two redefined Th1-inducing conditions to test the role of IFN-γ versus the role of IL-12 in silencing the Il4 gene in Th1 cells. We showed that IFN-γ contributes more to the silenced Il4 gene state than IL-12 and that IFN-γ is more potent than IL-12 in inducing T-bet and Runx3 mRNA expression. IFN-γ completely depended on STAT1 signaling and partially depended on T-bet to silence the IL-4-producing potential. In contrast, IL-12 depended on T-bet to partially suppress the IL-4-producing potential.
Materials and Methods
Animals and cell cultures
C57BL/6 mice, BALB/c mice, and STAT4−/− mice on BALB/c background (Kaplan and others 1996) were purchased from The Jackson Laboratory (Bar Harbor, ME). STAT1−/− mice on the 129 background (Meraz and others 1996) were purchased from Taconic Inc (Hudson, NY) and backcrossed to C57BL/6 background mice (three generations). T-bet−/− mice on C57BL/6 background (Szabo and others 2002) were purchased from The Jackson Laboratory and maintained in the animal facility of National Jewish Medical and Research Center (Denver, CO). Naïve CD4+ T cells were purified from the spleen and lymph nodes with the CD4+ CD62L+ T Cell Isolation Kit (Miltenyi Biotec Inc.). Purified naïve CD4+ T cells (0.2 × 106 cells) were stimulated with irradiated T-cell-depleted APC (1 × 106 cells) in 2 mL of complete RPMI medium supplemented with IL-2 (30 U/mL), anti-CD3 (2C11, 3 μg/mL) antibody, and anti-CD28 antibody (3 μg/mL) for 3–11 days. For Th1-cell priming using the IL-12 combinations, IL-12 (10 ng/mL, BD), anti-IL-4 antibody (11B11, 10 μg/mL), and anti-IFN-γ antibody (XMG, 10 μg/mL) were added (referred to as the IL-12 conditions); for Th1-cell priming using the IFN-γ combinations, IFN-γ (20 ng/mL, BD), anti-IL-4 antibody (10 μg/mL), and anti-IL-12 antibody (C17.8, 10 μg/mL) were used (referred to as the IFN-γ conditions); and for Th2-cell priming, IL-4 (5 ng/mL, BD), anti-IL-12 antibody (10 μg/mL), and anti-IFN-γ antibody (10 μg/mL) were added (referred to as the Th2-inducing conditions). In the control group, anti-IL-4 (10 μg/mL), anti-IL-12 (10 μg/mL), and anti-IFN-γ (10 μg/mL) antibodies were added (referred to as the neutralized conditions). All animal protocols were approved by the Institutional Animal Care and Users Committees of Loyola University Chicago and National Jewish Medical and Research Center.
Intracellular staining (ICS)
Primed cells were stimulated with PMA and ionomycin in the presence of 2 μM of monensin (Calbiochem). After 6 h of stimulation, cells were fixed with 4% paraformaldehyde and stained with APC-labeled anti-CD4, FITC-labeled anti-IFN-γ, and PE-labeled anti-IL-4 monoclonal antibodies (BD), as described previously (Zhang and others 2001). Samples were collected using FACScan or FACScalibur (BD) and analyzed with FlowJo software (Tree Star Inc). A CD4+ gate was used for analyzing all FACS data and dead cells were excluded by low Forward Scatter.
ELISA
At the end of cultures, T cells were washed and stimulated with PMA (50 ng/mL) and ionomycin (1 μm) at a concentration of 106 cells/mL of complete medium overnight. The IL-4 protein concentration in the supernatants was measured by using commercial ELISA detection kits (BD PharMingen).
Real-time PCR
Total RNA was isolated with STAT60 (Tel-test) and cDNA was synthesized by reverse transcription. The amounts of T-bet and Runx3 mRNA were quantified by real-time PCR using specific primers and SYBR® Green PCR Master Mix (Applied Biosystems). The amount of mRNA was expressed as an amount relative to that of a housekeeping gene, Hprt1. 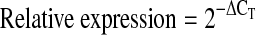 , where
, where 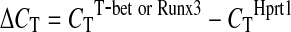 . The primers used were as follows: T-bet forward: 5′-CCTGTTGTGGTCCAAGTT-3′, T-bet reverse, 5′-TTTCCACACTGCACCCACTT-3′; Runx3 forward, 5′-ATCCATGCCCATCAAACCAA-3′, Runx3 reverse, 5′-GGTAAGTTAGGACTGATCAG-3′; Hprt1 forward, 5′-CTCATGGACTGATTATGGACAGGAC-3′, and Hprt1 reverse, 5′-GCAGGTCAGCAAAGA ACTTATAGCC-3′ (Marques and others 2006).
