Abstract
Transglutaminase (TG) plays important and diverse roles in mammals, such as blood coagulation and formation of the skin barrier, by catalyzing protein crosslinking. In invertebrates, TG is known to be involved in immobilization of invading pathogens at sites of injury. Here we demonstrate that Drosophila TG is an important enzyme for cuticle morphogenesis. Although TG activity was undetectable before the second instar larval stage, it dramatically increased in the third instar larval stage. RNA interference (RNAi) of the TG gene caused a pupal semi-lethal phenotype and abnormal morphology. Furthermore, TG-RNAi flies showed a significantly shorter life span than their counterparts, and approximately 90% of flies died within 30 days after eclosion. Stage-specific TG-RNAi before the third instar larval stage resulted in cuticle abnormality, but the TG-RNAi after the late pupal stage did not, indicating that TG plays a key role at or before the early pupal stage. Immediately following eclosion, acid-extractable protein from wild-type wings was nearly all converted to non-extractable protein due to wing maturation, whereas several proteins remained acid-extractable in the mature wings of TG-RNAi flies. We identified four proteins—two cuticular chitin-binding proteins, larval serum protein 2, and a putative C-type lectin—as TG substrates. RNAi of their corresponding genes caused a lethal phenotype or cuticle abnormality. Our results indicate that TG-dependent protein crosslinking in Drosophila plays a key role in cuticle morphogenesis and sclerotization.
Introduction
In mammals, TG fulfills a variety of essential functions by catalyzing isopeptide bond formation between Lys and Gln residues to form ε-(γ-glutamyl) lysine bonds between appropriate substrates in a Ca2+-dependent manner [1]–[3]. For example, plasma TG (factor XIII) stabilizes noncovalently associated fibrin polymers through covalent crosslinking of substituent fibrin monomers [4], and TG-1 (keratinocyte TG) crosslinks several proteins to form a thick layer of insoluble proteins, resulting in the formation of a cornified cell envelope [2]. In invertebrates, such as the crayfish Pacifastacus leniusculus and Drosophila, hemolymph coagulation depends on TG-mediated crosslinking of specific clotting proteins [5]–[9]. In the horseshoe crab Tachypleus tridentatus, a proteolytic coagulation cascade leads to the conversion of coagulogen into insoluble coagulin polymers, which are in turn stabilized by TG-mediated crosslinking with TG substrates including proxin and stablin, resulting in immobilization of invading pathogens at sites of injury [10]–[13]. On the other hand, in the nematode parasite Onchocerca volvulus, TG-catalyzed crosslinking is important for the molting of third-stage larvae [14]. TGase activity is also important in hemocyte homeostasis in the hematopoietic tissue of P. leniusculus [15]. Recently, Wang et al. provided proof for an immune function for Drosophila TG: Drosophila larvae with reduced TG levels exhibit increased mortality after septic injury and are more susceptible to a natural infection involving entomopathogenic nematodes and their symbiotic bacteria [16].
Arthropod cuticles function principally as an exoskeletal covering of the total body surface and are highly organized structures produced by extracellular secretion from the epidermis. Cuticles consist of chitin filaments, proteins, lipids, and inorganic substances, which are modified by sclerotic processes such as the oxidative incorporation of o-diphenols into the cuticle matrix [17]–[20]. We have observed TG-dependent crosslinking of cuticle proteins in the horseshoe crab and characterized one of the cuticle proteins specifically expressed in epidermis, which we designated caraxin [21], [22]. Crosslinked caraxin forms an elaborate mesh of honeycomb structures, suggesting that the mesh plays a role in promoting wound healing and sclerotization at sites of injury. Thus, as in the case of mammalian skin, TG-dependent protein crosslinking may be involved in the initial stage of host defense in the sub-cuticular epidermis of arthropods. The TG family comprises eight members in mammals, with each member performing diverse physiological functions [3]. In contrast, genome annotations in Drosophila have identified only a single TG gene that is predicted to encode a protein of 87 kDa (CG7356).
Here we characterized Drosophila TG biochemically and genetically, and demonstrated that the invertebrate TG is involved in cuticle morphogenesis and sclerotization in vivo. The epidermis-barrier function against invading pathogens and the wound-repair pathway appear to be evolutionarily well conserved between mammals and Drosophila, indicating that Drosophila would likely serve as a sophisticated model system for elucidation of the molecular mechanisms underlying mammalian skin disorders.
Results
Stage-specific expression pattern of TG and effects of wounding on TG expression
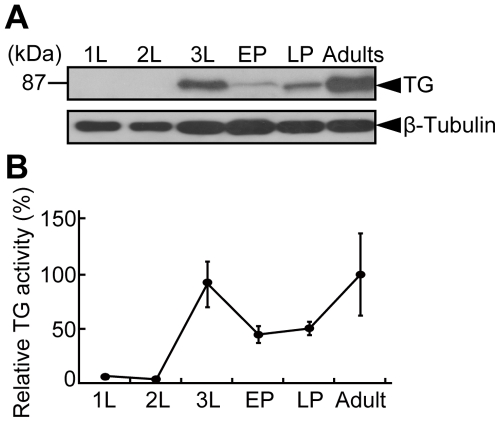
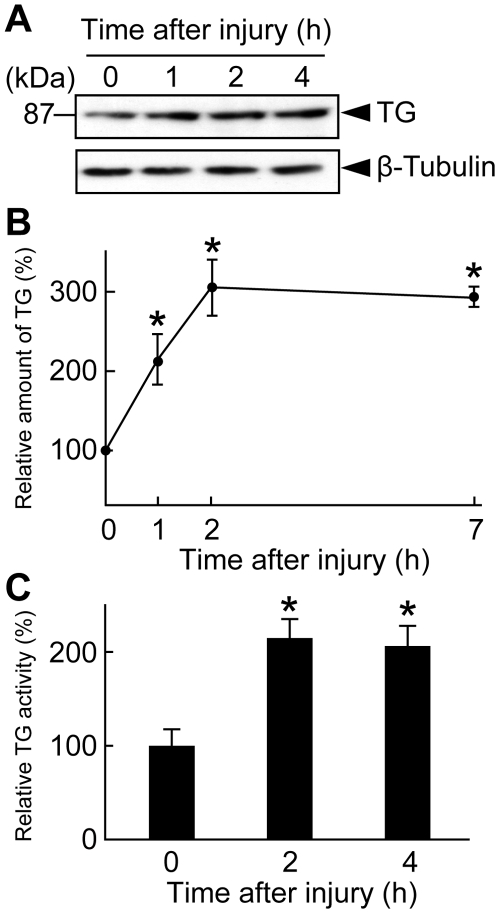
The amount of TG antigen and the TG enzyme activity in the extract of whole body were evaluated at different developmental stages. TG activity was significantly different between developmental stages (F 5, 12 = 15.178, P<0.0001 by the ANOVA analysis) (Figure 1B). Although neither TG antigen nor TG activity were detectable at the first instar larva (1L) or second instar larva (2L) stage, both were clearly detected after the third instar larva (3L) stage (Figures 1A and 1B). The differences in TG activity between 1L and 3L or 2L and 3L were significant at 5% level (the Bonferroni correction for multiple comparisons). TG activity in adult flies was equivalent to that of 3L (Figure 1B). To evaluate whether TG expression is induced in response to stimulation by wounding, adult flies were injured by a steel pin at the abdominal sternite. TG antigen increased at 1 h after injury (Figure 2A). Consistent with this observation, an approximately threefold increase in TG antigen was observed by ELISA at 2 h after injury, with the antigen level persisting for at least 4 days (Figure 2B: F 3, 8 = 24.182, P = 0.0002). The differences in TG antigen between 0 h and 1 h, 0 h and 2 h, or 0 h and 7 h were significant at 5% level. Moreover, an increase in TG activity in response to wounding was observed by TG enzymatic activity assay, further supporting the hypothesis that TG is involved in wound repair in Drosophila (Figure 2C: F 2, 6 = 8.8906, P = 0.01606). The differences in TG activity between 0 h and 2 h or 0 h and 4 h were significant at 5% level.
