Abstract
Chronic infection with hepatitis C virus (HCV) is a major global health problem. One way HCV may evade the host immune response is by inhibiting the production of type I interferon (IFN). In addition, the standard treatment for chronic HCV infection involves treatment with IFN-α (or its pegylated derivative), alone or in combination with ribavirin. Therefore, it is believed that an important reason that most HCV-infected individuals progress from acute to chronic infection is due to a defect in the host response. In this study, we examined the host response to HCV infection in a cohort of patients enrolled in the UTHSC Cooperative HCV Research Center by determining levels of biologically active IFN in the sera of patients. We found that 15 of 35 enrolled HCV-infected patients show serum levels of IFN (ranging from 2 to 40 IU/mL) before initiation of therapy. Uninfected individuals do not have circulating levels of IFN. Basal IFN levels do not correlate with the clinical response to therapy, nor do they reflect the age, sex, or race of patients. These results suggest that the differential response of patients most likely reflects a defect in the later stages of the host innate immune response, such as the cellular response to endogenous or exogenous IFN. In contrast, the early stage of the host immune response in vivo of many HCV-infected patients (∼40%) is intact as determined by IFN production.
Introduction
Hepatitis C virus (HCV) is estimated to infect ∼200 million people worldwide, causing a spectrum of liver diseases that varies from the asymptomatic carrier state to end-stage liver disease. HCV-infected patients develop various liver diseases, including chronic hepatitis, cirrhosis, liver failure, and even hepatocellular carcinoma. Only a fraction of HCV-infected individuals spontaneously clear the viral infection, while the majority of HCV-infected individuals (70%–80%) develop a chronic infection. Hepatitis C virus is a member of the Flaviviridae family of enveloped, single-stranded, positive-sense RNA viruses. Several structural and nonstructural proteins of HCV have been shown to antagonize the host innate immune response that is normally triggered by viral infection (Hiscott and Lin 2006). Viral RNA is a potent inducer of the host immune response and is recognized by specific Toll-like receptors (TLRs) or by the cytoplasmic helicases retinoic-acid inducible gene I (RIG-I) and melanoma differentiation associated protein 5 (MDA5) (Meylan and others 2006). Rapid induction of type I interferons (IFNs), IFNα, IFNβ and IFNω, is a central event in establishing the host innate antiviral response that is downstream of TLR-dependent and -independent pathways. Interferon acts in a paracrine fashion to regulate gene expression that results in the induction of an antiviral state (Pfeffer and others 1998). HCV control of the innate antiviral response, especially at the level of type I IFN production, may provide a cellular foundation for viral persistence (Gale and Foy 2005).
The combination of IFNα or its pegylated derivative (peg-IFN) with the antiviral drug, ribavirin, is the current treatment of choice for HCV-infected patients (Reyes 2001). Interferon α has antiviral activity against a diverse variety of RNA and DNA viruses. When IFNα has been utilized alone as a monotherapy in chronically infected HCV patients, the success rate is ∼20%. Peg-IFNα, which has an improved half-life over standard IFNα, appears to have a somewhat higher success rate. However, it is unknown why IFNα therapy causes a sustained virological response in only a fraction of the patient population, as determined by the clearance of HCV. Moreover, several studies have identified specific cohorts of patients that have a relatively low response to these therapeutic regimens. For example, several studies established that the response rate of African Americans (AAs) is significantly lower than non-Hispanic whites (Castellino and others 2004; Fleckenstein 2004; Muir and others 2004). This finding is of major health concern in the United States, since AAs account for ∼22% of the HCV-infected patients.
In this report, we examined the initial host response to HCV infection in vivo. Serum samples were collected from patients infected with HCV genotype 1 prior to commencing combination IFN/ribavirin therapy at the UTHSC-Hepatitis C Cooperative Research Center. We found that IFN levels (ranging from 2 to 40 IU/mL) are detectable before the onset of therapy in ∼40% of the HCV-infected patients. Interferon could not be detected in the sera from healthy volunteers. Interferon levels did not correlate with the racial background, sex, or the ultimate clinical response of the patients to therapy. Moreover, although previous trials indicate that patients with exogenous IFN produce neutralizing antibodies against IFN, we were unable to detect the presence of neutralizing antibodies produced against IFN. These results suggest that HCV does not circumvent an early stage of the host innate antiviral response, that is, the production of type I IFNs, in HCV-infected individuals. Furthermore, our results suggest that the differential response of HCV-infected patients to IFN therapy may reflect differences at the level of IFN signaling and/or induction of IFN's biological actions.
