Abstract
Human papillomaviruses (HPV) are small DNA viruses that target stratified keratinocytes for infection. A subset of HPV types infect epithelia in the genital tract and are the causative agents of cervical as well as other anogenital cancers. Interferon treatment of existing genital HPV lesions has had mixed results. While HPV proteins down-regulate the expression of interferon-inducible genes, interferon treatment ultimately induces their high-level transcription after a delay. Cells containing complete HPV genomes that are able to undergo productive replication upon differentiation are sensitive to interferon-induced growth arrest, while cells from high-grade cancers that only express E6 and E7 are resistant. Recent studies indicate this sensitivity is dependent upon the binding of the interferon-inducible factor, p56, to the E1 replication protein. The response to interferon by HPV proteins is complex and results from the action of multiple viral proteins.
Introduction
Human papillomaviruses (HPV) are small, double-stranded DNA viruses that induce hyperproliferative lesions of cutaneous and mucosal epithelial tissues (Howley 1996; zur Hausen 2002). HPVs infect stratified epithelia and establish infections that can persist for decades. For these infections to persist, papillomaviruses have evolved strategies to evade the effects of the innate immune system during the initial phases of infection as well as long-term surveillance by the adaptive immune system. Over 100 different viral types have been identified and, of these, ∼30 specifically infect epithelial cells in the genital tract (zur Hausen 2002). Genital papillomavirus infections are passed through sexual contact and are the most common sexually transmitted viral infections. It is currently estimated that ∼20 million Americans are infected and that 70% of sexually active persons will acquire a genital HPV infection during their lifetime (Centers for Disease Control and Prevention [updated 2009]).
Genital HPV are categorized as high- and low-risk virus types based on their potential to induce malignant transformation. The high-risk viral types, including HPV-16, 18, 31, 33, 45, are associated at a high frequency with the development of malignant lesions and are the causative agents of cervical cancer. Over 99% of cervical tumors contain HPV DNA (Walboomers and others 1999; zur Hausen 2002). In contrast, low-risk HPVs such as HPV-6 and 11 induce only benign warts in the genital tract, which are at low risk for progression to malignancy. HPV genomes are found as extrachromosomal elements or episomes in precancerous cervical lesions, while genomes are often found integrated into host DNA in cervical carcinomas (Parkin and others 2001).
Initial infection with genital HPVs results in low-grade lesions called dysplasias or cervical intraepithelial neoplasia grade I and most are cleared by the immune system within 1 or 2 years (Jenson and others 1991; Hopfl and others 2000). However, some infections can persist for extended periods as they are not effectively cleared by the immune system. Persistence of high-risk HPV infections is the major risk factor for the development of cervical cancer and other genital malignancies (zur Hausen 1996). Infection with high-risk HPV combined with other risk factors such as immunosuppression, cigarette smoking and coinfection with human immunodeficiency virus can lead to the development of cervical cancer.
Cervical cancer is the second most prevalent cancer worldwide and it is estimated that 470,000 new cases are diagnosed each year (Parkin and others 2001). Although most cases of cervical cancer are found outside of the United States in economically disadvantaged nations, ∼11,000 U.S. women are diagnosed yearly and ∼5,000 die of the disease (Parkin and others 2001). In 2006, the FDA approved an HPV vaccine. Gardasil (Merck & Co.) is a prophylactic vaccine and is, therefore, not effective in treating those already infected with HPV. The Gardasil vaccine consists of recombinant virus-like particles (VLPs) that are spontaneously self-assembled upon expression of L1, the major viral capsid protein. VLPs do not contain viral DNA and trigger an antibody response that protects against infection. The vaccine protects against infection with HPV types 16 and 18, which are responsible for 70% of cervical cancer cases, as well as with HPV-6 and 11, which cause 90% of genital warts cases (Centers for Disease Control and Prevention [updated 2009]).
The Human Papillomavirus Life Cycle
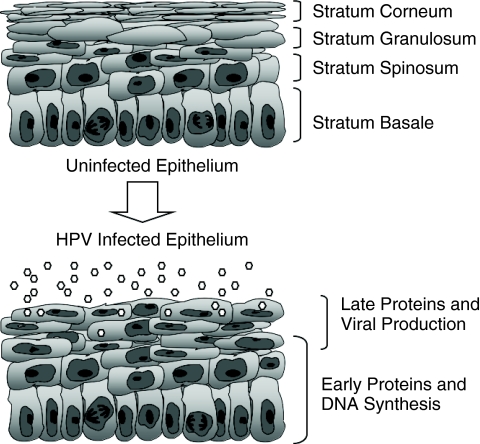
HPVs are nonenveloped viruses that replicate in the nuclei of the infected host cell (Longworth and Laimins 2004b). The productive life cycle of HPVs is directly linked to differentiation of the infected epithelial cell (Fig. 1). Infection by papillomaviruses is believed to occur through microtraumas in the epithelium, which exposes cells in the basal layer to entry by viruses. The receptor that mediates viral entry remains unknown although heparin sulfate is likely responsible for the initial attachment of virions to cells (Howley 1996). Once the virus has entered into keratinocytes in the basal layer, HPV genomes are established in the nucleus as replicating extrachromosomal episomes and a low level of HPV expression occurs. This viral gene expression allows for the stable maintenance of viral episomes at approximately 20 to 100 copies per cell. As these infected basal cells divide, viral genomes are replicated along with host chromosomes and distributed equally to daughter cells. One of the infected daughter cells detaches from the basal layer, migrates toward the suprabasal regions, and undergoes differentiation. The other daughter cell remains in the basal layer to provide for further cell division (Stubenrauch and others 1998; Fehrmann and Laimins 2003; Longworth and Laimins 2004b). Normally, uninfected keratinocytes exit the cell cycle as they detach from the basement membrane and in many keratinocytes this results in degradation of nuclei in suprabasal cells. However, HPV-positive cells remain active in the cell cycle after leaving the basal layer through the action of viral proteins. Upon differentiation, suprabasal HPV-positive cells are induced to reenter S phase and activate expression of cellular factors necessary for replication to induce viral DNA amplification (Cheng and others 1995; Flores and others 2000). Concurrent with DNA amplification, late viral proteins including capsid proteins are expressed resulting in the assembly of infectious virions. Following assembly, mature viruses are released from the upper layers of the epithelium through shedding (Howley 1996; Longworth and Laimins 2004b). The mechanisms that regulate the differentiation-dependent phase of the viral life cycle are only now beginning to be understood.