. The primers used were as follows: T-bet forward: 5′-CCTGTTGTGGTCCAAGTT-3′, T-bet reverse, 5′-TTTCCACACTGCACCCACTT-3′; Runx3 forward, 5′-ATCCATGCCCATCAAACCAA-3′, Runx3 reverse, 5′-GGTAAGTTAGGACTGATCAG-3′; Hprt1 forward, 5′-CTCATGGACTGATTATGGACAGGAC-3′, and Hprt1 reverse, 5′-GCAGGTCAGCAAAGA ACTTATAGCC-3′ (Marques and others 2006).
Statistical analysis
All of the error bars in this report represent the standard deviation. The difference between two samples was analyzed with Student's t-test.
Results
The roles of IFN-γ and IL-12 in silencing the IL-4-producing potential in Th1 cells
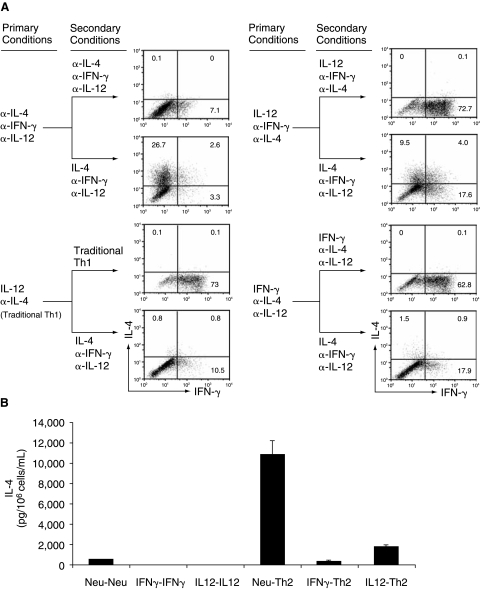
Under traditional Th1-inducing conditions (without the addition of anti-IFN-γ antibody), the IL-12-primed CD4+ T cells could produce IFN-γ, which then might silence the IL-4-producing potential through an auto-feedback mechanism. To clarify the role of IL-12 and IFN-γ in silencing the Il4 gene, we primed naïve CD4+ T cells with the IL-12 conditions (addition of IL-12, anti-IL-4 antibody, and anti-IFN-γ antibody) or the IFN-γ conditions (addition of IFN-γ, anti-IL-4 antibody, and anti-IL-12 antibody) for 11 days. We then reprimed the resultant cells under the original conditions or under Th2-inducing conditions for 5 days to test whether the IL-4-producing potential was suppressed or silenced. We defined the suppression percentage mediated by IFN-γ or IL-12 as the following formula: Suppression (%) = (Percentage of IL-4 positive in the “neutralized→Th2” group)−(Percentage of IL-4 positive in the “IFN-γ or IL-12→Th2” group)/(Percentage of IL-4 positive in the “neutralized→Th2”group) × 100%. We found that nearly half of the IL-12-primed CD4+ T cells could differentiate into IL-4-producing cells when Th2-inducing conditions were provided, while only <5% of the IFN-γ-primed Th1 cells retained the potential to differentiate into IL-4-producing cells (Fig. 1A and Table 1). In contrast, when cultured in the presence of anti-IL-4, anti-IL-12, and anti-IFN-γ antibodies, CD4+ T cells retained the full potential (comparable to that of naïve CD4+ T cells) to differentiate into IL-4-producing cells (Table 1). ELISA measurements confirmed the results obtained from using intracellular staining (ICS), but also indicated that IL-12 is a more potent factor in suppressing the IL-4-producing potential. The IL-12-treated Th1 cells were found to produce only 14% as much IL-4 compared with CD4+ T cells that were cultured under the neutralized conditions (Fig. 1B). The ELISA measurements indicated an 86% suppression of IL-4 production, whereas ICS showed only a 54% suppression. The difference in these measurements might be attributable to the inherent nature of the two techniques as ICS measures the percentage of cells that are able to produce IL-4 while ELISA measures the amount of IL-4 produced by cells that are capable of producing it. Regardless, these results demonstrate that IFN-γ is the major factor in silencing the IL-4-producing potential in Th1 cells and that IL-12 is a secondary contributor in this process.
FIG. 1.