Figure 1. Stage-specific expression of TG.
(A) The wild-type flies were collected at indicated developmental stages and homogenized. TG antigens were detected by Western blotting (upper panel). β-tubulin was detected by Western blotting as a control (lower panel) with a mouse anti-tubulin antibody. (B) TG activity was assayed by the incorporation of Bi-PA into N, N'-dimethylcasein. The means ± S. D. of three independent experiments were plotted.
Figure 2. The effect of wounding on TG expression.
Wild-type flies were injured using a steel pin. Flies were collected at the indicated times and homogenized. (A) TG antigens at the indicated times were detected by Western blotting (upper panel). β-tubulin was detected by Western blotting as control (lower panel). (B) The amount of TG after the wounding was determined by enzyme-linked immunosorbent assay. The means ± S. D. of three independent experiments were plotted. A significant difference (versus 0 h) is indicated by asterisk (P<0.05 after Bonferroni correction). (C) TG activities were measured by the monodansylcadaverine incorporation at 1 and 4 h after wounding. The means ± S. D. of three independent experiments were plotted. A significant difference (versus 0 h) is indicated by asterisk (P<0.05).
Phenotypes of TG-RNAi flies
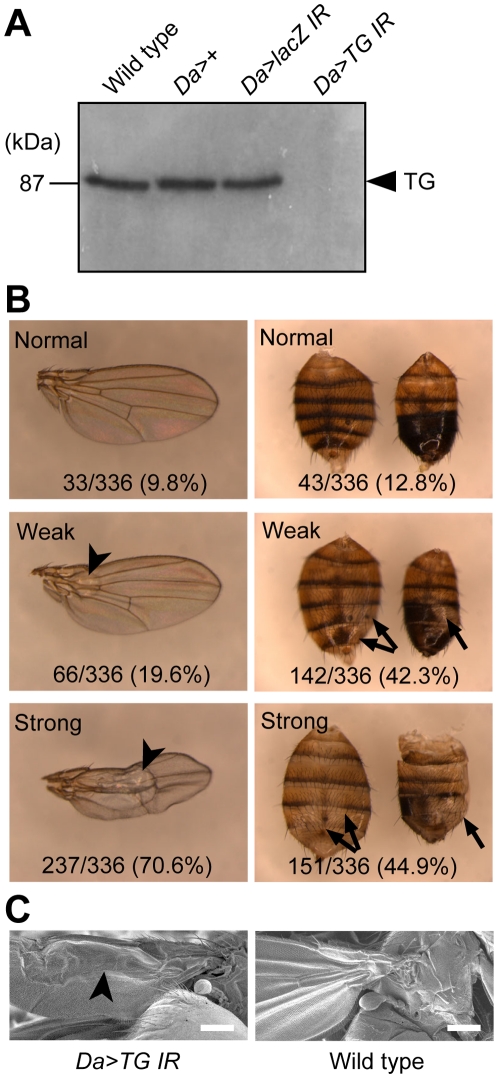
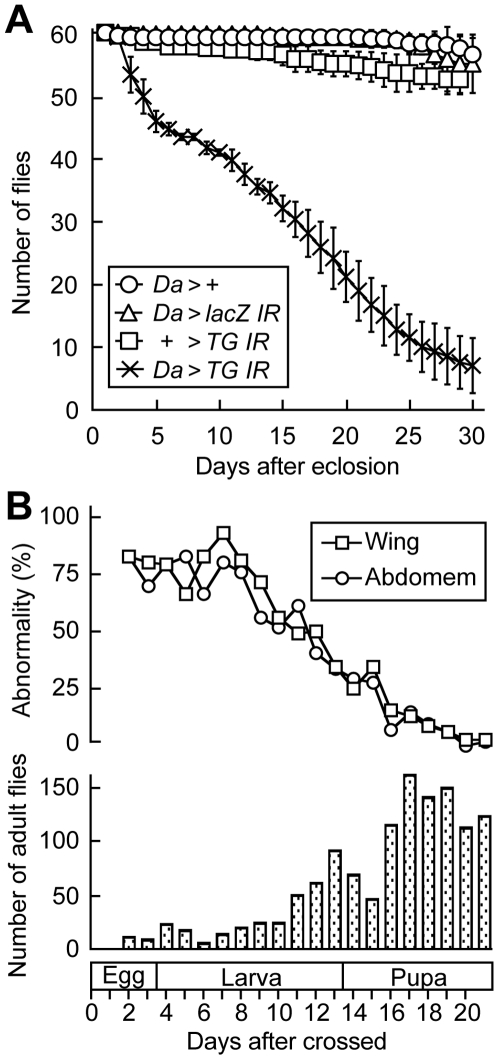
We next characterized the phenotypes of TG-RNAi flies using ubiquitously expressed driver (Da-GAL4>UAS-TG IR). No TG antigen was detected in the extract from whole body of TG-RNAi flies by western blotting (Figure 3A, Da>TG IR). TG-RNAi revealed a pupal semi-lethal phenotype, with an eclosion rate for TG-RNAi flies that was about 20% that of controls expressing a LacZ-RNAi construct (Da>lacZ IR). After eclosion, about 90% of TG-RNAi flies exhibited abnormal cuticle morphologies of the wings and the abdominal tergite (Figure 3B). Wing formation is executed by well-defined stages; upon pupation the imaginal wing discs evaginate, and subsequent epithelial cell expansion without further cell proliferation causes the wings to become compactly folded within the confines of the pupal case and to spread within 1 h after eclosion [23], [24]. TG-RNAi flies, however, failed to expand their wings, causing the wings to blister (Figures 3B and 3C, arrowheads). In addition, several melanized segments on the abdominal tergite of adult flies failed to develop in TG-RNAi flies (Figure 3B, arrows). TG-RNAi flies showed a significantly shorter life span than did their wild-type counterparts, with approximately 90% of the flies dying within 30 days of eclosion (Figure 4A; χ2 = 11084.091, d.f. = 1. P<0.0001). In contrast, control flies, Da-GAL4>+, Da-GAL4>UAS-LacZ IR, and +>UAS-TG IR, developed normally into adults and did not display any cuticle abnormalities.
Figure 3. Phenotypes of TG-RNAi flies.