FIG. 1.
Diagram showing normal and human papillomavirus (HPV) infected stratified epithelia. Normal keratinocytes are actively dividing only in the basal layer, and many suprabasal epithelial cells lose nuclei as they differentiate. HPV-positive cells remain active in the cell cycle throughout all epithelial layers and induce late viral functions as well as virion production in suprabasal layers.
The HPV Genome
All HPV genomes contain approximately 8 open-reading frames (ORFs) that are expressed from polycistronic mRNAs transcribed from a single strand of DNA (Howley 1996). HPV gene products are divided into early and late proteins and their expression is directed from 2 major promoters. The early promoter directs expression of early HPV proteins and initiates upstream of the E6 ORF. Transcription of the early promoter occurs in undifferentiated cells and prior to productive replication. The early HPV proteins include E1, E2, E1^E4, E5, E6, and E7. Late viral transcription is triggered upon differentiation of the host cell and is activated from start sites located within the E7 ORF. Late genes expressed from the late promoter include those encoding the capsid proteins L1 and L2 as well as E1^E4 and E5 (Howley 1996; Longworth and Laimins 2004b). Because activation of late transcription is so tightly linked to epithelial cell differentiation, it is thought that differentiation-specific cellular factors regulate this process (del Mar Pena and Laimins 2001).
HPV Proteins: E6 and E7
The high-risk E6 and E7 proteins are selectively retained and expressed in cervical cancers. These proteins act cooperatively to mediate immortalization and transformation. The E7 proteins are ∼100 amino acids in size and are found predominately in the nucleus of HPV-infected cells (Longworth and Laimins 2004b). High-risk E7 proteins by themselves can immortalize NIH 3T3 mouse cells (Riley and others 2003) but require the presence of E6 to efficiently immortalize human keratinocytes (Munger and others 1989). All high-risk E7 proteins contain 3 conserved regions: CR1, CR2, and CR3. CR1 and CR2 domains have sequence homology to similar domains of the adenoviral E1A protein (Phelps and others 1988; Phelps and others 1992).
The CR2 domain of E7 contains an LXCXE motif that is important for binding the retinoblastoma (Rb) family of cellular proteins, while the CR3 domain contains 2 zinc fingerlike motifs (Longworth and Laimins 2004b). The Rb family of proteins includes Rb, p107, and p130, and these factors are important for regulation of the cell cycle. Rb family members regulate transition through the G1 phase of the cell cycle by acting as negative regulators of the E2F family of transcriptional activators, which control expression of S-phase specific genes (Edmonds and Vousden 1989; Jewers and others 1992; Berezutskaya and others 1997; Wang and others 2001). The binding of E7 to Rb family members results in the constitutive activation of genes involved in cell cycle progression (Edmonds and Vousden 1989; Weintraub and others 1995). The ability to bind Rb family members extends to the low-risk E7 proteins, which bind at 10-fold less affinity than their high-risk counterparts (Ciccolini and others 1994; Schmitt and others 1994). Rb family members also control cell cycle exit that normally occurs during epithelial differentiation. The disruption of Rb function by E7 allows for epithelial cells to remain active in the cell cycle upon differentiation, which is necessary for productive viral replication (Chellappan and others 1992).
E7 proteins can also increase the activity of other cell cycle regulators responsible for driving cell cycle progression. E7 associates with cyclins A (directly) and E (indirectly via p107) as well as the cyclin-dependent kinase (cdk) inhibitors, p21 and p27 (Davies and others 1993; Tommasino and others 1993; Zerfass-Thome and others 1996; Funk and others 1997; Jones and others 1997; Ruesch and Laimins 1998). A third group of proteins that bind high-risk E7 are the class I histone deacetylases (HDACs) (Brehm and others 1998; Longworth and Laimins 2004b). HDACs are transcriptional corepressors that remove acetyl groups from the lysine-rich N-terminal tails of histone proteins (Longworth and Laimins 2004b). E7 proteins bind to HDACs resulting in increased E2F-mediated transcription and S-phase progression (Longworth and others 2005). HPV-31 genomes harboring mutations that block the binding of E7 to HDACs result in an inability to maintain viral DNA as episomes and have a limited lifespan (Longworth and Laimins 2004a).