The roles of IFN-γ and IL-12 in silencing the IL-4-producing potential in Th1 cells. (A) Naïve CD4+ T cells from C57BL/6 mice were stimulated under the neutralized conditions, traditional Th1 conditions, IL-12 conditions, or IFN-γ conditions. On day 11, the primed cells were reprimed under the indicated secondary conditions for 5 days. The resultant cells were stimulated with PMA and ionomycin (P&I) and analyzed for IL-4 and IFN-γ protein by ICS. (B) The resultant cells were stimulated with P&I overnight at a concentration of 106 cells in 1 mL of complete medium. IL-4 protein in the supernatants was measured by ELISA. These data are representative of four independent experiments with similar results.
Table 1.
Analysis of IL-4-Producing Potential
| |
IL-4 positive (%)a |
Suppression (%)b |
||||
|---|---|---|---|---|---|---|
| Mouse strain | Naïve→Th2c | Neutralized→Th2 | IFN-γ→Th2 | IL-12→Th2 | IFN-γ-mediated | IL-12-mediated |
| WT | 17.7 ± 8.5d | 17.0 ± 4.5 | 0.8 ± 0.5e | 7.8 ± 1.2f | 95.3 | 54.1 |
| STAT1−/− | 17.2 ± 4.5 | 17.8 ± 4.3 | 8.7 ± 3.0 | 0.2 | 51.0 | |
| STAT4−/− | 13.1 ± 1.5 | 1.3 ± 0.6 | 12.7 ± 1.4 | 90.0 | 3.0 | |
| T-bet−/− | 32.0 ± 9.1 | 10.8 ± 2.3g | 27.8 ± 6.0 | 66.3 | 13.1 | |
IL-4 positive (%) indicates the percentage of IL-4 positive and IFN-γ negative cells within CD4+ cells.
Suppression (%) = (Percentage of IL-4 positive in the “Neutralized→Th2” group) − (Percentage of IL-4 positive in the “IFN-γ or IL-12→Th2” group)/(Percentage of IL-4 positive in the “Neutralized→Th2”group) × 100%.
“Naïve→Th2” indicates that naïve CD4+ T cells were primed under Th2-inducing conditions for 5 days; “Neutralized→Th2,” “IFN-γ→Th2,” or “IL-12→Th2” indicates that naïve CD4+ T cells were cultured in the indicated primary conditions for 11 days, then switched to the indicated secondary conditions for 5 days.
Mean ± SD derived from at least three independent experiments with number of mice used ranging from 3 to 9.
Value of P < 0.001 compared with “Neutralized→Th2” group (WT).
Value of P < 0.001 compared with “Neutralized→Th2” group (WT).
Value of P < 0.05 compared with “Neutralized→Th2” group (T-bet−/−).
Because the IFN-γ-mediated silencing effect may be affected by genetic background (Seder and others 1993; Darnell and others 1994; Bradley and others 1996; Wenner and others 1996), we further examined the IFN-γ effect on silencing the IL-4-producing potential in Th1 cells derived from BALB/c mice. We showed that the IFN-γ-primed CD4+ T cells also failed to differentiate into IL-4-producing cells (data not shown). These data indicate that IFN-γ can exert a similar silencing effect on the IL-4-producing potential in Th1 cells in the context of the C57BL/6 and the BALB/c genetic background.
IFN-γ completely depends on STAT1 while IL-12 completely depends on STAT4 to silence/suppress the IL-4-producing potential in Th1 cells
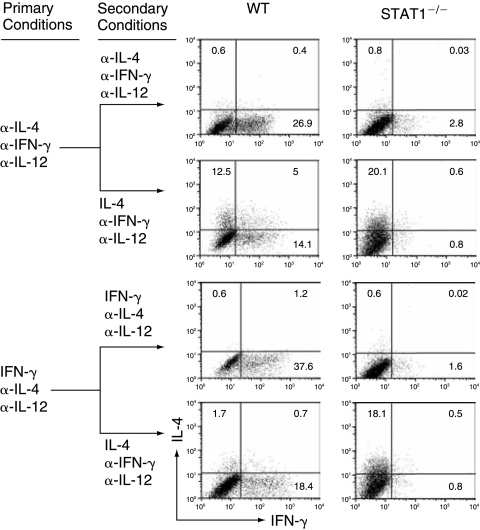
The IFN-γ-mediated effects have been reported to be either STAT1 dependent or STAT1 independent (Darnell and others 1994; Ramana and others 2002). To determine the role of STAT1 in the IFN-γ-mediated silencing of the IL-4-producing potential, we primed naïve CD4+ T cells, prepared from STAT1−/− (B6 × 129 background) and WT control mice (B6 × 129 background), with either the neutralized conditions or the IFN-γ conditions. The IFN-γ-treated STAT1−/− CD4+ T cells expressed IL-4 at levels similar to that of anti-IFN-γ-treated STAT1−/− CD4+ T cells (Fig. 2 and Table 1). Similar results were obtained using WT and STAT1−/− on the 129 background mice (data not shown). These results together suggest that IFN-γ completely depends on STAT1 to silence the IL-4-producing potential.
