Abstract
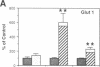
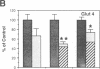
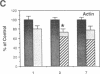
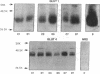
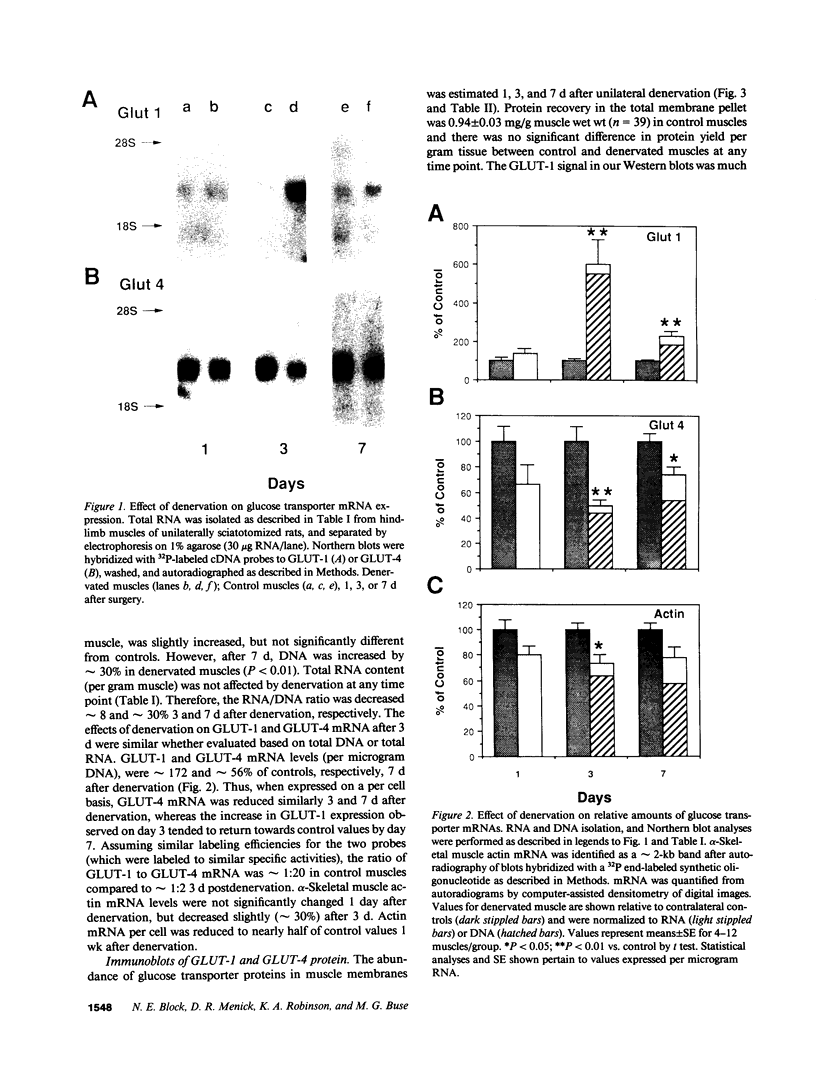
Denervation rapidly (within 24 h) induces insulin resistance of several insulin-responsive pathways in skeletal muscle, including glucose transport; resistance is usually maximal by 3 d. We examined the effect of denervation on the expression of two glucose transporter isoforms (GLUT-1 and GLUT-4) in rat hindlimb muscle; GLUT-4 is the predominant species in muscle. 1 d postdenervation, GLUT-1 and GLUT-4 mRNA and protein concentrations were unchanged. 3 and 7 d postdenervation, GLUT-4 mRNA and protein (per microgram DNA) were decreased by 50%. The minor isoform, GLUT-1 mRNA increased by approximately 500 and approximately 100%, respectively, on days 3 and 7 while GLUT-1 protein increased by approximately 60 and approximately 100%. The data suggest that the insulin resistance of glucose transport early after denervation does not reflect a decrease in total glucose transporter number; however, decreased GLUT-4 expression may contribute to its increased severity after 3 d. Parallel decreases in GLUT-4 mRNA and GLUT-4 protein postdenervation are consistent with pretranslational regulation; GLUT-1 expression may be regulated pre- and posttranslationally. The cell type(s) which overexpress GLUT-1 postdenervation need to be identified. Nervous stimuli and/or contractile activity may modulate the expression of GLUT-1 and GLUT-4 in skeletal muscle tissue.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUSE M. G., BUSE J. The effect of denervation and insulin on the penetration of D-xylose into rat hemidiaphragms. Diabetes. 1961 Mar-Apr;10:134–141. doi: 10.2337/diab.10.2.134. [DOI] [PubMed] [Google Scholar]
- Babij P., Booth F. W. Alpha-actin and cytochrome c mRNAs in atrophied adult rat skeletal muscle. Am J Physiol. 1988 May;254(5 Pt 1):C651–C656. doi: 10.1152/ajpcell.1988.254.5.C651. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Kayano T., Buse J. B., Burant C. F., Takeda J., Lin D., Fukumoto H., Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990 Mar;13(3):198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- Berger J., Biswas C., Vicario P. P., Strout H. V., Saperstein R., Pilch P. F. Decreased expression of the insulin-responsive glucose transporter in diabetes and fasting. Nature. 1989 Jul 6;340(6228):70–72. doi: 10.1038/340070a0. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science. 1987 Mar 20;235(4795):1495–1498. doi: 10.1126/science.3029870. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Lemmon S. K., Treutelaar M. K., Buse M. G. Insulin resistance of denervated rat muscle: a model for impaired receptor-function coupling. Am J Physiol. 1984 Nov;247(5 Pt 1):E657–E666. doi: 10.1152/ajpendo.1984.247.5.E657. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Buse M. G. In vitro and in vivo activation of the insulin receptor kinase in control and denervated skeletal muscle. J Biol Chem. 1986 Jul 5;261(19):8985–8993. [PubMed] [Google Scholar]