Materials and Methods
Patient selection
Adult (AA) and Caucasian (C) patients with compensated chronic HCV who previously have not been treated with any form of IFN and/or ribavirin were enrolled and treated at the UTHSC General Clinical Research Center as part of our clinical trial entitled “Racial Differences in HCV-Host Interaction.” Patients were required to be adult, AA or C with genotype 1A or 1B chronic hepatitis C with positive HCV RNA and no prior attempt at treatment. A liver biopsy was required with the histological diagnosis of chronic hepatitis. Patients with histological diagnosis of cirrhosis were enrolled if they did not have symptomatic portal hypertension and if they had a neutrophil count > 1500 per mm3, platelet count of 85,000 per mm3, albumin level > 3.0 g per dL and serum creatinine < 1.4 mg per dL. All patients signed written informed consent specific for this protocol before entry into the study. Exclusion criteria included any cause of chronic liver disease other than HCV, human immunodeficiency virus (HIV) infection, active hemolytic anemia, evidence of decompensated cirrhosis with ascites, bleeding varices or portosystemic encephalopathy. In addition, patients with any known preexisting medical conditions that could interfere with participation such as uncontrolled seizure disorders, poorly controlled diabetes, serious pulmonary disease, immunologically mediated disease, gout or any medical condition likely to require steroids during the course of the study were excluded from this study. Patients with cardiac ischemia, significant arrhythmia, cardiac failure, active substance abuse, retinal abnormalities, organ transplantation, HIV infection, or serious psychiatric disease were also excluded.
Treatment regimen
All patients were treated with standard weight-based therapy with 1.5 μg per kg of pegylated IFNα2b subcutaneously once per week and 13 mg per kg of ribavirin PO daily for up to 48 weeks. Therapy was discontinued after 24 weeks if patients did not have a negative HCV RNA level. Use of erythropoietin was allowed.
Patients who completed at least 12 weeks of therapy were classified as Sustained Viral Responders (SVR) if they cleared virus on treatment and remained virus-free for 6 months after completion of therapy. Nonresponders (NR) were defined as patients who never cleared virus. Patients who initially had a negative HCV RNA level upon treatment but subsequently became positive again for HCV RNA (either during continued treatment or within 6 months after completing therapy) were defined as Relapsers (R).
Patient demographics
Table 1 presents the results from the initial 35 patients enrolled in the study. Twenty-one were self-identified as AA and 14 as C. Twenty-two were female (10 C, 12 AA) and 13 were male (4 C, 9 AA). Age range was from 20 to 67 years. Thirty-four were either genotype 1A or 1B. A single patient was found to be infected with genotype 2B of HCV and hence did not receive therapy as part of the study.
Table 1.
Baseline Characteristics of Study Population
| Ref. no. | Age | Race | Sex | Genotype | Years of infection | Response | IFN (U/mL) |
|---|---|---|---|---|---|---|---|
| 697-01 | 46 | C | F | 1A | 9 | SVR | 0 |
| 697-02 | 36 | C | F | 1A | 18 | SVR | 0 |
| 697-03 | 20 | C | F | 1A | 20 | SVR | 0 |
| 697-04 | 48 | C | F | 1A | 30 | NR | 0 |
| 697-05 | 44 | AA | F | 1B | 24 | Un | 4 |
| 697-06 | 50 | C | F | 1A | 32 | R | 40 |
| 697-07 | 51 | AA | M | 1A | 30 | NR | 0 |
| 697-08 | 36 | AA | M | 1B | 5 | R | 40 |
| 697-09 | 28 | AA | F | 1A | Un | SVR | 0 |
| 697-10 | 47 | AA | F | 1A | 29 | Un | 4 |
| 697-11 | 35 | AA | F | 1A | 20 | NR | 0 |
| 697-12 | 40 | AA | F | 1A | ∼15 | SVR | 0 |
| 697-13 | 43 | C | F | 1A | ∼9 | SVR | 0 |
| 697-14 | 46 | C | M | 1A | 15 | NR | 4 |
| 697-15 | 56 | AA | F | 1A | 30 | Un | 0 |
| 697-16 | 45 | AA | F | 1B | ∼11-14 | SVR | 0 |
| 697-17 | 45 | AA | F | 1A | 16 | SVR | 0 |
| 697-18 | 45 | C | M | 1A | 26 | SVR | 10 |
| 697-19 | 43 | AA | F | 1A | 25 | Un | 0 |
| 697-20 | 39 | C | F | 1A | 23 | SVR | 0 |
| 697-21 | 50 | AA | F | 1A | 20+ | NR | 40 |
| 697-22 | 65 | AA | M | 1B | 30 | Un | 0 |
| 697-23 | 54 | C | F | 1A | 25 | R | 16 |
| 697-24 | 67 | C | F | 1B | 22 | R | 0 |
| 697-25 | 44 | AA | M | 1B | ∼18 | NR | 20 |
| 697-26 | 40 | AA | M | 1A | 10 | R | 0 |
| 697-27 | 50 | AA | M | 1A | 10 | R | 0 |
| 697-28 | 45 | C | M | 1A | 25 | SVR | 20 |
| 697-29 | 42 | AA | M | 1B | 20 | Un | 20 |
| 697-30 | 51 | AA | M | 1A | 18 | NR | 12 |
| 697-31 | 46 | C | F | 1B | 30 | NR | 0 |
| 697-32 | 46 | AA | F | 2B | 20 | Un | 10 |
| 697-33 | 61 | AA | F | 1A | 30 | NR | 16 |
| 697-34 | 43 | C | M | 1A | >15 | SVR | 24 |
| 697-35 | 49 | AA | M | 1A | 28 | Un | 0 |
Patients who did not complete the clinical trial are listed as having an unknown (Un) response to therapy.