(A) TG antigen from whole body extract of adult TG-RNAi flies was detected by Western blotting. w1118, Da-GAL4>+ and Da-GAL4>UAS-LacZ IR were used as controls. Da, Da-GAL4. (B) Phenotypes of TG-RNAi flies for the wing (left panels) and abdominal cuticle (right panels) were classified into three grades depending on the extent of observed abnormality. The ratios of abnormal flies to total adult flies are indicated. Each fly was laid at 25°C. (C) Scanning electron microscopy of TG-RNAi fly. Scale bar = 200 µm.
Figure 4. The life span of the Da-GAL4>UAS-TG IR flies.
(A) The life span of the RNAi flies was compared with those of the control flies, Da-GAL4>+, Da-GAL4>UAS-lacZ IR and +>UAS-TG IR. Sixty adult flies were collected and maintained at 25°C. The number of surviving flies was recorded daily. The means ± S. D. of four independent experiments were plotted. Da, Da-GAL4. (B) Phenotypes of Tub-GAL80ts; Da-GAL4>UAS-TG IR. Tub-GAL80ts; Da-GAL4 flies were crossed with the UAS-TG IR flies in 20 vials and maintained at 18°C. The suppression of TG by RNAi was triggered by increasing the temperature to 29°C. The ratios flies with abnormal wings (square) and abnormal abdominal cuticles (circle) to total adult flies are indicated (upper panel). The number of adult flies born from each vial is indicated (lower panel).
In order to investigate the role of TG at different developmental stages, we used a temperature-sensitive mutant (Tub-GAL80ts; Da-GAL4) in which the driver function of GAL4 is repressed by ubiquitously expressed GAL80 at 18°C and de-repressed at 29°C, thereby enabling selective temporal expression of the UAS-TG IR. To obtain the temperature-sensitive flies, Tub-GAL80ts; Da-GAL4 flies were crossed with UAS-TG IR flies in 20 vials for 2 days at 18°C, and then, each vial containing eggs was transferred to 29°C at one-day interval. After 21 days of the crossing, the numbers of matured flies in the vials were counted. The stage-specific RNAi before 3L significantly increased the frequency of lethality, and about 75% of the adult flies had abnormalities in the formation of their wings and abdominal stripes (Figure 4B). In contrast, the RNAi after LP had no effect on cuticle abnormality (Figure 4B), indicating that the importance of TG in cuticle morphogenesis is particularly pronounced prior to the EP stage.
Identification of TG substrates
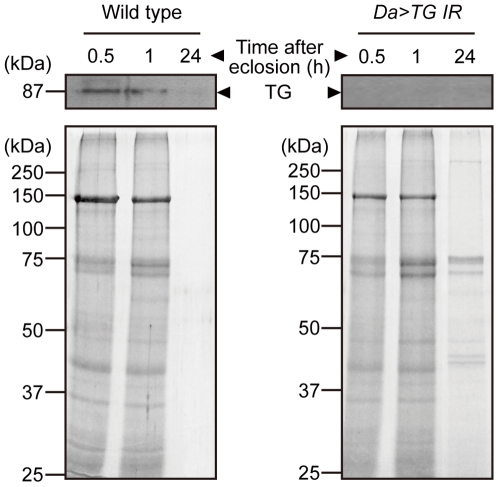
To identify TG substrates, cuticle proteins were extracted with 10% acetic acid from the wings of wild-type (w1118) and Da-GAL4>UAS-TG IR flies after eclosion. In wild-type flies, proteins that were acid-extractable immediately after eclosion (0.5 h) disappeared by 24 h post-eclosion, raising the possibility that during wing maturation these proteins are crosslinked to generate non-acid-extractable forms (Figure 5, Wild type). In contrast, several proteins were still extractable from the wings of TG-RNAi flies at 24 h post-eclosion, implying that they are candidates for TG substrates (Figure 5, Da>TG IR). These proteins were subjected to mass spectrometry, resulting in the identification of 12 proteins (Table 1).
Figure 5. Identification of TG substrates associated with cuticle formation.
The wings of wild-type and Da-GAL4>UAS-TG IR flies were collected at indicated times after eclosion. Wing proteins were extracted and subjected to SDS-PAGE. TG antigen was detected by Western blotting (upper panels). Loaded proteins were stained with Coomassie Brilliant Blue R-250 (lower panels). Da, Da-GAL4.
Table 1. Acid-extractable wing proteins from TG-RNAi flies identified by mass spectrometry.
| Nominal molecular weight | CG number | Protein name | MASCOT score | Sequence coverage (%) | Phenotype with Da-GAL4 | Phenotype with MS-GAL4 |
| 374386 | CG11064 | RFABP | 5117 | 54 | Lethal | NP |
| 124155 | CG9299 | Cpr76Bd | 974 | 32 | Faded melanization (See Fig. 6) | NP |
| 83409 | CG6806 | LSP2 | 2248 | 60 | Faded melanization (See Fig. 6) | NP |
| 72964 | CG6186 | Transferrin 1 | 1826 | 82 | Lethal | NP |
| 50573 | CG13214 | Cpr47Ef | 985 | 36 | Lethal | NP |
| 48802 | CG1780 | Imaginal disc growth factor 4 | 1036 | 70 | NP | NP |
| 29372 | CG10287 | Gasp | 931 | 85 | NP | NP |
| 26900 | CG3244 | Clect27 | 461 | 48 | Lethal | Anterior crossvein loss (See Fig. 6) |
| 26735 | CG15884 | Cpr97Eb | 586 | 74 | Lethal | Curled wing (See Fig. 6) |
| 25455 | CG1469 | Ferritin 2 light chain | 1307 | 76 | Lethal | NP |
| 23302 | CG2216 | Ferritin 1 heavy chain | 1325 | 79 | Lethal | NP |
| 19100 | CG15008 | Cpr64Ac | 573 | 80 | NP | NP |
RFABP, retinoid and fatty acid binding protein; NP, no phenotypic difference observed.
RNAi of putative TG substrates
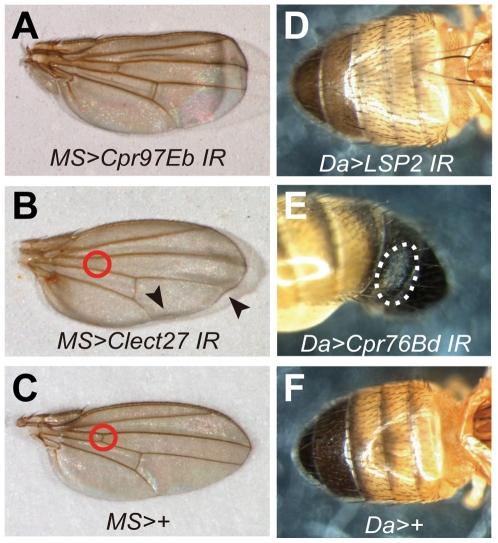
We characterized the phenotypes of flies in which RNAi targeted each of these 12 genes in Table 1. Knocking down experiments of seven genes including CG11064, CG6186, CG13214, CG3244, CG15884, CG1469, and CG2216 resulted in a lethal phenotype, and those of CG9299 (Cpr76Bd) and CG6806 (larval serum protein 2, LSP2) resulted in abnormal cuticle morphology. In contrast, those of the other three genes resulted in neither lethality nor phenotypic abnormality. Since the Da-GAL4 driver promoted a lethal phenotype for the seven genes, we used an MS1096-GAL4 driver, for which expression is restricted to the wing disc. The wing disc-restricted knocking down resulted in phenotypic abnormality for CG15884 and CG3244, which respectively coded Cpr97Eb protein and a putative C-type lectin with a molecular mass of 27 kDa (tentatively designated Clect27). MS1096-GAL4>UAS-Cpr97Eb IR flies exhibited curled wings (Figure 6A), whereas, MS1096-GAL4>UAS-Clect27 IR flies had a wrinkled morphology (Figure 6B, arrowheads) and lacked the anterior crossvein (Figure 6B, a circle). Several melanized segments on the abdominal tergite in the adult flies were faded and variegated in Da-GAL4>UAS-LSP2 IR and Da-GAL4>UAS-Cpr76Bd IR flies, respectively (Figures 6D and 6E).