- Charron M. J., Kahn B. B. Divergent molecular mechanisms for insulin-resistant glucose transport in muscle and adipose cells in vivo. J Biol Chem. 1990 May 15;265(14):7994–8000. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C., Solomon S. S., Jallepalli P., Heckemeyer C., Finnern J., Powers A. Glucose intolerance due to insulin resistance in patients with spinal cord injuries. Diabetes. 1980 Nov;29(11):906–910. doi: 10.2337/diab.29.11.906. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M., McCall A. L., Lodish H. F. Distribution of glucose transporter messenger RNA transcripts in tissues of rat and man. J Clin Invest. 1987 Feb;79(2):657–661. doi: 10.1172/JCI112864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehner S. C., Davies A., Baldwin S. A., Lienhard G. E. The blood-nerve barrier is rich in glucose transporter. J Neurocytol. 1988 Apr;17(2):173–178. doi: 10.1007/BF01674204. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Garcia de Herreros A., Birnbaum M. J. The regulation by insulin of glucose transporter gene expression in 3T3 adipocytes. J Biol Chem. 1989 Jun 15;264(17):9885–9890. [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Birnbaum M. J. Pretranslational suppression of an insulin-responsive glucose transporter in rats with diabetes mellitus. Science. 1989 Jul 7;245(4913):60–63. doi: 10.1126/science.2662408. [DOI] [PubMed] [Google Scholar]
- Gatchalian C. L., Schachner M., Sanes J. R. Fibroblasts that proliferate near denervated synaptic sites in skeletal muscle synthesize the adhesive molecules tenascin(J1), N-CAM, fibronectin, and a heparan sulfate proteoglycan. J Cell Biol. 1989 May;108(5):1873–1890. doi: 10.1083/jcb.108.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Hall Z. W. Immunological evidence for a change in subunits of the acetylcholine receptor in developing and denervated rat muscle. Neuron. 1988 Apr;1(2):117–125. doi: 10.1016/0896-6273(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Gunning P., Mohun T., Ng S. Y., Ponte P., Kedes L. Evolution of the human sarcomeric-actin genes: evidence for units of selection within the 3' untranslated regions of the mRNAs. J Mol Evol. 1984;20(3-4):202–214. doi: 10.1007/BF02104727. [DOI] [PubMed] [Google Scholar]
- Hansson H. A., Rozell B., Skottner A. Rapid axoplasmic transport of insulin-like growth factor I in the sciatic nerve of adult rats. Cell Tissue Res. 1987 Feb;247(2):241–247. doi: 10.1007/BF00218305. [DOI] [PubMed] [Google Scholar]
- Haspel H. C., Rosenfeld M. G., Rosen O. M. Characterization of antisera to a synthetic carboxyl-terminal peptide of the glucose transporter protein. J Biol Chem. 1988 Jan 5;263(1):398–403. [PubMed] [Google Scholar]
- Haspel H. C., Wilk E. W., Birnbaum M. J., Cushman S. W., Rosen O. M. Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem. 1986 May 25;261(15):6778–6789. [PubMed] [Google Scholar]
- Henriksen E. J., Rodnick K. J., Mondon C. E., James D. E., Holloszy J. O. Effect of denervation or unweighting on GLUT-4 protein in rat soleus muscle. J Appl Physiol (1985) 1991 May;70(5):2322–2327. doi: 10.1152/jappl.1991.70.5.2322. [DOI] [PubMed] [Google Scholar]