The high- and low-risk E6 proteins are ∼150 amino acids in size and are localized to both nuclear and cytoplasmic compartments. E6 proteins have 2 zinc-binding domains containing 4 Cys-X-X-Cys motifs (Cole and Danos 1987; Barbosa and others 1989) and act as oncoproteins. Expression of E6 alone can immortalize human mammary epithelial cells and transform NIH3T3 cells (Kiyono and others 1998; Liu and others 1999). However, E6 requires the coexpression of E7 to efficiently immortalize human keratinocytes (Hawley-Nelson and others 1989). E6 has been reported to bind over 12 different proteins (zur Hausen 2002). The best characterized binding interaction is with the p53 tumor suppressor protein. E6 binds to p53 in a trimeric complex with the E3 cellular ubiquitin ligase, E6AP, causing the rapid degradation of p53 through the 26S proteasome (Scheffner and others 1990; Werness and others 1990). p53 is activated upon DNA damage through various modifications and regulates transcription of genes involved in cell cycle control and apoptosis. The E6-mediated down-regulation of p53 abrogates the proapoptotic activities of p53 and allows for viral replication (Howley 1996).
High-risk E6 can also interfere with p53-mediated cell cycle regulation through its interaction with a p53 coactivator, p300/CBP (Patel and others 1999; Zimmermann and others 1999), which is independent of E6's ability to degrade p53 (Zimmermann and others 1999; Thomas and Chiang 2005). p300/CBP proteins are histone acetyltransferases that modulate transcription through the acetylation of histones at target promoters. Acetylation of histones rearranges chromatin into a more open and assessable configuration, leading to the activation of target genes (Zimmermann and others 1999; Chan and La Thangue 2001; Grossman 2001; Vo and Goodman 2001; Thomas and Chiang 2005). In addition, p53 itself can be acetylated by p300/CBP at several lysine residues in the C-terminus and this results in a more stable p53 protein with increased transcriptional activity (Fu and others 2004; Yang 2004). In HPV-infected cells, E6 forms a complex with both p53 and p300/CBP and blocks acetylation of p53 by p300/CBP (Zimmermann and others 1999). The ability of E6 to block this acetylation of p53 and its subsequent activation represents another mechanism by which HPV has evolved to block cell cycle arrest.
Additional binding partners of E6 include members of the PDZ domain containing family including MUPP-1, hDlg, hScribble, and MAGI. PDZ-binding proteins are involved in cell signaling and cell–cell adhesion (Kim 1997). Binding of these proteins occurs at the extreme C-terminus of E6 and results in the degradation of the PDZ proteins (Lee and others 2000; Nakagawa and Huibregtse 2000; Thomas and others 2002). Transgenic mice expressing E6 mutants that lack the PDZ-binding domain do not develop epidermal hyperplasias as seen with wild-type E6 (Nguyen and others 2003). Furthermore, HPV-31 genomes harboring mutations in the E6 PDZ-binding domain showed defects in growth, early transcription, as well as episomal maintenance (Lee and Laimins 2004). Another function of high-risk E6 important for immortalization is the activation of the catalytic subunit of telomerase, hTERT (Klingelhutz and others 1996). As will be discussed, E6 proteins have also been shown to interact with members of the innate immune response pathway, such as IRF-3 (Ronco and others 1998).
E1 and E2 Replication Proteins
The E1 and E2 replication proteins are among the first to be expressed upon viral infection. These proteins form a complex and bind to sequences at the viral origin of replication. Once bound, they recruit cellular polymerases and accessory proteins to mediate replication (Mohr and others 1990; Frattini and Laimins 1994; Conger and others 1999). E1 proteins are expressed at low levels upon viral infection and function in origin recognition by binding to AT-rich sequences in the HPV origin (Frattini and Laimins 1994; Muller and others 1997; Chen and Stenlund 2001). The E1 protein also exhibits DNA helicase activity, such that it separates viral DNA strands ahead of the replication complex (Hughes and Romanos 1993). Interestingly, E1 can only bind HPV origins weakly by itself and requires complex formation with E2 to assemble onto the origin. E2 also modulates the activity of cellular transcription factors to regulate viral transcription from the early promoter (Cripe and others 1987).
Late Proteins: E1^E4, E5, L1, and L2
The E5 and E1^E4 proteins are expressed primarily in the differentiation-dependent phase of the viral life cycle (Howley 1996). The E4 ORF lacks an AUG translation initiator codon and uses the corresponding sequence in the E1 ORF to initiate translation. High-risk E1^E4 proteins can induce the collapse of keratin networks when overexpressed in cells, suggesting a role for E1^E4 proteins in viral egress (Doorbar and others 1991). Additionally, overexpression of E1^E4 proteins from HPV-11 and 16 induces a G2 arrest in various cell types (Davy and others 2002). HPV E5 is a small, hydrophobic membrane protein that localizes to endosomal membranes, the Golgi, and occasionally to the plasma membrane (Conrad and others 1993). In bovine papillomavirus, the E5 ORF encodes the viral oncoprotein that possesses strong transforming activity (Petti and others 1991). E5 also regulates the activation of late viral functions in cells induced to differentiate, suggesting a primary role for E5 in the productive phase of the viral life cycle (Fehrmann and others 2003; Genther and others 2003). More recently, E5 was shown to associate with the B-cell-associated protein 31 (Bap31) and this interaction is important for the proliferative capacity of HPV-positive cells upon differentiation (Regan and Laimins 2008). Viral capsids are composed of L1 and L2 and the expression of these proteins is restricted to the highly differentiated, suprabasal cells. HPV virions are icosahedral in structure and are composed of 360 L1 monomers that are assembled into 72 pentameric structures called capsomeres (Chen and others 2000; Modis and others 2002).
