Abstract
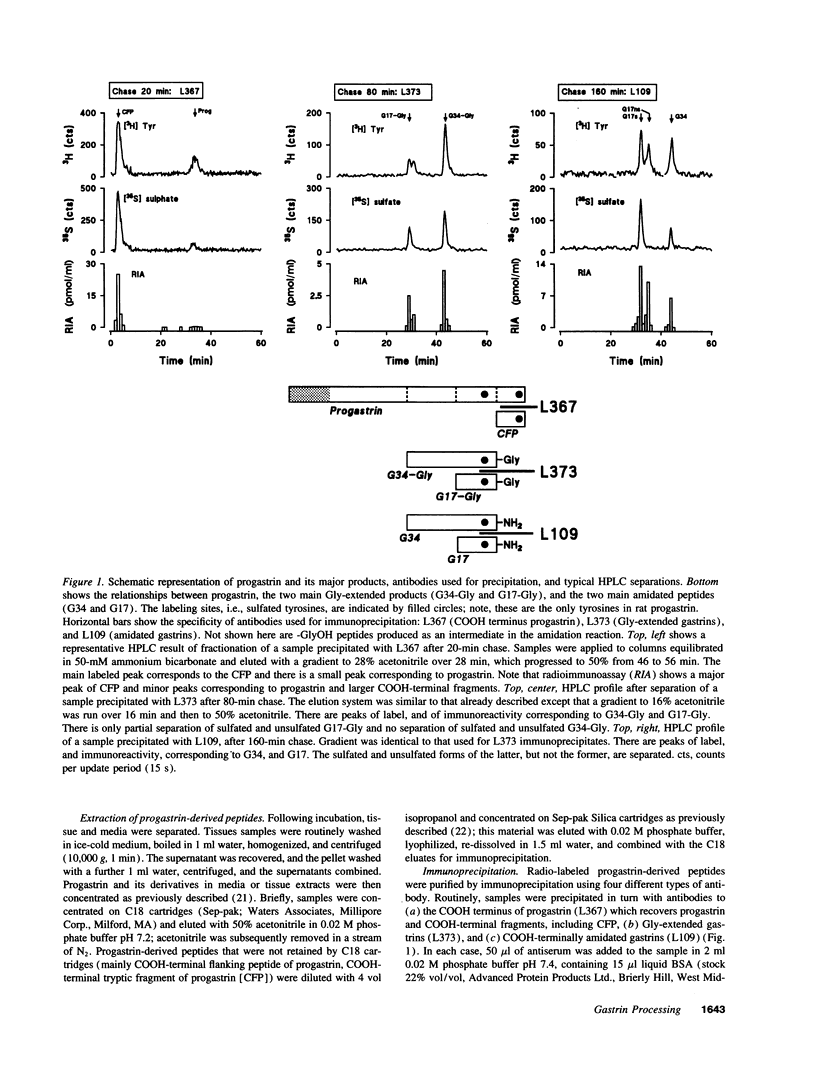
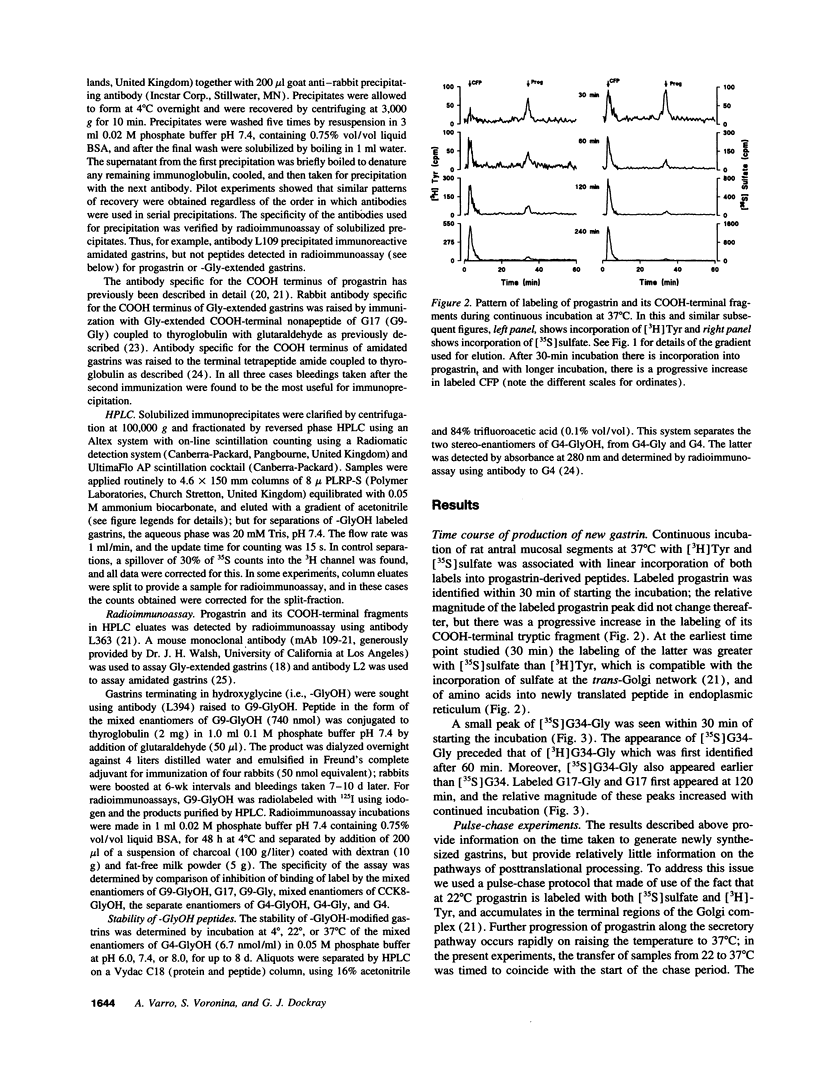
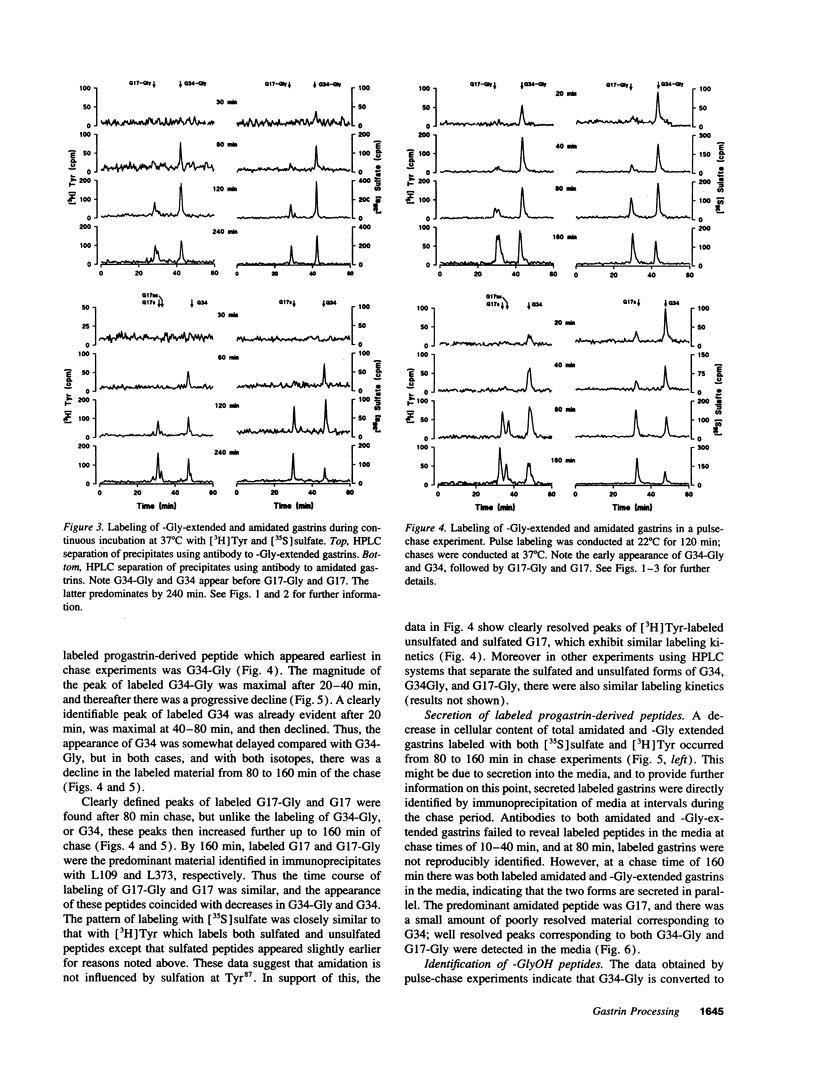
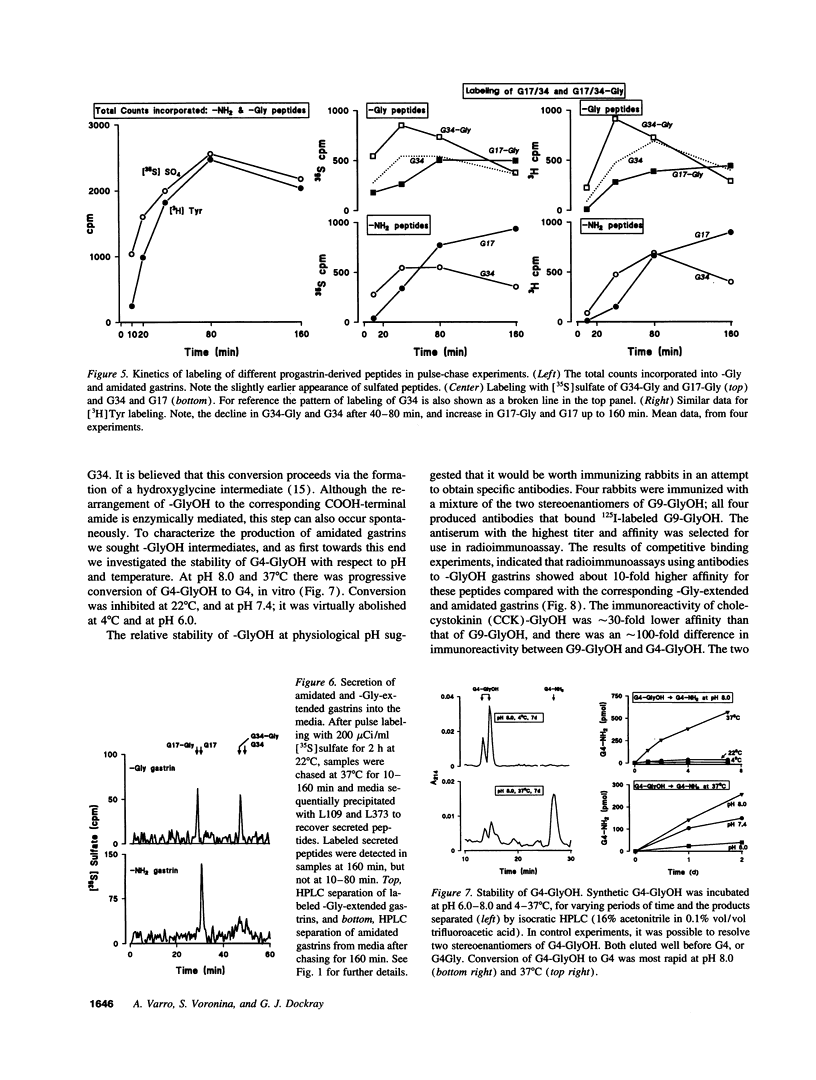
The precursor of the acid-stimulating hormone gastrin gives rise to multiple peptides differing markedly in biological activity, but the relevant biosynthetic pathways are poorly understood. We have used antibodies to amidated gastrins, gastrins with COOH-terminal glycine (Gly) gastrins with COOH-terminal hydroxyglycine (GlyOH) and to the COOH terminus of progastrin, to immunoprecipitate peptides labeled with [35S]sulfate or [3H]tyrosine during incubation of rat antral mucosa in vitro. Labeled progastrin was detectable after 30 min of continuous incubation with isotopic precursors, G34 and G34-Gly after 60 min, and G17 and G17-Gly after 120 min. Pulse chase experiments indicated that progastrin is converted to G34-Gly which then follows one of two pathways: (a) hydroxylation of COOH-terminal Gly and conversion to G34 followed by cleavage yielding G17, or (b) cleavage to G17-Gly. The kinetics of G17-Gly and G17 labeling were similar, suggesting that G17-Gly is a product in its own right, and not simply an intermediate in G17 synthesis. Since the two peptides are reported to have distinct biological activities, they appear to be alternative mature products of progastrin processing.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma T., Taggart R. T., Walsh J. H. Effects of bombesin on the release of glycine-extended progastrin (gastrin G) in rat antral tissue culture. Gastroenterology. 1987 Aug;93(2):322–329. doi: 10.1016/0016-5085(87)91022-5. [DOI] [PubMed] [Google Scholar]
- Baldwin G. S., Casey A., Mantamadiotis T., McBride K., Sizeland A. M., Thumwood C. M. PCR cloning and sequence of gastrin mRNA from carcinoma cell lines. Biochem Biophys Res Commun. 1990 Jul 31;170(2):691–697. doi: 10.1016/0006-291x(90)92146-q. [DOI] [PubMed] [Google Scholar]