- Hiraki Y., Garcia de Herreros A., Birnbaum M. J. Transformation stimulates glucose transporter gene expression in the absence of protein kinase C. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8252–8256. doi: 10.1073/pnas.86.21.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Charron M. J., Lodish H. F., Cushman S. W., Flier J. S. Differential regulation of two glucose transporters in adipose cells from diabetic and insulin-treated diabetic rats. J Clin Invest. 1989 Aug;84(2):404–411. doi: 10.1172/JCI114180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Flier J. S. Regulation of glucose-transporter gene expression in vitro and in vivo. Diabetes Care. 1990 Jun;13(6):548–564. doi: 10.2337/diacare.13.6.548. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Rossetti L., Lodish H. F., Charron M. J. Decreased in vivo glucose uptake but normal expression of GLUT1 and GLUT4 in skeletal muscle of diabetic rats. J Clin Invest. 1991 Jun;87(6):2197–2206. doi: 10.1172/JCI115254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen R. G., Sheng Z. H., Yang J., Chen L. Q., Rogart R. B., Barchi R. L. Primary structure and expression of a sodium channel characteristic of denervated and immature rat skeletal muscle. Neuron. 1990 Feb;4(2):233–242. doi: 10.1016/0896-6273(90)90098-z. [DOI] [PubMed] [Google Scholar]
- Kasanicki M. A., Pilch P. F. Regulation of glucose-transporter function. Diabetes Care. 1990 Mar;13(3):219–227. doi: 10.2337/diacare.13.3.219. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Tanaka M., Akamatsu Y. Regulation of glucose transport activity and expression of glucose transporter mRNA by serum, growth factors and phorbol ester in quiescent mouse fibroblasts. Biochim Biophys Acta. 1989 Mar 27;980(1):100–108. doi: 10.1016/0005-2736(89)90205-8. [DOI] [PubMed] [Google Scholar]
- Klip A., Pâquet M. R. Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care. 1990 Mar;13(3):228–243. doi: 10.2337/diacare.13.3.228. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Young D. A., Holloszy J. O. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987 Nov 16;224(1):224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- Koivisto U. M., Martinez-Valdez H., Bilan P. J., Burdett E., Ramlal T., Klip A. Differential regulation of the GLUT-1 and GLUT-4 glucose transport systems by glucose and insulin in L6 muscle cells in culture. J Biol Chem. 1991 Feb 5;266(4):2615–2621. [PubMed] [Google Scholar]
- Mudd L. M., Werner H., Shen-Orr Z., Roberts C. T., Jr, LeRoith D., Haspel H. C., Raizada M. K. Regulation of rat brain/HepG2 glucose transporter gene expression by phorbol esters in primary cultures of neuronal and astrocytic glial cells. Endocrinology. 1990 Jan;126(1):545–549. doi: 10.1210/endo-126-1-545. [DOI] [PubMed] [Google Scholar]
- Murray M. A., Robbins N. Cell proliferation in denervated muscle: identity and origin of dividing cells. Neuroscience. 1982 Jul;7(7):1823–1833. doi: 10.1016/0306-4522(82)90040-9. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Pitton G., Saggin L., Ausoni S., Sartore S., Lømo T. Embryonic and neonatal myosin heavy chain in denervated and paralyzed rat skeletal muscle. Dev Biol. 1988 May;127(1):1–11. doi: 10.1016/0012-1606(88)90183-2. [DOI] [PubMed] [Google Scholar]