Figure 6. Phenotypes of TG substrate RNAi flies.
Phenotypes of the MS1096-GAL4>UAS-Cpr97Eb IR (A), MS1096-GAL4>UAS-Clect27 IR (B), Da-GAL4>UAS-LSP2 IR (D) and Da-GAL4>UAS-Cpr76Bd IR (E) flies. The control flies, MS-GAL4>+ (C) and Da-GAL4>+ (F), are also indicated. Each fly was laid at 25°C. MS, MS1096-GAL4; Da, Da-GAL4.
Characterization of recombinant proteins of the putative TG substrates
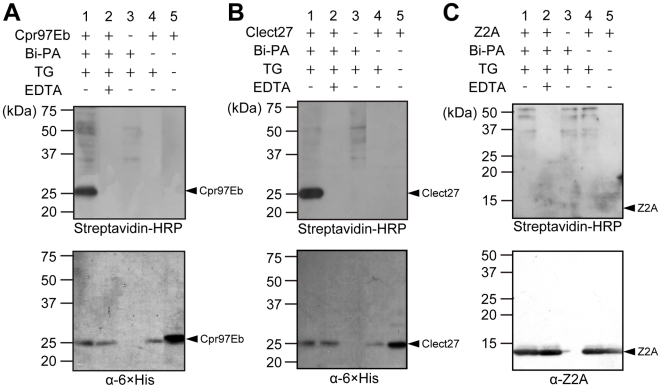
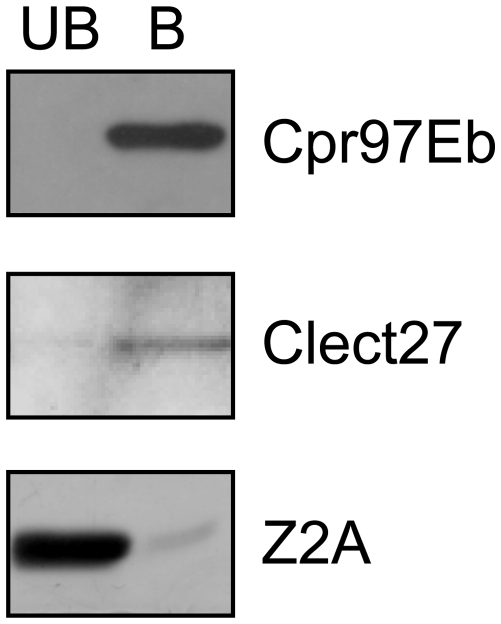
In order to determine whether the putative TG substrates including Cpr76Bd, LSP2, Cpr97Eb, and Clect27 could serve as TG substrates, recombinant forms of these proteins were generated in E. coli with C-terminal His-tags. However, expression levels for Cpr76Bd and LSP2 in E. coli were too low to obtain their recombinant forms. After purification by nickel affinity and ion-exchange chromatography, Cpr97Eb and Clect27 were incubated with 5-biotinamidopentylamine (Bi-PA) in the presence of TG, subjected to SDS-PAGE, and analyzed by western blotting with biotinylated streptavidin-horseradish peroxidase (HRP). Incorporation of Bi-PA into Crp97Eb and Clect27 proteins was observed (lane 1 in Figures 7A and 7B), which was inhibited by EDTA, indicating that TG activity is dependent on the presence of divalent cations (lane 2 in Figures 7A and 7B). Bi-PA was also incorporated into several unknown proteins derived from TG fraction used (lane 3 in Figures 7A and 7B). Intermolecular reaction to form a homopolymer of Cpr97Eb or Clect27 protein by TG was not observed in the absence of Bi-PA, suggesting that Cpr97Eb and Clect27 proteins may undergo heterotypic TG-dependent crosslinking (lane 4 in Figures 7A and 7B). In addition, Clect27 and Cpr97Eb proteins exhibited binding activity to chitin (Figure 8).
Figure 7. TG-dependent incorporation of Bi-PA to Cpr97Eb and Clect27 proteins.
Cpr97Eb (A) and Clect27 (B) proteins were incubated with or without Bi-PA in the presence of TG. The incorporation of Bi-PA was detected with biotinylated streptavidin-HRP (upper panel). Z2A (C) is a recombinant version of horseshoe crab β-1,3-D-glucan-binding protein [43], which was used as negative control for the TG-dependent incorporation. Loaded recombinant proteins were detected by Western blotting with an anti-6×His tag antibody or anti-Z2A antibody (lower panels).
Figure 8. Binding of Cpr97Eb and Clect27 proteins to chitin.
Cpr97Eb and Clect27 proteins were mixed with chitin, and unbound (UB) and bound (B) fractions were subjected to SDS-PAGE. The bound fraction was eluted by 2% SDS. Z2A was used as negative control for chitin binding. Proteins were detected by Coomassie Brilliant Blue R-250 staining (Clect27 and Z2A) or by anti-6×His tag antibody (Cpr97Eb).
Measurements of transcuticular water loss of TG-RNAi flies
We hypothesized that cuticular abnormalities of TG-RNAi flies cause transcuticular water loss. To estimate water content, wet and dry weights of whole body of adult TG-RNAi flies at 3 and 15 days after eclosion were measured. The wet/dry ratios of TG-RNAi flies were respectively 3.30±0.72 and 3.17±0.26, which were consistent with those of the wild type flies (3.20±0.06 and 3.46±0.05, respectively), indicating that the shorter life span of TG-RNAi flies is not caused by transcuticular water loss: no significant difference between TG-RNAi files and the wild type flies was found by the G-test analysis (G = 8.13×10−5, d.f. = 1, P = 0.993 at 3 days after eclosion; G = 0.00278, d.f. = 1, P = 0.958 at 15 days after eclosion).