- Baldwin G. S., Zhang Q. X. Measurement of gastrin and transforming growth factor alpha messenger RNA levels in colonic carcinoma cell lines by quantitative polymerase chain reaction. Cancer Res. 1992 Apr 15;52(8):2261–2267. [PubMed] [Google Scholar]
- Berson S. A., Yllow R. S. Nature of immunoreactive gastrin extracted from tissues of gastrointestinal tract. Gastroenterology. 1971 Feb;60(2):215–222. [PubMed] [Google Scholar]
- Brand S. J., Klarlund J., Schwartz T. W., Rehfeld J. F. Biosynthesis of tyrosine O-sulfated gastrins in rat antral mucosa. J Biol Chem. 1984 Nov 10;259(21):13246–13252. [PubMed] [Google Scholar]
- Calam J., Dockray G. J., Walker R., Tracy H. J., Owens D. Molecular forms of gastrin in peptic ulcer: comparison of serum and tissue concentrations of G17 and G34 in gastric and duodenal ulcer subjects. Eur J Clin Invest. 1980 Jun;10(3):241–247. doi: 10.1111/j.1365-2362.1980.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Ciccotosto G. D., Shulkes A. Pharmacokinetics and organ specific metabolism of glycine-extended and amidated gastrin in sheep. Am J Physiol. 1992 Nov;263(5 Pt 1):G802–G809. doi: 10.1152/ajpgi.1992.263.5.G802. [DOI] [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Dockray G. J., Best L., Taylor I. L. Immunochemical characterization of gastrin in pancreatic islets of normal and genetically obese mice. J Endocrinol. 1977 Feb;72(2):143–151. doi: 10.1677/joe.0.0720143. [DOI] [PubMed] [Google Scholar]
- Dockray G. J., Vaillant C., Hopkins C. R. Biosynthetic relationships of big and little gastrins. Nature. 1978 Jun 29;273(5665):770–772. doi: 10.1038/273770a0. [DOI] [PubMed] [Google Scholar]
- Dockray G. J., Varro A., Desmond H., Young J., Gregory H., Gregory R. A. Post-translational processing of the porcine gastrin precursor by phosphorylation of the COOH-terminal fragment. J Biol Chem. 1987 Jun 25;262(18):8643–8647. [PubMed] [Google Scholar]