- Seider M. J., Nicholson W. F., Booth F. W. Insulin resistance for glucose metabolism in disused soleus muscle of mice. Am J Physiol. 1982 Jan;242(1):E12–E18. doi: 10.1152/ajpendo.1982.242.1.E12. [DOI] [PubMed] [Google Scholar]
- Shih H. T., Wathen M. S., Marshall H. B., Caffrey J. M., Schneider M. D. Dihydropyridine receptor gene expression is regulated by inhibitors of myogenesis and is relatively insensitive to denervation. J Clin Invest. 1990 Mar;85(3):781–789. doi: 10.1172/JCI114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivitz W. I., DeSautel S. L., Kayano T., Bell G. I., Pessin J. E. Regulation of glucose transporter messenger RNA in insulin-deficient states. Nature. 1989 Jul 6;340(6228):72–74. doi: 10.1038/340072a0. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Lawrence J. C., Jr Insulin action in denervated rat hemidiaphragms. Decreased hormonal stimulation of glycogen synthesis involves both glycogen synthase and glucose transport. J Biol Chem. 1984 Feb 25;259(4):2201–2207. [PubMed] [Google Scholar]
- Snow M. H. A quantitative ultrastructural analysis of satellite cells in denervated fast and slow muscles of the mouse. Anat Rec. 1983 Dec;207(4):593–604. doi: 10.1002/ar.1092070407. [DOI] [PubMed] [Google Scholar]
- Sowell M. O., Boggs K. P., Robinson K. A., Dutton S. L., Buse M. G. Effects of insulin and phospholipase C in control and denervated rat skeletal muscle. Am J Physiol. 1991 Feb;260(2 Pt 1):E247–E256. doi: 10.1152/ajpendo.1991.260.2.E247. [DOI] [PubMed] [Google Scholar]
- Sowell M. O., Dutton S. L., Buse M. G. Selective in vitro reversal of the insulin resistance of glucose transport in denervated rat skeletal muscle. Am J Physiol. 1989 Sep;257(3 Pt 1):E418–E425. doi: 10.1152/ajpendo.1989.257.3.E418. [DOI] [PubMed] [Google Scholar]
- Tordjman K. M., Leingang K. A., James D. E., Mueckler M. M. Differential regulation of two distinct glucose transporter species expressed in 3T3-L1 adipocytes: effect of chronic insulin and tolbutamide treatment. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7761–7765. doi: 10.1073/pnas.86.20.7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turinsky J. Dynamics of insulin resistance in denervated slow and fast muscles in vivo. Am J Physiol. 1987 Mar;252(3 Pt 2):R531–R537. doi: 10.1152/ajpregu.1987.252.3.R531. [DOI] [PubMed] [Google Scholar]
- Turinsky J. Glucose and amino acid uptake by exercising muscles in vivo: effect of insulin, fiber population, and denervation. Endocrinology. 1987 Aug;121(2):528–535. doi: 10.1210/endo-121-2-528. [DOI] [PubMed] [Google Scholar]
- Weiland M., Schürmann A., Schmidt W. E., Joost H. G. Development of the hormone-sensitive glucose transport activity in differentiating 3T3-L1 murine fibroblasts. Role of the two transporter species and their subcellular localization. Biochem J. 1990 Sep 1;270(2):331–336. doi: 10.1042/bj2700331. [DOI] [PMC free article] [PubMed] [Google Scholar]