Abbreviations: AA, African American; C, Caucasian; IFN, interferon; SVR, sustained viral response.
Collection of serum samples
Before the initiation of therapy, blood was collected using a Vacutainer Blood Collection Set (BD) into polyethylene tubes according to protocols approved by the Institutional Review Board at the UTHSC. After the blood was allowed to clot (22°C for 10–20 min), serum samples were collected at 4°C after centrifugation (800g), aliquoted into 0.5 mL microcentrifuge tubes, and stored at −80°C for subsequent analysis.
Assays for IFN bioactivity
Serum samples were collected from previously untreated patients before onset of therapy and 5 healthy volunteers, and assayed for the antiviral activity of IFN. Cultures of human CaKi cells (highly IFN-sensitive renal cell carcinoma cells) were plated at 1 × 104 cells per well of 96-well micro-titer plates. After 24 h, the cells were incubated overnight with sequential 2-fold dilutions of serum samples, or with an National Institutes of Health (NIH) IFN standard for reference, followed by infection with vesicular stomatitis virus (VSV) at a multiplicity of infection of 0.1 plaque-forming units per mL. At 24 h after infection, the ability of IFN to protect cells against the cytopathic effect of VSV was determined (Nanus and others 1990). The sensitivity for this antiviral assay is routinely ∼1 IU per mL. The sera were also assayed for IFN biological activity by a highly sensitive assay for the antiproliferative activity of type I IFNs (Kessler and others 1988). Aliquots (25 μL) of serum samples were added to 5 × 104 Daudi lymphoblastoid cells in 0.475 mL of media in 24-well multicluster plates. For comparison human IFNα was added to wells at amounts varying from 1 to 100 IU in 25 μL of media, and after 3 days cell counts were made using a Coulter Counter. Each experiment was performed at least 3 times with duplicate wells used for each variable. The sensitivity for this antiproliferative assay is routinely ∼5 IU per mL.
Assay for neutralizing antibodies against IFN
Aliquots (25 μL) of serum samples were gently mixed in 24-well multicluster plates with 25 IU of human IFNα in a total volume of 75 μL for 4 h at 4°C. As positive controls, 3 antisera obtained from the NIH that neutralize the antiproliferative activity of multiple type I IFNs were used: NIH26-501-568 (anti-leukocyte IFN Ab), NIH30-501-553 (anti-lymphoblastoid IFN Ab), and NIH37-501-572 (anti-IFNα2B Ab). In addition, 2 antisera obtained from the NIH, which fail to neutralize the biological activity of multiple type I IFNs, were used as negative controls: NIH27-501-568 (control for anti-leukocyte IFN Ab) and NIH31-501-553 (control for anti-lymphoblastoid IFN Ab). Following the 4-h incubation, Daudi lymphoblastoid cells were added to the wells at a density of 5 × 104 cells per 0.4 mL of media. After 3 days, cell counts were determined in a Coulter Counter. The experiments were performed at least twice with duplicate wells used for each variable. In parallel assays, we also measured anti-IFN antibody (Ab) levels by neutralization of antiviral activity.
Statistical analysis
The data on IFN production in the different patient subgroups were subjected to nonparametric Mann–Whitney analysis using Graphpad InStat 3 software.