FIG. 2.
IFN-γ completely depends on STAT1 to silence the IL-4-producing potential in Th1 cells. Naïve CD4+ T cells from WT (129 × B6) or STAT1−/− (129 × B6) mice were stimulated under the neutralized conditions or under the IFN-γ combinations. On day 11, the primed cells were reprimed as indicated. IL-4 and IFN-γ expression was measured by ICS. The data are representative of three independent experiments with similar results.
Because STAT4 has been shown to be pivotal in many of IL-12-mediated functions (Kaplan and others 1996) and STAT1 has been shown to enhance IL-12 responsiveness by up-regulating the IL-12 receptor β2 chain (Mullen and others 2001; Afkarian and others 2002), we assessed the role of both STAT1 and STAT4 in IL-12-mediated suppression of the IL-4-producing potential. We showed that the IL-12-treated WT and STAT1−/− CD4+ T cells retained the reduced capacity to express IL-4 after switching to Th2-inducing conditions (~50% of both WT and STAT1−/− CD4+ treated with anti-IL-12 and anti-IFN-γ, Fig. 3A and Table 1). The IL-12-treated STAT4−/− CD4+ T cells expressed IL-4 at levels comparable to that of WT or STAT4−/− CD4+ T cells treated with anti-IL-12, anti-IFN-γ, and anti-IL-4 antibodies (Fig. 3B). These results document that IL-12 completely depends on STAT4 but does not depend on STAT1 to suppress the IL-4-producing potential in Th1 cells.
FIG. 3.
IL-12 completely depends on STAT4 and does not depend on STAT1 to partially suppress the IL-4-producing potential in Th1 cells. (A) Naïve CD4+ T cells from WT (129 × B6) and STAT1−/− (129 × B6) mice were primed and reprimed as indicated. IL-4 and IFN-γ expression was measured by ICS. (B) Naïve CD4+ T cells prepared from WT control (Balb/c) and STAT4−/− mice were primed and reprimed as indicated. IL-4 and IFN-γ expression was measured by ICS. These data are representative of three independent experiments with similar results.
Role of T-bet in IFN-γ- and IL-12-mediated silencing/suppression of the IL-4-producing potential in Th1 cells
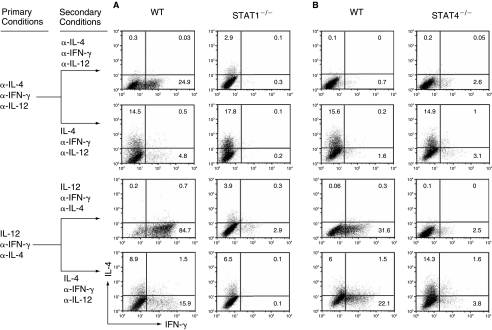
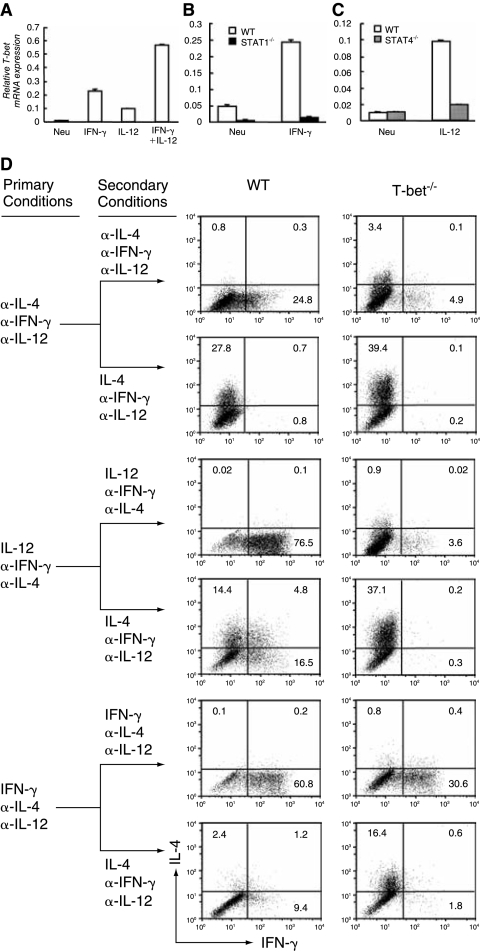
To determine the role of T-bet in both IFN-γ-mediated and IL-12-mediated silencing/suppression of the IL-4-producing potential, we first measured the induction of T-bet mRNA by IFN-γ and IL-12 in WT, STAT1−/−, and STAT4−/− CD4+ T cells. IFN-γ-induced T-bet mRNA expression increased 25-fold and such induction was completely dependent on STAT1 (Fig. 4A and B), while IL-12 completely depended on STAT4 to up-regulate T-bet mRNA expression by 10-fold (Fig. 4A and C). IFN-γ and IL-12 synergistically up-regulated levels of T-bet mRNA in T cells (Fig. 4A). Thus, we conclude that both IFN-γ and IL-12 are capable of inducing T-bet expression. Besides T-bet, Eomes, a paralog