Discussion
In this study, RNAi of the TG gene product using the ubiquitously expressed Da-GAL4 driver caused a pupal semi-lethal phenotype and abnormal morphology at 25°C, (Figures 3 and 4), indicating that TG is important for Drosophila development and morphogenesis. Recently, Wang et al. reported that a TG-RNAi strain with reduced expression of TG using an ACt5C-GAL4 driver showed no morphological defects at 22°C [16]. This discrepancy in the phenotypes for TG-RNAi flies of the two strains may be due to a more severe reduction level of TG in our RNAi flies by the different driver at higher temperature. The temperature dependence of GAL4-UAS expression system is widely accepted, and by altering the temperature, a wide range of expression levels of any responder can be achieved [25]. For example, to obtain information about the function of Drosophila POMT1 (protein O-mannosyltransferase-1), the Act5C-GAL4/UAS-POMT-1-IR fly was raised at 25°C and 28°C, and the fly showed a viability of 19% at 25°C, but 0% at 28°C, indicating that the knockdown is more effective at 28°C [26]. Interestingly, Wang et al. reported that the TG-knockdown flies increase mortality after entomopathogenic nematode infections, suggesting TG-dependent clot formation works as an important effector by helping to prevent septic infections [16]. Here we have demonstrated an increase of TG antigen and a concomitant increase in TG activity as a result of wounding, a finding that also suggests that TG is involved in the early phase of the innate immune reaction (Figure 2). TG-RNAi flies showed a significantly shorter life span than their counterparts (Figure 4A). TG-1 knockout mice die of severe dehydration due to high transepidermal water loss within 4–5 h after birth, caused by defective skin barrier development [27]. However, TG-RNAi flies did not appear to die of transcuticular water loss, suggesting that TG may have another pivotal function for survival.
We identified four putative TG substrates, namely Cpr97Eb, Cpr76Bd, LSP2, and CG3244 (here designated Clect27) by mass spectrometry and evaluated their functions by RNAi (Table 1). The Da-GAL4 driver promoted lethal phenotypes for Crp97Eb and Clect27 knockdowns, whereas the wing disc-restricted driver promoted abnormal wing morphologies (Figures 6A and 6B). The TG-dependent incorporation of Bi-PA into Crp97Eb and Clect27 proteins further implicated these proteins as potential TG substrates in vivo (Figure 7). Crp97Eb protein contains a Rebers and Riddiford consensus sequence, which is found in arthropod cuticular chitin-binding proteins [17], [18], [21], [28]. As expected, recombinant Cpr97Eb protein exhibited chitin-binding activity (Figure 8). Cpr97Eb gene is strongly expressed during pupal wing morphogenesis [29], and the morphological defect of the wing disc-restricted RNAi flies in this study suggests that Cpr97Eb protein is involved in the TG-dependent crosslinking required for cuticle morphogenesis and sclerotization. Clect27 protein is a putative galactose-binding C-type lectin based on the amino acid sequence with unknown physiological function. Clect27 gene expression shows a highly localized pattern in the wing disc [30]. The wing disc-restricted Clect27-RNAi flies lacked anterior crossveins (Figure 6B, a circle) and recombinant Clect27 protein exhibited chitin-binding activity (Figure 8). Taken together, these findings suggest that Clect27 protein expressed in the wing may bind to the cuticle and be crosslinked by TG, and raise the possibility that a defect in this process may underlie the observed lack of the anterior crossvein in the wings of wing disc-restricted Clect27-RNAi flies.
RNAi of Cpr76Bd and LSP2 resulted in faded or variegated black lines on the abdominal tergite, suggesting that these TG substrates are associated with melanin formation in the cuticle (Figures 6D and 6E). Although the physiological function of Cpr76Bd protein remains unknown, it contains Rebers and Riddiford consensus sequence, suggesting a cuticular chitin-binding activity. LSP2 is one of the major protein in hemolymph at 3L, and transcription of LSP2 gene is controlled by 20-hydroxyecdysone [31], [32]. The LSP2 homo-hexamer is synthesized in the fat body and secreted into hemolymph [33], [34]. LSPs act mainly as storage proteins that provide energy and amino acids during metamorphosis [35], [36]. Injection of a larval serum protein, calliphorin from the blowfly Calliphora vicina labeled with [14C]phenylalanine, into larvae showed that the relatively high portion of calliphorin and/or calliphorin-derived phenylalanines are incorporated into SDS-insoluble portion of adult cuticle, suggesting the possible conversion of calliphorin into tanned insoluble proteins, and/or hydroxylation of phenylalanine to tyrosine involved in cuticular sclerotization [37]. Moreover, fractionation of sclerotized pupal cuticle showed that calliphorin forms covalent and non-covalent links with other cuticle components. Thus, LSP2 may be incorporated into cuticle by TG activity and involved in melanization, although the order of events in cuticle hardening due to TG and melanization remains to be clarified.
TG requires Ca2+ for activation [3]. Horseshoe crab TG is stored in hemocytes as a latent form under the low concentration of Ca2+ (∼1 µM) in cytosol and secreted in response to stimulation by lipopolysaccharides [10]. The secreted TG is activated immediately by Ca2+ in hemolymph plasma (∼10 mM), which is very important for the crosslinking of coagulin with TG substrates at injured sites to stop bleeding and to immobilize invading microbes [12], and the activated TG also catalyzes crosslinking of cuticular chitin-binding proteins secreted from the sub-cuticular epithelial cells [22]. Although in Drosophila, tissue localization of TG remains unknown, TG must be regulated timely and spatially by the Ca2+ concentration. Upon eclosion, the wings are expanded by blood pressure, a process that is completed within one hour [24]. TG secreted from cells in response to appropriate stimulation must be activated by Ca2+ in hemolymph plasma and transferred into the whole parts of the wings, and TG must crosslink several proteins to support wing maturation. Indeed, TG antigen existed in the wings of wild type flies immediately after eclosion (Figure 5, Wild type). In TG-RNAi flies, several proteins remained intact without crosslinking in the wings, resulting in wing blisters. This indicates that TG plays a critical role in the hardening steps during wing formation. During wing formation in Drosophila, an appropriate programmed cell death in epidermal cells is required prior to tanning and hardening [38]. In mammals, TG plays an important role in apoptosis to prevent the leakage of cytosolic components by protein crosslinking [39], [40]. Drosophila TG may be also involved in an apoptotic step in wing formation.
Mice lacking TG-1 display a defective skin-barrier function and deficient wound repair [27]. A Drosophila transcription factor grainy head regulates enzymes, such as dopa decarboxylase and tyrosine hydroxylase, both of which catalyze the production of quinones, leading to covalent crosslinking between cuticle proteins and cuticular structural components [41]. Mice lacking grainy head-like 3, a homologue of Drosophila grainy head, display the same defects as in the case of the TG-1 knockout mice, accompanied by reduced expression of TG-1 [42]. The epidermis-barrier function and the wound-repair pathway seem to be evolutionarily well conserved between mammals and Drosophila, suggesting that Drosophila could serve as a sophisticated model system to elucidate the molecular mechanisms underlying mammalian skin disorders.
Materials and Methods
Fly stocks
Flies were maintained on the standard Drosophila medium at 18, 25 or 29°C. Flies, white (w1118), Da-GAL4 and Tub-GAL80ts; Da-GAL4 were obtained from the Bloomington Stock Center. MS1096-GAL4 strain was a gift from Dr. Ulrich Theopold at Stockholm University. UAS-TG IR, UAS-Cpr76Bd IR, UAS-Clect27 IR, and UAS-LSP2 IR flies were obtained from Dr. Ryu Ueda at the National Institute of Genetics, Mishima, Japan. UAS-Cpr97Eb IR flies were obtained from the Vienna Drosophila RNAi Center. UAS-TG strain was gift from Drs. Koji Ikura and Akira Ichikawa. Strain w1118 was used as the wild type.