Results
The presence of bioactive IFN in serum samples from HCV-infected individuals
Serum samples were collected from patients before the beginning of therapy, and assayed for IFN activity by an antiviral microassay that measures the inhibition of the cytopathic effects of VSV. The antiviral assay routinely detects IFN titers as low as 1 IU per mL. However, this assay does not distinguish between type I (IFNα and IFNβ) and type II IFN (IFNγ). Therefore, to determine type I IFN selectively, all serum samples that were identified as having detectable IFN titers were reassayed by a highly sensitive microassay for IFN's antiproliferative activity using Daudi lymphoblastoid cells (Kessler and others 1988). The human Daudi lymphoblastoid cell line is highly sensitive to the antiproliferative action of type I IFNs but it is resistant to the antiproliferative action of IFNγ at concentrations as high as 10,000 IU per mL (Pfeffer and Tamm 1983). All positive samples for antiviral activity were found to have equivalent IFN titers when assayed by this highly sensitive bioassay of IFN's antiproliferative assay, indicating that the IFN titers represent the production of type I IFNs. The lower limit of detection of this assay is ∼ 5 IU per mL. It is of interest that commercial enzyme-linked immunosorbent assay (ELISA)-based assays for type I IFNs were found to be less sensitive to either bioassay with limits of detection > 50 IU per mL (data not shown).
Serum samples from 35 HCV-infected patients were assayed for IFN activity and the results tabulated in Table 1. Fifteen of the thirty-five (∼43%) enrolled HCV-infected patients were found to have basal serum levels of IFN (ranging from 4 to 40 IU/mL) before initiation of therapy. In contrast, serum samples collected from 5 healthy volunteers were found not to contain detectable levels of IFN (data not shown). Similar IFN levels were determined when serum samples were assayed by antiviral or antiproliferative assays. The finding of IFN in the sera of HCV-infected patients suggests that a significant fraction of these patients mount the initial phase of the host antiviral innate response, that is, type I IFN production. Moreover, all the patients in this study are chronically infected with HCV, and thus did not clear HCV before treatment. Thus, our results also indicate that the ability to mount this initial innate antiviral response does not translate into the ability to curb the viral infection.
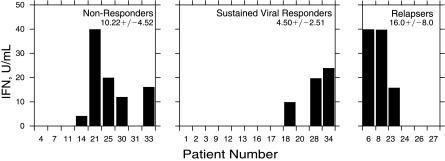
We next examined whether there was any relationship between IFN production in HCV-infected patients and the subsequent clinical response to combined IFN/ribavirin therapy (Fig. 1). Interferon titers were found in 55% of patients found to be NRs (5/9); 50% of the patients that relapsed (R) after an initial antiviral response to IFN/ribavirin (3/6); and in 25% of patients with a sustained viral response (SVR) to therapy (3/12). None of the differences in IFN production within the patient subgroups were found to be significant by nonparametric statistical analysis. Of the 35 patients initially enrolled, 27 patients (77%) eventually completed this arm of the clinical study.
FIG. 1.
Interferon (IFN) production according to the clinical response.
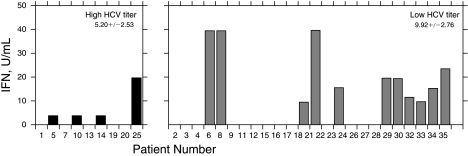
We also examined whether there was a relationship between viral load and IFN production (Fig. 2). Twenty-five of the 34 HCV genotype 1 patients enrolled in this study had viral titers below 850,000 IU per mL as determined by quantitative PCR, and 9 had titers ≥ 850,000 IU copies per mL. Forty-four percent of the patients with lower viral load had detectable IFN levels in their sera, equivalent to the percentage of patients with a higher viral load. In addition, there was a similar variation of IFN levels detected in each group, varying from 4 to 40 U per mL. Thus, there was no relationship between viral load and IFN production.
FIG. 2.
Interferon (IFN) production according to hepatitis C virus (HCV) titer.
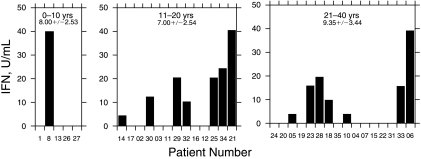
The presence of IFN in the serum of HCV-infected patients did not correlate with the length of known HCV infection (Fig. 3). Detectable serum IFN levels were present in 20% of patients infected with HCV for <10 years (1/5); 50% of patients infected for between 11 and 20 years (6/12); and 44% of patients infected for >21 years (8/18). Thus, IFN levels did not correlate with the length of HCV infection.