of T-bet, has also been shown to contribute to the regulation of IFN-γ production in CD4+ T cells (Pearce and others 2003; Suto and others 2006). To test the role of Eomes in both IFN-γ-mediated and IL-12-mediated silencing/suppression of the IL-4-producing potential, we quantified Eomes mRNA by real-time PCR and found that the Eomes was not up-regulated by IFN-γ alone, by IL-12 alone, or in combination (data not shown). Thus, it does not appear that Eomes plays a substantial role in IFN-γ- or IL-12-mediated suppression of the IL-4-producing potential in Th1 cells.
FIG. 4.
The role of T-bet in the IFN-γ-mediated and IL-12-mediated silencing/suppression of the IL-4-producing potential in Th1 cells. (A–C) Naïve CD4+ T cells from WT control, STAT1−/−, or STAT4−/− mice were stimulated with anti-CD3/anti-CD28 plus APC under the neutralized conditions, the IFN-γ conditions, the IL-12 conditions, or the combination of IFN-γ and IL-12 conditions. After 5 days of culture, total RNA was isolated from the resultant cells and used for cDNA synthesis. The amount of T-bet mRNA was quantified by real-time PCR and expressed as the amount relative to that of Hprt1. The error bars indicate the standard deviation of duplicate measurements. (D) Naïve CD4+ T cells from WT control and T-bet−/− mice were stimulated under the neutralized conditions, the IL-12 conditions, or the IFN-γ conditions before they were reprimed under the same conditions or switched to Th2-inducing conditions. IL-4 and IFN-γ proteins were measured by ICS. These data are representative of three independent experiments with similar results.
To definitively assess the role of T-bet in IFN-γ-mediated and in IL-12-mediated silencing/suppression of the IL-4-producing potential, we primed naïve CD4+ T cells from WT and T-bet−/− mice with the neutralized conditions, the IFN-γ-priming conditions, or the IL-12-priming conditions for 11 days, and then tested the commitment status of the primed cells by providing them with Th2-inducing conditions. The resulting cells were stimulated with PMA and ionomycin. We found that in the absence of T-bet, the IL-12-primed CD4+ T cells fully retained their potential to develop into IL-4-producing cells, whereas the IFN-γ-primed CD4+ T cells retained only 34% of their potential to differentiate into IL-4-producing cells (Fig. 4D, Table 1). Similar results were obtained when the resultant cells were stimulated by anti-CD3 and anti-CD28 antibodies plus irradiated APC or by immobilized anti-CD3 and anti-CD28 antibodies (data not shown). These results demonstrate that T-bet is required for IL-12 to silence the IL-4-producing potential in Th1 cells but only partially responsible for the IFN-γ-mediated silencing of the IL-4-producing potential in Th1 cells.