HPV and Interferon
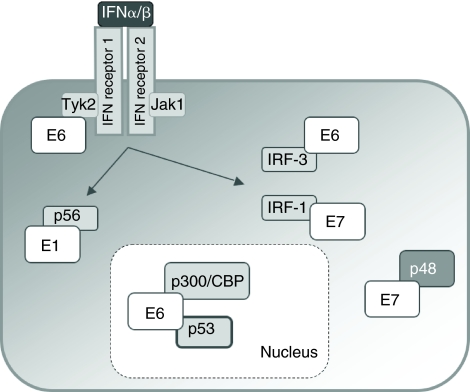
Recent studies have demonstrated that HPV proteins target aspects of the innate immune system (Fig. 2). Normal keratinocytes constitutively express low levels of interferons in the absence of viral infection (Bielenberg and others 1999). HPV proteins modulate the response to interferon in several ways. First, the levels of interferon-inducible genes are reduced in HPV-infected cells from that seen in normal keratinocytes. Microarray analyses have demonstrated that the expression of MxA, 2′–5′ oligoadenylate synthetase 2, as well as Stat-1 is reduced in HPV-positive cells. The addition of interferon still induces expression of these interferon-inducible genes but at initially reduced rates. Following 24 h of exposure to interferon, the levels of expression, however, increases to that seen in normal cells (Chang and Laimins 2000).
FIG. 2.
Cartoon diagramming the multiple ways human papillomavirus (HPV) proteins interfere with the response to interferon. E6 targets Tyk2 as well as IRF-3 and p300/p53. E7 binds to p48 and IRF-1. E1 binds to p56.
Additional studies have demonstrated that HPV proteins directly target components of the innate immune system to inhibit their action. The E6 oncoprotein has been shown to bind to interferon regulatory factor-3 (IRF-3), thereby interfering with its ability to act as a transcriptional activator (Ronco and others 1998). In addition, E6 has been reported to bind the Tyk2 kinase of the Jak-Sat pathway and inhibit its function (Li and others 1999). In a similar manner, E7 has been shown to bind to IRF-1 and block its functions (Park and others 2000). E7 has also been shown to bind to p48, a component of the interferon-stimulated gene factor 3 (ISGF3) complex, blocking the translocation of this complex to the nucleus and its ability to activate gene expression in response to interferon (Barnard and McMillan 1999). These interactions may provide a mechanism by which HPV proteins suppress expression of interferon-inducible genes.
PKR is commonly targeted by viral proteins and similar effects are seen in HPV infections. The levels of total and phosphorylated PKR are reduced in HPV-positive cells through the synergistic action of E6 and E7. This reduction of PKR activity leads to a reduced level of eIF2α phosphorylation following infection with VSV (Hebner and others 2006). HPV-18 E6 has been shown to directly interfere with phosphorylation of eIF2α leading to inhibition of translation regulation (Kazemi and others 2004). In addition to blocking PKR activity, the HPV E6 protein induces the relocalization of PKR to tight cytoplasmic clusters that correspond to P-bodies. P-bodies are sites of mRNA storage as well as degradation and the relocalization of PKR to these sites may contribute to the inhibition of PKR activity (Hebner and others 2006).
Since IFN induction is a potent antiviral defense mechanism, IFN therapy has been suggested as a treatment for HPV infections. Unfortunately, variable results have been observed in clinical trials (Bornstein and others 1997; Garcia-Millian and others 1999; Gonzalez-Sanchez and others 2001). Interferons have been used successfully in treating patients with genital warts induced by low-risk HPV types but show mixed results in treating low-grade lesions and cancers induced by high-risk HPVs (zur Hausen 2002). Early studies using mouse cells containing episomal copies of bovine papillomavirus DNA demonstrated that treatment with interferon caused a complete elimination of viral DNA and reversion to a nontransformed phenotype (Turek and others 1982). Long-term treatment of human keratinocytes that stably maintained HPV episomes resulted in growth arrest and apoptosis (Chang and others 2002). A small population of cells survived and these were found to contain only integrated copies of high-risk HPV-31 DNA. Interferon treatment of cells derived from high-grade cervical cancers that contained only integrated copies of HPV had no effect on their growth properties (Chang and others 2002). Similar effects have also been reported using cells that maintained episomal copies of HPV-16 (Herdman and others 2006). This suggests that interferon treatment may select for cells with integrated copies of HPV DNA making it an undesirable method for treating patients with either high- or low-grade HPV infections.
Recent studies have determined that expression of the interferon-inducible protein p56 blocks HPV replication by binding the E1 replication protein and sequestering it to the cytoplasm (Terenzi and others 2008). This block in replication of cells containing episomal copies of HPV may result in either loss of viral DNAs or integration into host chromosomes. Cells with integrated copies of HPV sequences are usually found in high-grade lesions suggesting this may be important for progression of HPV-induced disease perhaps through increased expression of E6/E7 oncoproteins. The interaction of p56 with E1 sheds light onto why treatment with interferon causes cells with viral episomes to loose the ability to maintain the DNA extrachromosomally but has no effect on cells with integrated copies of HPV sequences.