- Eipper B. A., Milgram S. L., Husten E. J., Yun H. Y., Mains R. E. Peptidylglycine alpha-amidating monooxygenase: a multifunctional protein with catalytic, processing, and routing domains. Protein Sci. 1993 Apr;2(4):489–497. doi: 10.1002/pro.5560020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysselein V. E., Maxwell V., Reedy T., Wünsch E., Walsh J. H. Similar acid stimulatory potencies of synthetic human big and little gastrins in man. J Clin Invest. 1984 May;73(5):1284–1290. doi: 10.1172/JCI111330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley G. G., Koski R. A., Melhem M. F., Pipas J. M., Meisler A. I. Expression of the gastrin gene in the normal human colon and colorectal adenocarcinoma. Cancer Res. 1993 Jun 15;53(12):2919–2926. [PubMed] [Google Scholar]
- GREGORY R. A., TRACY H. J. THE CONSTITUTION AND PROPERTIES OF TWO GASTRINS EXTRACTED FROM HOG ANTRAL MUCOSA. Gut. 1964 Apr;5:103–114. [PMC free article] [PubMed] [Google Scholar]
- Gregory R. A., Tracy H. J. Isolation of two "big gastrins" from Zollinger-Ellison tumour tissue. Lancet. 1972 Oct 14;2(7781):797–799. doi: 10.1016/s0140-6736(72)92151-4. [DOI] [PubMed] [Google Scholar]
- Hilsted L., Rehfeld J. F. Alpha-carboxyamidation of antral progastrin. Relation to other post-translational modifications. J Biol Chem. 1987 Dec 15;262(35):16953–16957. [PubMed] [Google Scholar]
- Huebner V. D., Jiang R. L., Lee T. D., Legesse K., Walsh J. H., Shively J. E., Chew P., Azumi T., Reeve J. R., Jr Purification and structural characterization of progastrin-derived peptides from a human gastrinoma. J Biol Chem. 1991 Jul 5;266(19):12223–12227. [PubMed] [Google Scholar]
- Kochman M. L., DelValle J., Dickinson C. J., Boland C. R. Post-translational processing of gastrin in neoplastic human colonic tissues. Biochem Biophys Res Commun. 1992 Dec 15;189(2):1165–1169. doi: 10.1016/0006-291x(92)92326-s. [DOI] [PubMed] [Google Scholar]