FIG. 3.
Interferon (IFN) production according to length of hepatitis C virus (HCV) infection.
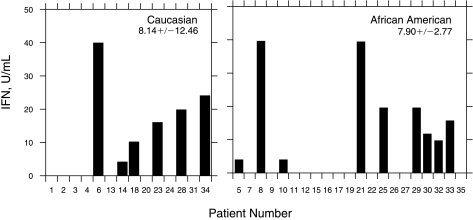
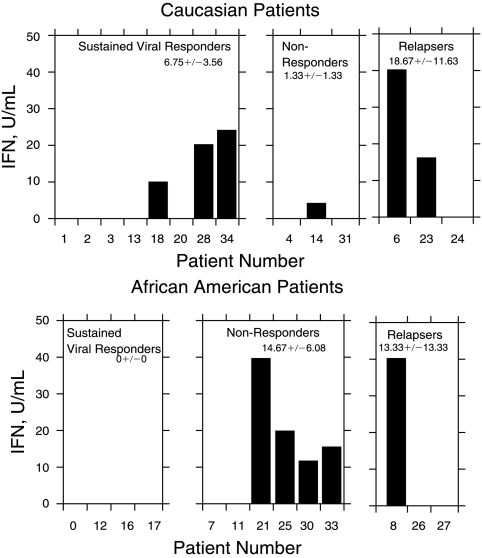
We have previously found a significant difference in the clinical response between AA and C patients to IFN monotherapy. Therefore, we next determined whether there was a relationship between the race of HCV-infected patients to IFN production. ∼43% of both AA (9/21) and C patients (6/14) had detectable serum IFN titers (Fig. 4). Thus, there was no racial difference in the induction of the innate immune response to viral infection (C = 8.14 ± 12.46 U/mL, AA = 7.90 ± 2.77 U/mL). We then compared the IFN response of the AA and C patients. There was no difference in the response between the different subgroups of C patients. However, there was a difference in the response within the subgroups of AA patients (Fig. 5). The AA sustained viral responders were found not to have detectable IFN production as compared to AA nonresponders (14.67 ± 6.08 U/mL). Moreover, comparison of the response between C and AA also yielded some interesting differences. The level of IFN production in Caucasian patients with a sustained viral response (6.75 ± 3.56 U/mL) was higher as compared to AA patients with a SVR (0 U/mL). In addition, AA nonresponders produced higher IFN levels (14.67 ± 6.08 U/mL) as compared to C non-responders (1.33 ± 1.33 U/mL).
FIG. 4.
Interferon (IFN) production according to race.
FIG. 5.
Interferon (IFN) production according to racial response.
Assay for anti-IFN antibodies
Treatment with exogenous IFN has previously been shown to induce production of neutralizing antibodies against IFN (anti-IFN). The development of anti-IFN in HCV-patients may play a role in inhibiting IFN's biological antiviral action. Since sera collected from some HCV-infected patients contain detectable IFN titers, serum samples were also assayed for levels of anti-IFN antibodies. Serum samples collected before onset of therapy were assayed for anti-IFN by a highly sensitive microassay for the neutralization of IFN's antiproliferative and antiviral activity (Improta and others 1992). In brief, serum samples were mixed with a fixed concentration of human IFNα, and then the IFN assayed for biological activity. As positive controls 3 antisera obtained from the NIH which neutralize the biological activity of multiple type I IFNs were used: NIH26-501-568 (anti-leukocyte IFN Ab), NIH30-501-553 (anti-lymphoblastoid IFN Ab), and NIH37-501-572 (anti-IFNα2B Ab). In addition, 2 antisera obtained from the NIH, which fail to neutralize the biological activity of multiple type I IFNs, were used as internal negative controls: NIH27-501-568 (control for anti-leukocyte IFN Ab) and NIH31-501-553 (control for anti-lymphoblastoid IFN Ab). Although the 3 positive control antisera (NIH26-501-568, NIH30-501-553, and NIH37-501-572) neutralized the antiviral and antiproliferative activity of different type I IFNs, we did not find detectable levels of anti-IFN antibodies in any of the serum samples collected before the onset of therapy from the 35 enrolled patients in our study.
Discussion
Approximately 3% of the world's population is infected with HCV, and ∼80% of HCV-infected individuals eventually become chronically infected. While long-term persistence results predominantly from evasion of the adaptive immune response to viral infection, evasion of host innate immune response is believed to be critical in establishing persistent HCV infection (Gale and Foy 2005). The IFN system is a key player in the innate immune response against viral infections, by inducing an antiviral state in the host against a variety of viral pathogens. Moreover, HCV is highly sensitive to treatment with type I IFNs, and IFN remains a mainstay of treatment of HCV infected patients (Strader and others 2004).