Preparation of polyclonal antibodies against TG
To prepare polyclonal antibodies, the full-length, the N-terminal region (residues 1–284), and the C-terminal region (515–776) of TG were expressed in E. coli strain BL21 (DE3) pLysS (Novagen). An expression level of the full-length of TG was too low to obtain the recombinant form as an antigen. Inclusion bodies containing the recombinant proteins of the N-terminal region and the C-terminal region of TG were isolated, subjected to SDS-PAGE under reducing conditions, and stained using negative staining. The protein bands corresponding to the recombinant proteins were excised from the gel and recovered by electroelution for the immunization of rabbits (Asahi Techno Glass, Chiba, Japan). An antibody titer of the anti-serum against the C-terminal region of TG did not rise after several boosts of the antigen. Therefore, an polyclonal antibody against the N-terminal region of TG was purified sequentially from the anti-serum by using protein A-Sepharose and antigen-conjugated Affi-Gel-10 (Bio-Rad Laboratories, Hercules, CA). The resulting antibody cross-reacted with horseshoe crab TG in hemocyte lysates by Western blotting.
Extraction of proteins from the whole body and wings
Flies were homogenized in 1% Nonidet P-40 in 50 mM Tris-acetate, pH 7.5, containing 150 mM NaCl, 1 mM EDTA and 1 mM phenylmethylsulfonyl fluoride, and centrifuged at 20,000× g at 4°C for 15 min to collect the supernatant. For wing protein extraction, wings were collected, washed with 70% ethanol, and homogenized in 10% acetic acid using a pellet mixer. After incubation at 4°C for 16 h, the homogenate was centrifuged at 20,000× g at 4°C for 15 min, after which the supernatant was lyophilized.
Western blotting
Samples were subjected to SDS-PAGE and transferred to PVDF membrane. After blocking with 5% dry milk, the membrane was incubated with the anti-TG antibody or anti-6×His tag antibody (Nacalai Tesque, Kyoto, Japan) and then with the secondary antibody (horseradish peroxidase-conjugated (HRP) goat anti-rabbit or -mouse IgG, Bio-Rad Laboratories), followed by development with Chemi-Lumi One (Nacalai Tesque). β-Tubulin was detected with a mouse anti-tubulin antibody (Chemicon International, Temecula, CA).
Enzyme-linked immunosorbent assay
Microtiter plates were coated with homogenates of 10 individuals (whole body) at 37°C for 1 h. After washing with 50 mM Tris-HCl, pH 7.5, containing 150 mM NaCl, wells were blocked with 5% dry milk in the same buffer. Plates were incubated with the anti-TG antibody at 37°C for 1 h and then with 5,000-fold diluted goat anti-rabbit IgG-HRP conjugate (Bio-Rad Laboratories), and developed using o-phenylenediamine substrate with detection at 490 nm.
TG activity assays
Microtiter plates were coated with 50 µl of N, N'-dimethylcasein (15 mg/ml; Sigma, St. Louis, MO) at 4°C overnight, and the wells were subsequently blocked with 0.5% dry milk in 0.1 M Tris-HCl, pH 8.5 at 37°C for 1 h and washed with 0.1 M Tris-HCl, pH 8.5. Reagents were added to each well as follows: 10 mM CaCl2, 10 mM dithiothreitol, 0.5 mM 5-biotinamidopentylamine (Bi-PA; Pierce Chemical, Rockford, IL), whole body lysate (2.5 mg protein), and 0.1 M Tris-HCl, pH 8.5 (total volume of 50 µl per well). The microtiter plates were incubated at 37°C for 1 h, and the reaction was stopped by washing with 200 mM EDTA followed by washing with 0.1 M Tris-HCl, pH 8.5. The biotinylated streptavidin-HRP conjugate (GE Healthcare, Buckinghamshire, UK) diluted 1∶200 with 0.5% dry milk in 0.1 M Tris-HCl, pH 8.5 was added to each well and incubated at 37°C for 1 h. The plate was washed once with 0.001% Triton X-100 followed by four washes with 0.1 M Tris-HCl, pH 8.5. The activity of horseradish peroxidase was detected with o-phenylenediamine at 490 nm.
TG activity was additionally assayed by fluorometric measurement of monodansylcadaverine incorporation into N, N'-dimethylcasein. Homogenates of eight individual flies were incubated with 50 mM Tris-acetate, pH 7.5, containing 10 mM CaCl2, 10 mM dithiothreitol, 0.5 mM monodansylcadaverine, and 0.04% of N, N'-dimethylcasein at 37°C for 30 min, after which the reaction was stopped by adding 10% trichloroacetic acid. The resulting precipitate was solublized with 50 mM Tris-acetate, pH 7.5, containing 8 M Urea and 0.5% SDS. The amount of the incorporated monodansylcadaverine was quantitated with a fluorescence spectrophotometer.
Statistical analyses
TG activity or the amount of TG antigen was analyzed by the ANOVA. The model of the ANOVA was as follows: Yij = u + Ai + eij, where Y is the TG activity, u is the overall mean, Ai is the ith developmental stage effect or injury time effect and eij is the error term. The developmental stage effect or injury time effect was considered as the fixed effect. Bonferroni correction for multiple comparisons was applied to evaluate the pairwise difference in average activity or average amount of TG antigen between developmental stages or injury times. The log-rank test was performed to compare survival in two groups (TG-RNAi and Da-GAL4>+ flies). Differences of transcuticular water loss between wild type and TG-RNAi flies were tested using the G test for goodness of fit.
Mass spectrometry
Proteins were subjected to SDS-PAGE and stained with Coomassie Brilliant Blue R-250. Protein bands were excised, digested with trypsin, and subjected to LC/MS/MS analysis. Peak lists obtained from the mass spectra were used to identify proteins using the Mascot search engine (Matrixscience).
Expression of recombinant Cpr97Eb and Clect27 in E. coli
To construct expression vectors, cDNA fragments were amplified by PCR. An amplimer encoding the entire Clect27 coding sequence were inserted into expression vector pET-22b (Novagen) between the NdeI and XhoI sites. An amplimer the entire Cpr97Eb coding sequence was inserted into pET-22b between the NcoI and XhoI sites. All constructs were verified by DNA sequencing. These constructs, which contain C-terminal His-tags, were expressed in the E. coli strain Origami B (DE3) (Novagen). Bacteria were cultured in Luria-Bertani medium, and expression was induced by the addition of isopropyl-β-D-thiogalactoside at a final concentration of 30 µM at 18°C for 20 h. Bacterial pellets were harvested by centrifugation and sonicated in 20 ml of 50 mM Tris-HCl, pH 8.2, containing 150 mM NaCl, 1% Nonidet P-40 and 1 mM phenylmethylsulfonyl fluoride. After sonication, supernatants were recovered by centrifugation and purified according to the manufacturer's protocol using Ni-NTA agarose. Eluates of Clect27 protein from Ni-NTA agarose were diluted with 50 mM Tris-HCl, pH 7.0, containing 100 mM NaCl and further purified on a DEAE Sepharose CL-6B column (1×2 cm). Proteins were eluted with a linear NaCl gradient (100–500 mM) in the same buffer.