Insights into the cooperative action of E6 and E7 in mediating resistance to interferon have focused on the acetylation status of p53. The high-risk E7 protein acts to increase p53 levels, while E6 accelerates its degradation through the binding of the cellular ubiquitin ligase E6AP (Longworth and Laimins 2004b). The expression of E7 sensitized keratinocytes to the growth-inhibitory effects of interferon and coexpression with E6 abrogated this effect (Hebner and others 2007). Treatment of E7-expressing cells with interferon ultimately resulted in growth arrest and cellular senescence through a process that is dependent upon acetylation of p53 by p300/CBP. The E6 protein binds p300/CBP and inhibits its acetylation of p53. Mutant forms of E6 that are unable to bind p300/CBP or p53 failed to block acetylation of p53 and were sensitive to growth arrest by interferon. Interestingly the binding of E6AP to E6 did not contribute to the inhibitory effects of interferon (Hebner and others 2007). This identified an important physiological role for E6 binding to p300/CBP in blocking growth arrest of human keratinocytes in the presence of interferon and so contributes to the persistence of HPV-infected cells.
In summary, HPV have evolved a number of strategies to overcome the effects of interferon. Treatment with interferon can result in selecting cells with integrated copies of HPV DNA making it an ineffective methodology unless it can be combined with other therapeutic agents.
Acknowledgments
This work was supported by grants from the NCI (R37CA74202) and NIAID (UO1 AI31494) to L.A.L. M.B. was supported by carcinogenesis training grant T32 CA009560-20.
References
- Barbosa MS. Lowy DR. Schiller JT. Papillomavirus polypeptides E6 and E7 are zinc-binding proteins. J Virol. 1989;63(3):1404–1407. doi: 10.1128/jvi.63.3.1404-1407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard P. McMillan NA. The human papillomavirus E7 oncoprotein abrogates signaling mediated by interferon-alpha. Virology. 1999;259(2):305–313. doi: 10.1006/viro.1999.9771. [DOI] [PubMed] [Google Scholar]
- Berezutskaya E. Yu B. Morozov A. Raychaudhuri P. Bagchi S. Differential regulation of the pocket domains of the retinoblastoma family proteins by the HPV16 E7 oncoprotein. Cell Growth Differ. 1997;8(12):1277–1286. [PubMed] [Google Scholar]
- Bielenberg DR. McCarty MF. Bucana CD. Yuspa SH. Morgan D. Arbeit JM. Ellis LM. Cleary KR. Fidler IJ. Expression of interferon-beta is associated with growth arrest of murine and human epidermal cells. J Invest Dermatol. 1999;112(5):802–809. doi: 10.1046/j.1523-1747.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- Bornstein J. Pascal B. Zarfati D. Goldshmid N. Abramovici H. Recombinant human interferon-beta for condylomata acuminata: a randomized, double-blind, placebo-controlled study of intralesional therapy. Int J STD AIDS. 1997;8(10):614–621. doi: 10.1258/0956462971918878. [DOI] [PubMed] [Google Scholar]
- Brehm A. Miska EA. McCance DJ. Reid JL. Bannister AJ. Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391(6667):597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention. Genital HPV infection—CDC fact sheet. 2009. http://www.cdc.gov/std/HPV/STDFact-HPV.htm http://www.cdc.gov/std/HPV/STDFact-HPV.htm
- Chan HM. La Thangue NB. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci. 2001;114(Pt 13):2363–2373. doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- Chang YE. Laimins LA. Microarray analysis identifies interferon-inducible genes and Stat-1 as major transcriptional targets of human papillomavirus type 31. J Virol. 2000;74(9):4174–4182. doi: 10.1128/jvi.74.9.4174-4182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YE. Pena L. Sen GC. Park JK. Laimins LA. Long-term effect of interferon on keratinocytes that maintain human papillomavirus type 31. J Virol. 2002;76(17):8864–8874. doi: 10.1128/JVI.76.17.8864-8874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan S. Kraus VB. Kroger B. Munger K. Howley PM. Phelps WC. Nevins JR. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci USA. 1992;89(10):4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. Stenlund A. The E1 initiator recognizes multiple overlapping sites in the papillomavirus origin of DNA replication. J Virol. 2001;75(1):292–302. doi: 10.1128/JVI.75.1.292-302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XS. Garcea RL. Goldberg I. Casini G. Harrison SC. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cell. 2000;5(3):557–567. doi: 10.1016/s1097-2765(00)80449-9. [DOI] [PubMed] [Google Scholar]
- Cheng S. Schmidt-Grimminger DC. Murant T. Broker TR. Chow LT. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 1995;9(19):2335–2349. doi: 10.1101/gad.9.19.2335. [DOI] [PubMed] [Google Scholar]
- Ciccolini F. Di Pasquale G. Carlotti F. Crawford L. Tommasino M. Functional studies of E7 proteins from different HPV types. Oncogene. 1994;9(9):2633–2638. [PubMed] [Google Scholar]
- Cole ST. Danos O. Nucleotide sequence and comparative analysis of the human papillomavirus type 18 genome. Phylogeny of papillomaviruses and repeated structure of the E6 and E7 gene products. J Mol Biol. 1987;193(4):599–608. doi: 10.1016/0022-2836(87)90343-3. [DOI] [PubMed] [Google Scholar]
- Conger KL. Liu JS. Kuo SR. Chow LT. Wang TS. Human papillomavirus DNA replication. Interactions between the viral E1 protein and two subunits of human DNA polymerase alpha/primase. J Biol Chem. 1999;274(5):2696–2705. doi: 10.1074/jbc.274.5.2696. [DOI] [PubMed] [Google Scholar]