T-bet is required for the IL-12-induced, not the IFN-γ-induced, up-regulation of Runx3 mRNA expression
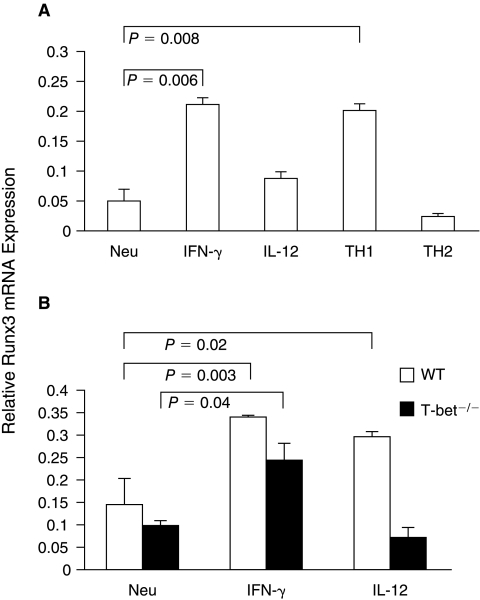
Recently, Runx3 has been reported to cooperate with T-bet to suppress Il4 gene transcription under Th2-inducing conditions (Djuretic and others 2007). To understand how Runx3 mRNA expression is regulated by IL-12 and IFN-γ, we analyzed Runx3 mRNA expression in CD4+ T cells treated with various conditions. We found that, compared to Runx3 mRNA expression in CD4+ T cells cultured under the neutralized conditions, Runx3 mRNA expression in CD4+ T cells treated with IFN-γ or IL-12 was up-regulated 4.2-fold (P < 0.05) or 1.8-fold, respectively (Fig. 5A). The IFN-γ-induced Runx3 mRNA expression (P < 0.05) was partially T-bet dependent, whereas the IL-12-induced Runx3 mRNA expression was mostly T-bet dependent (Fig. 5B).
FIG. 5.
IL-12 and IFN-γ up-regulate Runx3 mRNA expression in Th1 cells. (A) Naïve CD4+ T cells were prepared from C57BL/6 mice. They were used for RNA isolation or cultured under the neutralized conditions, IFN-γ conditions, IL-12 conditions, traditional Th1 or Th2 conditions. Runx3 mRNA was quantified by real-time PCR 5 days after priming. (B) Naïve CD4+ T cells from WT or T-bet−/− mice were cultured under the conditions indicated. Runx3 mRNA was quantified by real-time PCR. P values were analyzed by using Student's t-test. The data are representative of two or three independent experiments with similar results.
Discussion
How Th1 cells silence their Th2 cytokine-producing potential is an important issue in Th1 immunity. The roles of IL-12 and IFN-γ in silencing the IL-4-producing potential in Th1 cells remain unclear. Here we define “IL-4-producing potential in Th1 cells” as the ability to differentiate into Th2 cells when Th2-inducing conditions are provided. To address this problem, we used IFN-γ or IL-12 neutralizing antibodies and CD4+ T cells that are deficient in the key signaling molecule in the IFN-γ or IL-12 signaling pathway. This knockout approach allowed us to examine the role of IFN-γ or IL-12 in silencing/suppressing the IL-4-producing potential in the complete absence of STAT1, STAT4, or T-bet molecules. However, a deficiency of these molecules could affect Il4 gene expression indirectly due to developmental defects. For example, STAT1- or T-bet-deficient CD4+ T cells could show a higher tendency to differentiate into IL-4-producing cells or could even become precommitted to become IL-4-producing cells. This indirect effect, potentially mediated by developmental abnormalities using the knockout approach, was addressed in our study by using neutralizing antibodies.
In this report, the results obtained from both approaches are consistent. We found that the degree of suppression of the IL-4-producing potential in WT Th1 cells mediated by IL-12 plus anti-IFN-γ antibody was comparable to the degree of suppression observed in STAT1−/− Th1 cells mediated by IL-12. We also showed that activated WT CD4+ T cells, when cultured in the presence of anti-IL-12 and anti-IFN-γ antibodies, retained their full potential to differentiate into IL-4-producing cells, as did the STAT1−/− and T-bet−/− CD4+ T cells when cultured in the presence of anti-IL-12 antibody.
Committed Th1 cells do not produce IL-4 even when Th2-inducing conditions are provided. Previous work has demonstrated that repeated primings under Th1-inducing conditions are required for Th1 cells to become fully committed (Murphy and others 1996). The role of IFN-γ and IL-12 in silencing the IL-4-producing potential has not been distinguished thus far. Our findings that showed that the IFN-γ-STAT1-T-bet pathway is the major signaling pathway, which leads to silencing the IL-4-producing potential of Th1 cells, might provide an explanation for the previous observation: repeated primings stimulate IFN-γ production by first round-primed Th1 cells, which in turn effectively silences the Th2 cytokine-producing potential.