Incorporation of Bi-PA into Clect27 and Cpr97Eb proteins
Recombinant proteins were incubated with TG in 50 mM Tris-HCl, pH 8.5, containing 10 mM CaCl2, 10 mM dithiothreitol and 1 mM Bi-PA at 37°C for 1 h. Whole body extract of Da-GAL4>UAS-TG was used as the TG source. Following the reaction, aliquots were subjected to SDS-PAGE and electroblotted on PVDF membrane. After blocking with 5% dry milk, the membrane was incubated at room temperature for 1 h with the biotinylated streptavidin-HRP conjugate diluted 1∶1000 with 20 mM Tris-HCl, pH 7.5 containing 5% nonfat dry milk and 150 mM NaCl, followed by development with Chemi-Lumi One reagent.
Binding assay for chitin
Chitin-binding assays were performed as previously reported [43]. Briefly, proteins were mixed with chitin in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and incubated at 4°C for 10 min. Supernatants were separated by centrifugation and precipitates were washed with the same buffer. Protein bound to chitin was eluted with 2% SDS. Bound and unbound fractions were subjected to SDS-PAGE. Clect27 and Cpr97Eb were detected by staining with Coomassie Brilliant Blue R-250 and by Western blotting with an anti-6×His tag antibody, respectively.
Measurements of wet and dry weights of TG-RNAi flies
Twenty adult TG-RNAi or wild-type flies were anesthetized and weighed (wet weights). Flies were then dried with a centrifugal vacuum concentrator (model 78120KT, Labconco, Kansas city, MO) for 6 h and weighed (dry weights).
Optical and Scanning Electron Microscopies
Optical microscopic observation was performed with a Nikon SMZ 1000 microscope. For scanning electron microscopy, non-fixed samples without coating were directly observed by a Keyence VE-9800 scanning electron microscope.
Acknowledgments
We are grateful to Drs. R. Ueda (National Institute of Genetics, Mishima, Japan) and U. Theopold (Stockholm University, Stockholm, Sweden) for providing fly strains. We thank Dr. M. Matsumoto and M. Oda for the LC/MS/MS analysis (Kyushu University, Fukuoka, Japan), Drs. A. Ichikawa and K. Ikura (Kyoto Institute of Technology, Kyoto, Japan) for providing fly strains and helpful discussions, Mr. Y. Kinosita (Keyence corporation, Osaka, Japan) for the scanning electron microscopy, and Ms. N. Ichinomiya-Sato and Kengo Koba for technical assistance. We also thank Dr. John Kulman (Puget Sound Blood Center, Seattle, USA) and Dr. Hidenori Tachida (Kyushu University, Fukuoka, Japan) for helpful discussions and suggestion on this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the Kyushu University Interdisciplinary Program in Education and Project in Research Development (type E-2 No. 21415 to S.K.), by Grants-in-Aid for graduate students in Kyushu University (to T.S.), by the Japan Foundation for Applied Enzymology (http://www.mt-pharma.co.jp/jfae/index.html) (to S.K.), and by Takeda Science Foundation (http://www.takeda-sci.or.jp/) (to S.K.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Furie B, Furie BC. The molecular basis of blood coagulation. Cell. 1988;53:505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- 2.Kalinin A, Marekov LN, Steinert PM. Assembly of the epidermal cornified cell envelope. J Cell Sci. 2001;114:3069–3070. doi: 10.1242/jcs.114.17.3069. [DOI] [PubMed] [Google Scholar]
- 3.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 4.Lorand L. Factor XIII: structure, activation, and interactions with fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:291–311. doi: 10.1111/j.1749-6632.2001.tb03516.x. [DOI] [PubMed] [Google Scholar]
- 5.Kopacek P, Hall M, Söderhäll K. Characterization of a clotting protein, isolated from plasma of the freshwater crayfish Pacifastacus leniusculus. Eur J Biochem. 1993;213:591–597. doi: 10.1111/j.1432-1033.1993.tb17798.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang R, Liang Z, Hal M, Söderhäll K. A transglutaminase involved in the coagulation system of the freshwater crayfish, Pacifastacus leniusculus. Tissue localisation and cDNA cloning. Fish Shellfish Immunol. 2001;11:623–637. doi: 10.1006/fsim.2001.0341. [DOI] [PubMed] [Google Scholar]
- 7.Theopold U, Schmidt O, Söderhäll K, Dushay MS. Coagulation in arthropods: defense, wound closure and healing. Trends Immunol. 2004;25:289–294. doi: 10.1016/j.it.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson C, Korayem AM, Scherfer C, Loseva O, Dushay MS, et al. Proteomic analysis of the Drosophila larval hemolymph clot. J Biol Chem. 2004;279:52033–52041. doi: 10.1074/jbc.M408220200. [DOI] [PubMed] [Google Scholar]
- 9.Scherfer C, Karlsson C, Loseva O, Bidla G, Goto A, et al. Isolation and characterization of hemolymph clotting factors in Drosophila melanogaster by a pullout method. Curr Biol. 2004;14:625–629. doi: 10.1016/j.cub.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Osaki T, Kawabata S. Structure and function of coagulogen, a clottable protein in horseshoe crab. Cell Mol Life Sci. 2004;61:1257–1265. doi: 10.1007/s00018-004-3396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawasaki H, Nose T, Muta T, Iwanaga S, Shimohigashi Y, et al. Head-to-tail polymerization of coagulin, a clottable protein of horseshoe crab. J Biol Chem. 2000;275:35297–35301. doi: 10.1074/jbc.M006856200. [DOI] [PubMed] [Google Scholar]
- 12.Osaki T, Okino N, Tokunaga F, Iwanaga S, Kawabata S. Proline-rich cell surface antigens of horseshoe crab hemocytes are substrates for protein-crosslinking with a clotting protein coagulin. J Biol Chem. 2002;277:40084–40090. doi: 10.1074/jbc.M206773200. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda Y, Osaki T, Hashii T, Koshiba T, Kawabata S. A cysteine-rich protein from an arthropod stabilizes clotting mesh and immobilizes bacteria at injury site. J Biol Chem. 2007;282:33545–33552. doi: 10.1074/jbc.M705854200. [DOI] [PubMed] [Google Scholar]
- 14.Lustigman S, Brotman B, Huima T, Castelhano AL, Singh RN, et al. Transglutaminase-catalyzed reaction is important for molting of Onchocerca volvulus third-stage larvae. Antimicrob Agents Chemother. 1995;39:1913–1919. doi: 10.1128/aac.39.9.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin X, Söderhäll K, Söderhäll I. Transglutaminase activity in the hematopoietic tissue of a crustacean, Pacifastacus leniusculus, importance in hemocyte homeostasis. BMC Immunol. 2008;9:58. doi: 10.1186/1471-2172-9-58. doi: 10.1186/1471-2172-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Wilhelmsson C, Hyrsl P, Loof TG, Dobes P, et al. Pathogen entrapment by transglutaminase—a conserved early innate immune mechanism. PLoS Pathogens. 2010;6:e1000763. doi: 10.1371/journal.ppat.1000763. doi: 10.1371/journal.ppat.1000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willis JH. Cuticular proteins in insects and crustaceans. Am Zool. 1999;39:600–609. [Google Scholar]