- Lamers C. B., Walsh J. H., Jansen J. B., Harrison A. R., Ippoliti A. F., van Tongere J. H. Evidence that gastrin 34 is preferentially released from the human duodenum. Gastroenterology. 1982 Jul;83(1 Pt 2):233–239. [PubMed] [Google Scholar]
- Malstrom J., Stadil F., Rehfeld J. F. Gastrins in tissue. Concentration and component pattern in gastric, duodenal, and jejunal mucosa of normal human subjects and patients with duodenal ulcer. Gastroenterology. 1976 May;70(5 PT1):697–703. [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Nemeth J., Taylor B., Pauwels S., Varro A., Dockray G. J. Identification of progastrin derived peptides in colorectal carcinoma extracts. Gut. 1993 Jan;34(1):90–95. doi: 10.1136/gut.34.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahier J., Pauwels S., Dockray G. J. Biosynthesis of gastrin. Localization of the precursor and peptide products using electron microscopic-immunogold methods. Gastroenterology. 1987 May;92(5 Pt 1):1146–1152. [PubMed] [Google Scholar]
- Rehfeld J. F. The expression of progastrin, procholecystokinin and their hormonal products in pituitary cells. J Mol Endocrinol. 1988 Sep;1(2):87–94. doi: 10.1677/jme.0.0010087. [DOI] [PubMed] [Google Scholar]
- Seva C., Dickinson C. J., Yamada T. Growth-promoting effects of glycine-extended progastrin. Science. 1994 Jul 15;265(5170):410–412. doi: 10.1126/science.8023165. [DOI] [PubMed] [Google Scholar]
- Sugano K., Aponte G. W., Yamada T. Identification and characterization of glycine-extended post-translational processing intermediates of progastrin in porcine stomach. J Biol Chem. 1985 Sep 25;260(21):11724–11729. [PubMed] [Google Scholar]
- Sugano K., Park J., Dobbins W. O., Yamada T. Glycine-extended progastrin processing intermediates: accumulation and cosecretion with gastrin. Am J Physiol. 1987 Oct;253(4 Pt 1):G502–G507. doi: 10.1152/ajpgi.1987.253.4.G502. [DOI] [PubMed] [Google Scholar]