It is generally believed that IL-12 is critical in driving naïve CD4+ T cells to differentiate into IFN-γ-producing cells and in silencing the Il4 gene in committed Th1 cells (Hsieh and others 1993; Seder and Paul 1994; Neurath and others 2002), while IFN-γ is thought to up-regulate the expression of the IL-12Rβ2 chain and thus enhance IL-12 responsiveness (Hsieh and others 1993; Seder and Paul 1994; Neurath and others 2002). The direct effect of IFN-γ in Th1-cell development remains less clear. In this investigation, we used refined Th1-inducing conditions. We neutralized IL-12 when adding IFN-γ and neutralized IFN-γ when adding IL-12 to distinguish the exact effects mediated by IFN-γ and IL-12. Our work revealed that IFN-γ is the major factor in silencing the IL-4-producing potential. It acted directly to silence the IL-4-producing potential in the absence of IL-12 or STAT4 and mediated its silencing effect in the C57BL/6 strain as well as in the BALB/c strain. IL-12, on the other hand, only partially suppressed the IL-4-producing potential in Th1 cells. Our work reveals that the IL-12-STAT4 pathway and the IFN-γ-STAT1 pathway converge at the point of T-bet.
Published works that have focused on investigating the role of T-bet in suppressing the Il4 gene in Th2 cells have not come to an agreement. For instance, one study showed that T-bet overexpression achieved a 70% suppression of IL-4 expression (Szabo and others 2000), whereas other studies failed to observe any such suppression (Afkarian and others 2002). Recently, Rao and colleagues demonstrated that T-bet is not sufficient to suppress the Il4 gene in the presence of Th2-inducing factors; rather, it cooperates with Runx3 to silence the Il4 gene (Djuretic and others 2007). On the other hand, Naoe et al. reported that Cbfβ but not Runx3 is required for silencing the Il4 gene (Naoe and others 2007). Further investigation of whether T-bet or Runx 3 overexpression is sufficient to silence the IL-4-producing potential will enhance our understanding of Th1 commitment.
How T-bet can activate Ifng gene transcription and silence Il4 gene transcription in the same cell is not completely understood. The regulatory sequence(s) located within the Ifng gene and the Il4 gene might play deciding roles. For example, the regulatory region(s) of the Ifng gene and the Il4 gene could determine which cotranscription factor or transcription repressor would interact with T-bet. It has been reported that T-bet cooperates with Hlx to fully activate the Ifng gene (Mullen and others 2002) and that T-bet cooperates with Runx3 to silence the Il4 gene (Djuretic and others 2007). Although in vivo relevance of the present work needs further investigation, our study, which analyzed the signaling pathways that lead to silencing the IL-4-producing potential of Th1 cells, provides a connection between the external silencing stimuli and the silencing actions of T-bet that occur inside the nucleolus.
Acknowledgments
We thank Tom Startz for technical assistance and Leah Cho and J. D. Williams for manuscript assistance. We thank Dr. Manuel Diaz's support for Zan Huang's graduate study. This work is funded by NIH grants (RO1 AI48568 and RO1 AI068083) and a fund provided by National Jewish Medical and Research Center.
References
- Afkarian M. Sedy JR. Yang J. Jacobson NG. Cereb N. Yang SY. Murphy TL. Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- Agarwal S. Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- Ansel KM. Greenwald RJ. Agarwal S. Bassing CH. Monticelli S. Interlandi J. Djuretic IM. Lee DU. Sharpe AH. Alt FW. Rao A. Deletion of a conserved Il4 silencer impairs T helper type 1-mediated immunity. Nat Immunol. 2004;5:1251–1259. doi: 10.1038/ni1135. [DOI] [PubMed] [Google Scholar]
- Bacon CM. McVicar DW. Ortaldo JR. Rees RC. O'Shea JJ. Johnston JA. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinases by IL-2 and IL-12. J Exp Med. 1995a;181:399–404. doi: 10.1084/jem.181.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon CM. Petricoin EF., 3rd Ortaldo JR. Rees RC. Larner AC. Johnston JA. O'Shea JJ. Interleukin 12 induces tyrosine phosphorylation and activation of STAT4 in human lymphocytes. Proc Natl Acad Sci USA. 1995b;92:7307–7311. doi: 10.1073/pnas.92.16.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley LM. Dalton DK. Croft M. A direct role for IFN-gamma in regulation of Th1 cell development. J Immunol. 1996;157:1350–1358. [PubMed] [Google Scholar]
- Chua AO. Chizzonite R. Desai BB. Truitt TP. Nunes P. Minetti LJ. Warrier RR. Presky DH. Levine JF. Gately MK, et al. Expression cloning of a human IL-12 receptor component. A new member of the cytokine receptor superfamily with strong homology to gp130. J Immunol. 1994;153:128–136. [PubMed] [Google Scholar]