- Conrad M. Bubb VJ. Schlegel R. The human papillomavirus type 6 and 16 E5 proteins are membrane-associated proteins which associate with the 16-kilodalton pore-forming protein. J Virol. 1993;67(10):6170–6178. doi: 10.1128/jvi.67.10.6170-6178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripe TP. Haugen TH. Turk JP. Tabatabai F. Schmid PG., III Durst M. Gissmann L. Roman A. Turek LP. Transcriptional regulation of the human papillomavirus-16 E6-E7 promoter by a keratinocyte-dependent enhancer, and by viral E2 trans-activator and repressor gene products: implications for cervical carcinogenesis. EMBO J. 1987;6(12):3745–3753. doi: 10.1002/j.1460-2075.1987.tb02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. Hicks R. Crook T. Morris J. Vousden K. Human papillomavirus type 16 E7 associates with a histone H1 kinase and with p107 through sequences necessary for transformation. J Virol. 1993;67(5):2521–2528. doi: 10.1128/jvi.67.5.2521-2528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy CE. Jackson DJ. Wang Q. Raj K. Masterson PJ. Fenner NF. Southern S. Cuthill S. Millar JB. Doorbar J. Identification of a G(2) arrest domain in the E1 wedge E4 protein of human papillomavirus type 16. J Virol. 2002;76(19):9806–9818. doi: 10.1128/JVI.76.19.9806-9818.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Mar Pena LM. Laimins LA. Differentiation-dependent chromatin rearrangement coincides with activation of human papillomavirus type 31 late gene expression. J Virol. 2001;75(20):10005–10013. doi: 10.1128/JVI.75.20.10005-10013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorbar J. Ely S. Sterling J. McLean C. Crawford L. Specific interaction between HPV-16 E1-E4 and cytokeratins results in collapse of the epithelial cell intermediate filament network. Nature. 1991;352(6338):824–847. doi: 10.1038/352824a0. [DOI] [PubMed] [Google Scholar]
- Edmonds C. Vousden KH. A point mutational analysis of human papillomavirus type 16 E7 protein. J Virol. 1989;63(6):2650–2656. doi: 10.1128/jvi.63.6.2650-2656.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrmann F. Klumpp DJ. Laimins LA. Human papillomavirus type 31 E5 protein supports cell cycle progression and activates late viral functions upon epithelial differentiation. J Virol. 2003;77(5):2819–2831. doi: 10.1128/JVI.77.5.2819-2831.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrmann F. Laimins LA. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene. 2003;22(33):5201–5207. doi: 10.1038/sj.onc.1206554. [DOI] [PubMed] [Google Scholar]
- Flores ER. Allen-Hoffmann BL. Lee D. Lambert PF. The human papillomavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. J Virol. 2000;74(14):6622–6631. doi: 10.1128/jvi.74.14.6622-6631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frattini MG. Laimins LA. Binding of the human papillomavirus E1 origin-recognition protein is regulated through complex formation with the E2 enhancer-binding protein. Proc Natl Acad Sci USA. 1994;91(26):12398–12402. doi: 10.1073/pnas.91.26.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M. Wang C. Zhang X. Pestell RG. Acetylation of nuclear receptors in cellular growth and apoptosis. Biochem Pharmacol. 2004;68(6):1199–1208. doi: 10.1016/j.bcp.2004.05.037. [DOI] [PubMed] [Google Scholar]
- Funk JO. Waga S. Harry JB. Espling E. Stillman B. Galloway DA. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 1997;11(16):2090–2100. doi: 10.1101/gad.11.16.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Millian R. Santos A. Perea SE. Gonzalez-Cabanas R. Valenzuela C. Arana M. Molecular analysis of resistance to interferon in patients with laryngeal papillomatosis. Cytokines Cell Mol Ther. 1999;5(2):79–85. [PubMed] [Google Scholar]
- Genther SM. Sterling S. Duensing S. Munger K. Sattler C. Lambert PF. Quantitative role of the human papillomavirus type 16 E5 gene during the productive stage of the viral life cycle. J Virol. 2003;77(5):2832–2842. doi: 10.1128/JVI.77.5.2832-2842.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sanchez JL. Martinez-Chequer JC. Hernandez-Celaya ME. Barahona-Bustillos E. Andrade-Manzano AF. Randomized placebo-controlled evaluation of intramuscular interferon beta treatment of recurrent human papillomavirus. Obstet Gynecol. 2001;97(4):621–624. doi: 10.1016/s0029-7844(00)01201-1. [DOI] [PubMed] [Google Scholar]
- Grossman SR. p300/CBP/p53 interaction and regulation of the p53 response. Eur J Biochem. 2001;268(10):2773–2778. doi: 10.1046/j.1432-1327.2001.02226.x. [DOI] [PubMed] [Google Scholar]
- Hawley-Nelson P. Vousden KH. Hubbert NL. Lowy DR. Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8(12):3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebner C. Beglin M. Laimins LA. Human papillomavirus E6 proteins mediate resistance to interferon-induced growth arrest through inhibition of p53 acetylation. J Virol. 2007;81(23):12740–12747. doi: 10.1128/JVI.00987-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebner CM. Wilson R. Rader J. Bidder M. Laimins LA. Human papillomaviruses target the double-stranded RNA protein kinase pathway. J Gen Virol. 2006;87(Pt 11):3183–3193. doi: 10.1099/vir.0.82098-0. [DOI] [PubMed] [Google Scholar]
- Herdman MT. Pett MR. Roberts I. Alazawi WO. Teschendorff AE. Zhang XY. Stanley MA. Coleman N. Interferon-beta treatment of cervical keratinocytes naturally infected with human papillomavirus 16 episomes promotes rapid reduction in episome numbers and emergence of latent integrants. Carcinogenesis. 2006;27(11):2341–2353. doi: 10.1093/carcin/bgl172. [DOI] [PubMed] [Google Scholar]