- 18.Andersen SO, Peter MG, Roepstorff P. Cuticular sclerotization in insects. Comp Biochem Physiol. 1996;113B:689–705. [Google Scholar]
- 19.Sugumaran M. Unified mechanism for sclerotization of insect cuticle. Adv Insect Physiol. 1998;27:227–334. [Google Scholar]
- 20.Kramer KJ, Kanost MR, Hopkins TL, Jiang H, Zhu YC, et al. Oxidative conjugation of catechols with proteins in insect skeletal systems. Tetrahedron. 2001;57:385–392. [Google Scholar]
- 21.Iijima M, Hashimoto T, Matsuda Y, Nagai T, Yamano Y, et al. Comprehensive sequence analysis of horseshoe crab cuticular proteins and their involvement in transglutaminase-dependent crosslinking. FEBS J. 2005;272:4774–4786. doi: 10.1111/j.1742-4658.2005.04891.x. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda Y, Koshiba T, Osaki T, Suyama H, Arisaka F, et al. An arthropod cuticular chitin-binding protein endows injured sites with transglutaminase-dependent mesh. J Biol Chem. 2007;282:37316–37324. doi: 10.1074/jbc.M705731200. [DOI] [PubMed] [Google Scholar]
- 23.Baker JD, Truman JW. Mutation in the Drosophila glycoprotein hormone receptor, rickets, eliminate neuropeptide-induced tanning and selectively block a stereotyped behavioral program. J Exp Biol. 2002;205:2555–2565. doi: 10.1242/jeb.205.17.2555. [DOI] [PubMed] [Google Scholar]
- 24.Kiger JA, Jr, Natzle JE, Kimbrell DA, Paddy MR, Kleinhesselink K, et al. Tissue remodeling during maturation of the Drosophila wing. Dev Biol. 2007;301:178–191. doi: 10.1016/j.ydbio.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffy JB. GAL4 system in Drosophila: a fly geneticist's Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 26.Ichimiya T, Manya H, Ohmae Y, Yoshida H, Takahashi K, et al. The twisted abdomen phenotype of Drosophila POMT1 and POMT2 mutants coincides with their heterophilic protein O-mannosyltransferase activity. J Biol Chem. 2004;279:42638–42647. doi: 10.1074/jbc.M404900200. [DOI] [PubMed] [Google Scholar]
- 27.Matsuki M, Yamashita F, Ishida-Yamamoto A, Yamada K, Kinoshita C, et al. Defective stratum corneum and early neonatal death in mice lacking the gene for transglutaminase 1 (keratinocyte TG). Proc Natl Acad Sci USA. 1998;95:1044–1049. doi: 10.1073/pnas.95.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karouzou MV, Spyropoulos Y, Iconomidou VA, Cornman RS, Hamodrakas SJ, et al. Drosophila cuticular proteins with the R&R Consensus: Annotation and classification with a new tool for discriminating RR-1 and RR-2 sequences. Insect Biochem Mol Biol. 2007;37:754–760. doi: 10.1016/j.ibmb.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Ren N, Zhu C, Lee H, Adler PN. Gene Expression During Drosophila Wing Morphogenesis and Differentiation. Genetics. 2005;171:625–638. doi: 10.1534/genetics.105.043687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler JM, Jacobsen LT, Cain MD, Jarman GM, Hubank M, et al. Discovery of genes with highly restricted expression patterns in the Drosophila wing disc using DNA oligonucleotide microarrays. Development. 2003;130:659–670. doi: 10.1242/dev.00293. [DOI] [PubMed] [Google Scholar]
- 31.Roberts DB, Wolfe J, Akam ME. The developmental profiles of two major hemolymph proteins from Drosophila melanogaster. J Insect Physiol. 1977;23:871–878. doi: 10.1016/0022-1910(77)90013-0. [DOI] [PubMed] [Google Scholar]
- 32.Mousseron-Grall S, Kejzlarova-Lepesant J, Burmester T, Chihara C, Barray M, et al. Sequence, structure and evolution of the ecdysone-inducible Lsp-2 gene of Drosophila melanogaster. Eur J Biochem. 1997;245:191–198. doi: 10.1111/j.1432-1033.1997.00191.x. [DOI] [PubMed] [Google Scholar]
- 33.Akam ME, Roberts DB, Richards GP, Ashburner M. Drosophila: the genetics of two major larval proteins. Cell. 1978;13:215–225. doi: 10.1016/0092-8674(78)90190-3. [DOI] [PubMed] [Google Scholar]
- 34.Akam ME, Roberts DB, Wolfe J. Drosophila hemolymph proteins: purification, characterization, and genetic mapping of larval serum protein 2 in D. melanogaster. Biochem Genet. 1978;16:101–119. doi: 10.1007/BF00484387. [DOI] [PubMed] [Google Scholar]
- 35.Levenbook L, Bauer AC. The fate of the larval storage protein calliphorin during adult development of Calliphora vicina. Insect Biochem. 1984;14:77–86. [Google Scholar]
- 36.Telfer WH, Kunkel JG. The function and evolution of insect storage hexamers. Annu Rev Entomol. 1991;36:205–228. doi: 10.1146/annurev.en.36.010191.001225. [DOI] [PubMed] [Google Scholar]
- 37.König M, Agrawal OP, Schenkel H, Scheller K. Incorporation of calliphorin into the cuticle of the developing blowfly, Calliphora vicina. Roux's Arch Dev Biol. 1986;195:296–301. doi: 10.1007/BF00376062. [DOI] [PubMed] [Google Scholar]
- 38.Kimura K, Kodama A, Hayasaka Y, Ohta T. Activation of the cAMP/PKA signaling pathway is required for post-ecdysial cell death in wing epidermal cells of Drosophila melanogaster. Development. 2004;131:1597–1606. doi: 10.1242/dev.01049. [DOI] [PubMed] [Google Scholar]
- 39.Fesus L, Thomazy V, Falus A. Induction and activation of tissue transglutaminase during programmed cell death. FEBS Lett. 1987;224:104–108. doi: 10.1016/0014-5793(87)80430-1. [DOI] [PubMed] [Google Scholar]
- 40.Melino G, Annicchiarico-Petruzzelli M, Piredda L, Candi E, Gentile V, et al. Tissue transglutaminase and apoptosis: sense and antisense transfection studies with human neuroblastoma cells. Mol Cell Biol. 1994;14:6584–6596. doi: 10.1128/mcb.14.10.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mace KA, Pearson JC, McGinnis W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science. 2005;308:381–385. doi: 10.1126/science.1107573. [DOI] [PubMed] [Google Scholar]
- 42.Ting SB, Caddy J, Hislop N, Wilanowski T, Auden A, et al. A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science. 2005;308:411–413. doi: 10.1126/science.1107511. [DOI] [PubMed] [Google Scholar]
- 43.Ueda Y, Ohwada S, Abe Y, Shibata T, Iijima M, et al. Factor G utilizes a carbohydrate-binding cleft that is conserved between horseshoe crab and bacteria for the recognition of β-1,3-D-glucans. J Immunol. 2009;183:3810–3818. doi: 10.4049/jimmunol.0900430. [DOI] [PubMed] [Google Scholar]