The finding that HCV is sensitive to IFN but establishes persistent infection in many HCV-infected patients suggests that HCV has evolved mechanisms to circumvent detection by the innate antiviral defense system and hence antagonize the ability of HCV-infected cells to produce IFN. For example, the viral protease NS3/4A of HCV suppresses activation of the transcription factor, IFN regulatory factor 3. IFN regulatory factor 3 not only regulates type I IFN production, but also a number of IFN-responsive genes in response to viral infection or treatment with double-stranded RNA (dsRNA) (Foy and others 2003; Thimme and others 2006). The NS3/4A of HCV also cleaves the essential adaptor TRIF (Toll/interleukin receptor domain-containing adapter-inducing interferon), involved in TLR3-mediated signaling (Rosenberg 2001), as well as the signaling adaptor Cardif (also known as IPS-1, MAVS or VISA), which is involved in the cytosolic pathways of RIG-I and MDA5 (Rehermann and Nascimbeni 2005). This may indicate that both pathways play a role in controlling HCV infection, but in different sets of cells. For example, TLR3 has been shown to be essential for IFN production in plasmacytoid dendritic cells, whereas RIG-I triggers IFN secretion upon viral infection in conventional dendritic cells and other tissues (Wieland and Chisari 2005).
In the present studies we evaluated the ability of HCV-infected patients in an enrolled clinical trial to produce IFN. The serum of patients was analyzed before any therapy with exogenous IFN had commenced. Two assays were used to define IFN production. First, samples were assayed by a highly sensitive antiviral assay for IFN. Second, IFN positive samples were analyzed for sensitivity to IFN's antiproliferative action to define IFN levels as well as to determine whether the IFN levels determined were due to type I versus type II IFN production. This analysis demonstrated that a large fraction of HCV patients had significant serum levels of type I IFN before commencement of therapy with exogenous IFN. These results lead us to conclude that, although there are a number of ways that HCV can evade the immune system by blocking IFN production in vitro, HCV patients paradoxically produce IFN. The presence of IFN in the sera of HCV-infected patients is consistent with previous studies showing that hundreds of IFN-stimulated genes (ISGs) are induced in the livers of patients chronically infected with HCV (Chen and others 2005; Sarasin-Filipowicz and others 2008). However, although IFN is produced in HCV-infected patients, the source for IFNα/β production in response to HCV infection is apparently extrahepatic. For example, using paired liver biopsies and peripheral blood mononuclear cells (PBMCs) collected from patients chronically infected with HCV, induction of many ISGs was found in liver biopsies but the IFN genes, themselves, were not induced (Mihm and others 2004; Sarasin-Filipowicz and others 2008). In contrast, both induction of ISGs and various type I IFN subtypes were readily apparent in the paired PBMCs from these same patients. Moreover, chronic HCV infection in chimpanzees resulted in ISG induction but the expression of type I IFN genes was unaffected (Bigger and others 2001; Bigger and others 2004).
Taken together our results on IFN production as well as those of others on persistent ISG induction in chronically infected patients demonstrate that the endogenous IFN system is constantly activated in many patients. As shown in Figure 3, we detected IFN production in patients who have been infected with HCV as little as 5 years as well as those > 30 years. A number of recent studies have shown that patients with a high ISG expression before the initiation of IFN therapy seem to respond poorly to IFN therapy (Bigger and others 2001; Bigger and others 2004; Sarasin-Filipowicz and others 2008). Although this finding is counterintuitive, as one would expect an active IFN system would help eliminate the virus during therapy, it is supported by data from chimpanzees and human patients. In agreement with these findings, one small subset of the patients in our study, AA with a SVR did not have detectable levels of IFN production.