- Hopfl R. Heim K. Christensen N. Zumbach K. Wieland U. Volgger B. Widschwendter A. Haimbuchner S. Müller-Holzner E. Pawlita M. Pfister H. Fritsch P. Spontaneous regression of CIN and delayed-type hypersensitivity to HPV-16 oncoprotein E7. Lancet. 2000;356(9246):1985–1986. doi: 10.1016/S0140-6736(00)03315-8. [DOI] [PubMed] [Google Scholar]
- Howley PM. Papillomaviridae: the viruses and their replication. 3rd. Philadelphia, PA: Lippincott-Raven Publishers; 1996. pp. 947–978. [Google Scholar]
- Hughes FJ. Romanos MA. E1 protein of human papillomavirus is a DNA helicase/ATPase. Nucleic Acids Res. 1993;21(25):5817–5823. doi: 10.1093/nar/21.25.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenson AB. Kurman RJ. Lancaster WD. Tissue effects of and host response to human papillomavirus infection. Dermatol Clin. 1991;9(2):203–209. [PubMed] [Google Scholar]
- Jewers RJ. Hildebrandt P. Ludlow JW. Kell B. McCance DJ. Regions of human papillomavirus type 16 E7 oncoprotein required for immortalization of human keratinocytes. J Virol. 1992;66(3):1329–1335. doi: 10.1128/jvi.66.3.1329-1335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL. Alani RM. Munger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 1997;11(16):2101–2111. doi: 10.1101/gad.11.16.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi S. Papadopoulou S. Li S. Su Q. Wang S. Yoshimura A. Matlashewski G. Dever TE. Koromilas AE. Control of alpha subunit of eukaryotic translation initiation factor 2 (eIF2 alpha) phosphorylation by the human papillomavirus type 18 E6 oncoprotein: implications for eIF2 alpha-dependent gene expression and cell death. Mol Cell Biol. 2004;24(8):3415–3429. doi: 10.1128/MCB.24.8.3415-3429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK. Polarized signaling: basolateral receptor localization in epithelial cells by PDZ-containing proteins. Curr Opin Cell Biol. 1997;9(6):853–859. doi: 10.1016/s0955-0674(97)80088-9. [DOI] [PubMed] [Google Scholar]
- Kiyono T. Foster SA. Koop JI. McDougall JK. Galloway DA. Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396(6706):84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- Klingelhutz AJ. Foster SA. McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380(6569):79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- Lee C. Laimins LA. Role of the PDZ domain-binding motif of the oncoprotein E6 in the pathogenesis of human papillomavirus type 31. J Virol. 2004;78(22):12366–12377. doi: 10.1128/JVI.78.22.12366-12377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS. Glaunsinger B. Mantovani F. Banks L. Javier RT. Multi-PDZ domain protein MUPP1 is a cellular target for both adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J Virol. 2000;74(20):9680–9693. doi: 10.1128/jvi.74.20.9680-9693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. Labrecque S. Gauzzi MC. Cuddihy AR. Wong AH. Pellegrini S. Matlashewski GJ. Koromilas AE. The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak-STAT activation by interferon-alpha. Oncogene. 1999;18(42):5727–5737. doi: 10.1038/sj.onc.1202960. [DOI] [PubMed] [Google Scholar]
- Liu Y. Chen JJ. Gao Q. Dalal S. Hong Y. Mansur CP. Band V. Androphy EJ. Multiple functions of human papillomavirus type 16 E6 contribute to the immortalization of mammary epithelial cells. J Virol. 1999;73(9):7297–7307. doi: 10.1128/jvi.73.9.7297-7307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longworth MS. Laimins LA. The binding of histone deacetylases and the integrity of zinc finger-like motifs of the E7 protein are essential for the life cycle of human papillomavirus type 31. J Virol. 2004a;78(7):3533–3541. doi: 10.1128/JVI.78.7.3533-3541.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longworth MS. Laimins LA. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol Mol Biol Rev. 2004b;68(2):362–372. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longworth MS. Wilson R. Laimins LA. HPV31 E7 facilitates replication by activating E2F2 transcription through its interaction with HDACs. EMBO J. 2005;24(10):1821–1830. doi: 10.1038/sj.emboj.7600651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modis Y. Trus BL. Harrison SC. Atomic model of the papillomavirus capsid. EMBO J. 2002;21(18):4754–4762. doi: 10.1093/emboj/cdf494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr IJ. Clark R. Sun S. Androphy EJ. MacPherson P. Botchan MR. Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science. 1990;250(4988):1694–1699. doi: 10.1126/science.2176744. [DOI] [PubMed] [Google Scholar]
- Muller F. Giroglou T. Sapp M. Characterization of the DNA-binding activity of the E1 and E2 proteins and the E1/E2 complex of human papillomavirus type 33. J Gen Virol. 1997;78(Pt 4):911–915. doi: 10.1099/0022-1317-78-4-911. [DOI] [PubMed] [Google Scholar]
- Munger K. Phelps WC. Bubb V. Howley PM. Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S. Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol. 2000;20(21):8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen ML. Nguyen MM. Lee D. Griep AE. Lambert PF. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6's induction of epithelial hyperplasia in vivo. J Virol. 2003;77(12):6957–6964. doi: 10.1128/JVI.77.12.6957-6964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS. Kim EJ. Kwon HJ. Hwang ES. Namkoong SE. Um SJ. Inactivation of interferon regulatory factor-1 tumor suppressor protein by HPV E7 oncoprotein. Implication for the E7-mediated immune evasion mechanism in cervical carcinogenesis. J Biol Chem. 2000;275(10):6764–6769. doi: 10.1074/jbc.275.10.6764. [DOI] [PubMed] [Google Scholar]