- Van Solinge W. W., Nielsen F. C., Friis-Hansen L., Falkmer U. G., Rehfeld J. F. Expression but incomplete maturation of progastrin in colorectal carcinomas. Gastroenterology. 1993 Apr;104(4):1099–1107. doi: 10.1016/0016-5085(93)90279-l. [DOI] [PubMed] [Google Scholar]
- Varro A., Desmond H., Pauwels S., Gregory H., Young J., Dockray G. J. The human gastrin precursor. Characterization of phosphorylated forms and fragments. Biochem J. 1988 Dec 15;256(3):951–957. doi: 10.1042/bj2560951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varro A., Dockray G. J. Post-translational processing of progastrin: inhibition of cleavage, phosphorylation and sulphation by brefeldin A. Biochem J. 1993 Nov 1;295(Pt 3):813–819. doi: 10.1042/bj2950813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varro A., Henry J., Vaillant C., Dockray G. J. Discrimination between temperature- and brefeldin A-sensitive steps in the sulfation, phosphorylation, and cleavage of progastrin and its derivatives. J Biol Chem. 1994 Aug 12;269(32):20764–20770. [PubMed] [Google Scholar]
- Varro A., Nemeth J., Bridson J., Lee C., Moore S., Dockray G. J. Processing of the gastrin precursor. Modulation of phosphorylated, sulfated, and amidated products. J Biol Chem. 1990 Dec 15;265(35):21476–21481. [PubMed] [Google Scholar]
- Walsh J. H., Debas H. T., Grossman M. I. Pure human big gastrin. Immunochemical properties, disappearance half time, and acid-stimulating action in dogs. J Clin Invest. 1974 Aug;54(2):477–485. doi: 10.1172/JCI107783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. H., Isenberg J. I., Ansfield J., Maxwell V. Clearance and acid-stimulating action of human big and little gastrins in duodenal ulcer subjects. J Clin Invest. 1976 May;57(5):1125–1131. doi: 10.1172/JCI108379. [DOI] [PMC free article] [PubMed] [Google Scholar]