However, the ability of patients to produce IFN does not correlate with the patient's sex, race, or viral load. Most interestingly, the ability to produce IFN does not appear to correlate well with the patient's response to IFN therapy. This leads us to hypothesize that the nonresponsiveness of a subset of HCV-infected patients reflects a failure to respond to IFN. Thus, defects in the IFN-response pathway must be a major determinant in the IFN response. Our findings are entirely consistent with previous studies in patient-derived PBMCs that indicate that HCV-infected patients with a poor response to IFN therapy exhibit a relatively weak induction of ISGs, while patients with a strong response to IFN induce ISGs to higher levels (He and others 2006; Taylor and others 2007). These findings suggest that the failure to respond may reflect subtle differences in the IFN-signaling pathway. Interferons bind to high affinity cell surface receptors displayed ubiquitously on cells. In fact, type I IFN receptors have been found in nearly every nucleated cell type so far studied. Upon IFN binding, IFN activates the nonreceptor tyrosine kinases JAK1 and TYK2, which are constitutively associated with the intracellular domains of the 2 IFN receptor subunits, IFNAR1 and IFNAR2. IFNAR2 is the ligand-binding subunit of the receptor while IFNAR1 is primarily involved in signal transduction. The IFN-activated JAK kinases mediate the phosphorylation of various members of the STAT family of transcription factors, which then form various homo- and heterodimers that bind to specific promoter elements within IFN-responsive genes to drive their transcription (Darnell and others 1994). Interferons also activate pathways that lead to nuclear factor κB (NFκB) activity, and NFκB also regulates the expression of IFN-responsive pathways (Du and others 2007). Therefore, it is highly likely that defects anywhere in the IFN-signaling pathway will significantly affect the IFN response.
Our studies show that the type I IFN system is induced in nearly half of the patients chronically-infected with HCV, indicating that the host innate response is triggered by HCV infection. Double-stranded RNA, a potent IFN inducer, is an intermediate in the HCV life cycle. Moreover, the HCV viral genome RNA encodes regions of extensive secondary dsRNA that is also likely to induce IFN production (Gale and Foy 2005). Several findings are consistent with our finding of IFN production in HCV-infected patients. For example, IFN-responsive genes are induced in the livers of experimentally HCV-infected chimpanzees within a week of viral infection (Bigger and others 2001). Hepatitis C virus RNA is sufficient to trigger IFN production when introduced into cells in vitro (Sumpter and others 2005). Thus, our studies are the first to show that HCV induces type I IFN production in vivo, but suggest that the differential response of patients to IFN therapy reflects a defect in IFN-responsiveness rather than a failure to produce IFN in the innate antiviral response to HCV infection.
Acknowledgments
Supported by National Institutes of Health grant U19 AI066316, General Clinical Research Center Grant M01-RR00211, and by funds from the Muirhead Chair Endowment at the University of Tennessee Health Science Center. We thank Dennis Carrigan for excellent technical assistance during the initial phase of these studies.
References
- Bigger CB. Brasky KM. Lanford RE. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J Virol. 2001;75(15):7059–7066. doi: 10.1128/JVI.75.15.7059-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigger CB. Guerra B. Brasky KM. Hubbard G. Beard MR. Luxon BA. Lemon SM. Lanford RE. Intrahepatic gene expression during chronic hepatitis C virus infection in chimpanzees. J Virol. 2004;78(24):13779–13792. doi: 10.1128/JVI.78.24.13779-13792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino S. Lensing S. Riely C. Rai SN. Davila R. Hayden RT. Fleckenstein J. Levstik M. Taylor S. Dean PJ. Kippenbrock S. Pope J. Carr J. Strickland DK. Hudson MM. The epidemiology of chronic hepatitis C infection in survivors of childhood cancer: an update of the St Jude Children's Research Hospital hepatitis C seropositive cohort. Blood. 2004;103(7):2460–2466. doi: 10.1182/blood-2003-07-2565. [DOI] [PubMed] [Google Scholar]
- Chen L. Borozan I. Feld J. Sun J. Tannis LL. Coltescu C. Heathcote J. Edwards AM. McGilvray ID. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infection. Gastroenterology. 2005;128(5):1437–1444. doi: 10.1053/j.gastro.2005.01.059. [DOI] [PubMed] [Google Scholar]
- Darnell JEJ. Kerr IM. Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Du Z. Wei L. Murti A. Pfeffer SR. Fan M. Yang CH. Pfeffer LM. Non-conventional signal transduction by type 1 interferons: the NF-kappaB pathway. J Cell Biochem. 2007;102(5):1087–1094. doi: 10.1002/jcb.21535. [DOI] [PubMed] [Google Scholar]