- Parkin DM. Bray F. Ferlay J. Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- Patel D. Huang SM. Baglia LA. McCance DJ. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 1999;18(18):5061–5072. doi: 10.1093/emboj/18.18.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L. Nilson LA. DiMaio D. Activation of the platelet-derived growth factor receptor by the bovine papillomavirus E5 transforming protein. EMBO J. 1991;10(4):845–855. doi: 10.1002/j.1460-2075.1991.tb08017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps WC. Munger K. Yee CL. Barnes JA. Howley PM. Structure-function analysis of the human papillomavirus type 16 E7 oncoprotein. J Virol. 1992;66(4):2418–2427. doi: 10.1128/jvi.66.4.2418-2427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps WC. Yee CL. Munger K. Howley PM. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988;53(4):539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Regan JA. Laimins LA. Bap31 is a novel target of the human papillomavirus E5 protein. J Virol. 2008;82(20):10042–10051. doi: 10.1128/JVI.01240-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RR. Duensing S. Brake T. Munger K. Lambert PF. Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res. 2003;63(16):4862–4871. [PubMed] [Google Scholar]
- Ronco LV. Karpova AY. Vidal M. Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12(13):2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruesch MN. Laimins LA. Human papillomavirus oncoproteins alter differentiation-dependent cell cycle exit on suspension in semisolid medium. Virology. 1998;250(1):19–29. doi: 10.1006/viro.1998.9359. [DOI] [PubMed] [Google Scholar]
- Scheffner M. Werness BA. Huibregtse JM. Levine AJ. Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Schmitt A. Harry JB. Rapp B. Wettstein FO. Iftner T. Comparison of the properties of the E6 and E7 genes of low- and high-risk cutaneous papillomaviruses reveals strongly transforming and high Rb-binding activity for the E7 protein of the low-risk human papillomavirus type 1. J Virol. 1994;68(11):7051–7059. doi: 10.1128/jvi.68.11.7051-7059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubenrauch F. Lim HB. Laimins LA. Differential requirements for conserved E2 binding sites in the life cycle of oncogenic human papillomavirus type 31. J Virol. 1998;72(2):1071–1077. doi: 10.1128/jvi.72.2.1071-1077.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenzi F. Saikia P. Sen GC. Interferon-inducible protein, P56, inhibits HPV DNA replication by binding to the viral protein E1. EMBO J. 2008;27(24):3311–3321. doi: 10.1038/emboj.2008.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. Laura R. Hepner K. Guccione E. Sawyers C. Lasky L. Banks L. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene. 2002;21(33):5088–5096. doi: 10.1038/sj.onc.1205668. [DOI] [PubMed] [Google Scholar]
- Thomas MC. Chiang CM. E6 oncoprotein represses p53-dependent gene activation via inhibition of protein acetylation independently of inducing p53 degradation. Mol Cell. 2005;17(2):251–264. doi: 10.1016/j.molcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Tommasino M. Adamczewski JP. Carlotti F. Barth CF. Manetti R. Contorni M. Cavalieri F. Hunt T. Crawford L. HPV16 E7 protein associates with the protein kinase p33CDK2 and cyclin A. Oncogene. 1993;8(1):195–202. [PubMed] [Google Scholar]
- Turek LP. Byrne JC. Lowy DR. Dvoretzky I. Friedman RM. Howley PM. Interferon induces morphologic reversion with elimination of extrachromosomal viral genomes in bovine papillomavirus-transformed mouse cells. Proc Natl Acad Sci USA. 1982;79(24):7914–7918. doi: 10.1073/pnas.79.24.7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo N. Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276(17):13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- Walboomers JM. Jacobs MV. Manos MM. Bosch FX. Kummer JA. Shah KV. Snijders PJ. Peto J. Meijer CJ. Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Wang J. Sampath A. Raychaudhuri P. Bagchi S. Both Rb and E7 are regulated by the ubiquitin proteasome pathway in HPV-containing cervical tumor cells. Oncogene. 2001;20(34):4740–4749. doi: 10.1038/sj.onc.1204655. [DOI] [PubMed] [Google Scholar]
- Weintraub SJ. Chow KN. Luo RX. Zhang SH. He S. Dean DC. Mechanism of active transcriptional repression by the retinoblastoma protein. Nature. 1995;375(6534):812–815. doi: 10.1038/375812a0. [DOI] [PubMed] [Google Scholar]
- Werness BA. Levine AJ. Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Yang XJ. Lysine acetylation and the bromodomain: a new partnership for signaling. Bioessays. 2004;26(10):1076–1087. doi: 10.1002/bies.20104. [DOI] [PubMed] [Google Scholar]
- Zerfass-Thome K. Zwerschke W. Mannhardt B. Tindle R. Botz JW. Jansen-Durr P. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene. 1996;13(11):2323–2330. [PubMed] [Google Scholar]
- Zimmermann H. Degenkolbe R. Bernard HU. O'Connor MJ. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J Virol. 1999;73(8):6209–6219. doi: 10.1128/jvi.73.8.6209-6219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomavirus infections—a major cause of human cancers. Biochim Biophys Acta. 1996;1288(2):F55–F78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]