- Darnell JE., Jr Kerr IM. Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Djuretic IM. Levanon D. Negreanu V. Groner Y. Rao A. Ansel KM. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol. 2007;8:145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- Hsieh CS. Macatonia SE. Tripp CS. Wolf SF. O'Garra A. Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Hwang ES. Szabo SJ. Schwartzberg PL. Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- Jacobson NG. Szabo SJ. Weber-Nordt RM. Zhong Z. Schreiber RD. Darnell JE., Jr Murphy KM. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J Exp Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MH. Sun YL. Hoey T. Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- Lee GR. Fields PE. Flavell RA. Regulation of IL-4 gene expression by distal regulatory elements and GATA-3 at the chromatin level. Immunity. 2001;14:447–459. doi: 10.1016/s1074-7613(01)00125-x. [DOI] [PubMed] [Google Scholar]
- Marques VP. Goncalves GM. Feitoza CQ. Cenedeze MA. Fernandes Bertocchi AP. Damiao MJ. Pinheiro HS. Antunes Teixeira VP. dos Reis MA. Pacheco-Silva A. Saraiva Camara NO. Influence of TH1/TH2 switched immune response on renal ischemia-reperfusion injury. Nephron Exp Nephrol. 2006;104:e48–56. doi: 10.1159/000093676. [DOI] [PubMed] [Google Scholar]
- Meraz MA. White JM. Sheehan KC. Bach EA. Rodig SJ. Dighe AS. Kaplan DH. Riley JK. Greenlund AC. Campbell D. Carver-Moore K. DuBois RN. Clark R. Aguet M. Schreiber RD. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- Mullen AC. High FA. Hutchins AS. Lee HW. Villarino AV. Livingston DM. Kung AL. Cereb N. Yao TP. Yang SY. Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- Mullen AC. Hutchins AS. High FA. Lee HW. Sykes KJ. Chodosh LA. Reiner SL. Hlx is induced by and genetically interacts with T-bet to promote heritable T(H)1 gene induction. Nat Immunol. 2002;3:652–658. doi: 10.1038/ni807. [DOI] [PubMed] [Google Scholar]
- Murphy E. Shibuya K. Hosken N. Openshaw P. Maino V. Davis K. Murphy K. O'Garra A. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med. 1996;183:901–913. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naoe Y. Setoguchi R. Akiyama K. Muroi S. Kuroda M. Hatam F. Littman DR. Taniuchi I. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. J Exp Med. 2007;204:1749–1755. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath MF. Finotto S. Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med. 2002;8:567–573. doi: 10.1038/nm0602-567. [DOI] [PubMed] [Google Scholar]
- Pearce EL. Mullen AC. Martins GA. Krawczyk CM. Hutchins AS. Zediak VP. Banica M. DiCioccio CB. Gross DA. Mao CA. Shen H. Cereb N. Yang SY. Lindsten T. Rossant J. Hunter CA. Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- Presky DH. Yang H. Minetti LJ. Chua AO. Nabavi N. Wu CY. Gately MK. Gubler U. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramana CV. Gil MP. Schreiber RD. Stark GR. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 2002;23:96–101. doi: 10.1016/s1471-4906(01)02118-4. [DOI] [PubMed] [Google Scholar]
- Seder RA. Gazzinelli R. Sher A. Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder RA. Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- Suto A. Wurster AL. Reiner SL. Grusby MJ. IL-21 inhibits IFN-gamma production in developing Th1 cells through the repression of Eomesodermin expression. J Immunol. 2006;177:3721–3727. doi: 10.4049/jimmunol.177.6.3721. [DOI] [PubMed] [Google Scholar]
- Szabo SJ. Kim ST. Costa GL. Zhang X. Fathman CG. Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Szabo SJ. Sullivan BM. Peng SL. Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- Szabo SJ. Sullivan BM. Stemmann C. Satoskar AR. Sleckman BP. Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- Usui T. Preiss JC. Kanno Y. Yao ZJ. Bream JH. O'Shea JJ. Strober W. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med. 2006;203:755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner CA. Guler ML. Macatonia SE. O'Garra A. Murphy KM. Roles of IFN-gamma and IFN-alpha in IL-12-induced T helper cell-1 development. J Immunol. 1996;156:1442–1447. [PubMed] [Google Scholar]
- Xu X. Sun YL. Hoey T. Cooperative DNA binding and sequence-selective recognition conferred by the STAT aminoterminal domain. Science. 1996;273:794–797. doi: 10.1126/science.273.5276.794. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Apilado R. Coleman J. Ben-Sasson S. Tsang S. Hu-Li J. Paul WE. Huang H. Interferon gamma stabilizes the T helper cell type 1 phenotype. J Exp Med. 2001;194:165–172. doi: 10.1084/jem.194.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]