- Fleckenstein J. Chronic hepatitis C in African Americans and other minority groups. Curr Gastroenterol Rep. 2004;6(1):66–70. doi: 10.1007/s11894-004-0028-z. [DOI] [PubMed] [Google Scholar]
- Foy E. Li K. Wang C. Sumpter R., Jr Ikeda M. Lemon SM. Gale M., Jr Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science. 2003;300(5622):1145–1148. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- Gale M., Jr Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436(7053):939–945. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- He XS. Ji X. Hale MB. Cheung R. Ahmed A. Guo Y. Nolan GP. Pfeffer LM. Wright TL. Risch N., others Global transcriptional response to interferon is a determinant of HCV treatment outcome and is modified by race. Hepatology. 2006;44(2):352–359. doi: 10.1002/hep.21267. [DOI] [PubMed] [Google Scholar]
- Hiscott J. Lin R. Inhibition of the interferon antiviral response by hepatitis C virus. Expert Rev Clin Immunol. 2006;2:49–58. doi: 10.1586/1744666X.2.1.49. [DOI] [PubMed] [Google Scholar]
- Improta T. Pine R. Pfeffer LM. Interferon-g potentiates the antiviral activity and the expression of interferon-stimulated genes induced by interferon-a in U-937 cells. J Interferon Res. 1992;12:87–94. doi: 10.1089/jir.1992.12.87. [DOI] [PubMed] [Google Scholar]
- Kessler DS. Pine R. Pfeffer LM. Levy DE. Darnell JEJ. Cells resistant to interferon are defective in activation of a promoter-binding factor. EMBO J. 1988;7:3779–3783. doi: 10.1002/j.1460-2075.1988.tb03262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E. Tschopp J. Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442(7098):39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- Mihm S. Frese M. Meier V. Wietzke-Braun P. Scharf JG. Bartenschlager R. Ramadori G. Interferon type I gene expression in chronic hepatitis C. Lab Invest. 2004;84(9):1148–1159. doi: 10.1038/labinvest.3700135. [DOI] [PubMed] [Google Scholar]
- Muir AJ. Bornstein JD. Killenberg PG. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med. 2004;350(22):2265–2271. doi: 10.1056/NEJMoa032502. [DOI] [PubMed] [Google Scholar]
- Nanus DM. Pfeffer LM. Bander NH. Bahri S. Albino AP. Antiproliferative and antitumor effects of alpha interferon in renal cell carcinomas: correlation with the expression of a kidney associated differentiation antigen. Cancer Res. 1990;50:4190–4194. [PubMed] [Google Scholar]
- Pfeffer LM. Dinarello CA. Herberman RB. Williams BR. Borden EC. Bordens R. Walter MR. Nagabhushan TL. Trotta PP. Pestka S. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 1998;58(12):2489–2499. [PubMed] [Google Scholar]
- Pfeffer LM. Tamm I. Comparison of the effects of alpha and beta interferons on the proliferation and volume of human tumor cells (HeLa-S3, Daudi, P3HR-1) J Interferon Res. 1983;3:395–408. doi: 10.1089/jir.1983.3.395. [DOI] [PubMed] [Google Scholar]
- Rehermann B. Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5(3):215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- Reyes GR. Ribavirin: recent insights into antiviral mechanisms of action. Curr Opin Drug Discov Devel. 2001;4(5):651–656. [PubMed] [Google Scholar]
- Rosenberg S. Recent advances in the molecular biology of hepatitis C virus. J Mol Biol. 2001;313(3):451–464. doi: 10.1006/jmbi.2001.5055. [DOI] [PubMed] [Google Scholar]
- Sarasin-Filipowicz M. Oakeley EJ. Duong FH. Christen V. Terracciano L. Filipowicz W. Heim MH. Interferon signaling and treatment outcome in chronic hepatitis C. Proc Natl Acad Sci USA. 2008;105(19):7034–7039. doi: 10.1073/pnas.0707882105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader DB. Wright T. Thomas DL. Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39(4):1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- Sumpter R., Jr Loo YM. Foy E. Li K. Yoneyama M. Fujita T. Lemon SM. Gale M., Jr Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005;79(5):2689–2699. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MW. Tsukahara T. Brodsky L. Schaley J. Sanda C. Stephens MJ. McClintick JN. Edenberg HJ. Li L. Tavis JE. Howell C. Belle SH. Changes in gene expression during pegylated interferon and ribavirin therapy of chronic hepatitis C virus distinguish responders from nonresponders to antiviral therapy. J Virol. 2007;81(7):3391–3401. doi: 10.1128/JVI.02640-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimme R. Lohmann V. Weber F. A target on the move: innate and adaptive immune escape strategies of hepatitis C virus. Antiviral Res. 2006;69(3):129–141. doi: 10.1016/j.antiviral.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Wieland SF. Chisari FV. Stealth and cunning: hepatitis B and hepatitis C viruses. J Virol. 2005;79(15):9369–9380. doi: 10.1128/JVI.79.15.9369-9380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]