Abstract
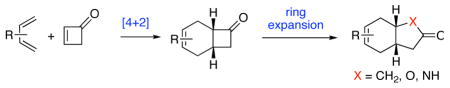
Cyclobutenone was employed as a dienophile in Diels-Alder cycloadditions which provided diverse complex cycloadducts in good yields. Experimental outcome indicated that cyclobutenone was more reactive in comparison with 2-cyclopentenone or 2-cyclohexenone. In addition, cycloadducts bearing strained cyclobutanone moiety were able to undergo regioselective ring expansions to produce corresponding cyclopentanones, lactones, and lactams, which otherwise were difficultly obtained by direct Diels-Alder reactions.
The power of the Diels-Alder cycloaddition reaction in organic synthesis is widely appreciated.1 The 6-membered rings created through Diels-Alder technology can be substructures of relatively simple targets or may be of value in building molecules of considerable complexity. Indeed, the Diels-Alder reaction has proven to be a valuable resource in reaching many natural products of novel architecture.2
Access to higher levels of complexity might well be facilitated if the dienophilic double bond in a Diels-Alder reaction is already housed in an existing ring. However, under strictly thermal conditions, parent cyclenones such as cyclohexenone and cyclopentenone are relatively unreactive unless the diene contains strategically placed activating groups. 3 While both of these dienophiles are known to undergo Lewis acid catalyzed Diels-Alder cycloaddition with less activated dienes, such applications require substrates which are stable to Lewis acids.
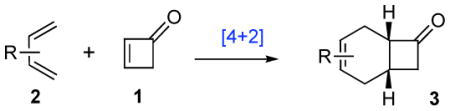
Remarkably, although cycloadditions of substituted cyclobutenones have been reported,4 we were unable to find a report of a Diels-Alder reaction of the parent cyclobutenone (1, Table 1). We anticipated that perhaps the ring strain of 1 might well serve to enhance its dienophilicity relative to corresponding cyclopentenones or cyclohexenones. Moreover, we could foresee that the products of such Diels-Alder reactions (cf. 3) could well represent particularly useful platforms for further elaborations (vide infra).
Table 1.
Diels-Alder reaction of various dienes with cyclobutenone.
 | ||||
|---|---|---|---|---|
| entry | diene | reaction condition | product | yielda (%) |
| 1b |
 2a |
CHCl3, 0 °C, 12 h |
 3a |
90% |
| 2 |
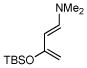 2b |
CHCl3, −40 °C, 2.5 h |
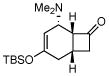 3b |
78% |
| 3 |
2c |
CHCl3, 45 °C, 12 h |
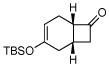 3c |
55% |
| 4c |
2d |
CHCl3, RT, 1 h |
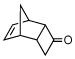 3d |
80% |
| 5 |
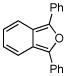 2e |
CHCl3, 45 °C, 12 h |
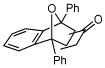 3e |
62% |
| 6 |
2f |
CHCl3, 45 °C, 12 h |
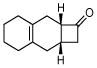 3f |
82% |
| 7 |
2g |
ZnCl2, CHCl3/CH3CN(1:1) 45°C, 12h |
3g |
81% |
| 8d |
2h |
ZnCl2, CHCl3/CH3CN(1:1) 45°C, 12 h |
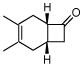 3h |
68% |
| 9 |
2i |
ZnCl2, CHCl3/CH3CN(1:1) 45 °C, 24 h |
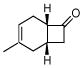 3i |
60% |
| 10 |
2j |
ZnCl2, CHCl3/CH3CN(1:1) 45 °C, 24 h |
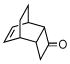 3j |
46% |
Isolated yield.
A mixture of cycloadducts (endo:exo= 4:1) was isolated in 90% combined yield. When the reaction was conducted at −30 °C for 48h, the endo:exo ratio was increased to 13:1 (52% yield based on 60% conversion).
The minor adduct was isolated in 8% yield.
Cycloadduct 3h was isolated in 40% yield in the absence of ZnCl2.
Sieja5 had actually described a preparation of cyclobutenone 1, following a very harsh Hunsdiecker-type decarboxylation-bromination of 3-oxo-cyclobutanecarboxylic acid 6 and subsequent elimination. We developed a modified version of the Sieja protocol in which a much milder Hunsdiecker-type decarboxylation-bromination was utilized, leading to gram-scale quantities of 1.7a,b Cyclobutenone (1) was found to be prone to polymerization when stored in the neat state, even at very low temperatures. Accordingly, it was stored as a solution in chloroform.
Activated acyclic dienes 2a-c were explored and found to be quite reactive in Diels-Alder reactions with 1 (Table 1). Dienes 2a, b, and d gave rise, predominantly, to endo products (entries 1, 2, 4). Not surprisingly, dienes 2c, 2e, and 2f require higher temperatures (45 °C) and longer reaction times to produce cycloadducts (entries 3, 5–6). The structure of 3e was confirmed by X-ray crystallographic analysis.7c Reactions of cyclobutenone with less reactive dienes 2g–j were conducted with Lewis acid activation (ZnCl2)8 to produce cycloadducts in moderate to good yields (entries 7–10).7a
In our estimation, 1 is quite similar in its Diels-Alder reactivity profile to maleic anhydride. This is remarkable in that vicinal di-activation is a common rate enhancing feature in Diels-Alder cycloadditions. While the magnitude of the effect could not have been predicted in advance, the rate enhancement with 1 must reflect the favorable effects of re-hybridization of two particularly strained sp2 carbons in the cycloaddition transition state. 9 By contrast, non-catalyzed Diels-Alder reactions of cyclopentenone or cyclohexenone as dienophiles typically require much higher temperatures (180–250 °C).10 In fact, in our hands, the uncatalyzed reaction of cyclopentadiene with cyclopentenone was only 40% complete after 36h at 150 °C. The results provided in Table 1 clearly demonstrate that cyclobutenone 1 is a far more reactive dienophile than are the analogous unsubstituted 5- and 6-membered cycloalkenones.
Given the now enhanced availability of 1 and its powerful dienophilicity, we explored some aspects of the chemistry of adducts 3 with a view to reaching otherwise inaccessible target structures via Diels-Alder logic (Table 2). For instance, treatment of cycloadduct 3f with trimethylsulfoxonium iodide and NaH in DMF (A) afforded the spiroepoxide11 which, upon exposure to lithium iodide, underwent rearrangement to produce cyclopentanone 4, apparently as a single regioisomer (entry 1). Thus, methylene insertion had occurred exclusively at the more substituted C–C bond, as consistent with the findings of Markó and co-workers.11 It is appropriate to note that 4 corresponds to the cycloadduct of a hypothetical Diels-Alder reaction of 2f and the non-functional dienophile, 3-cyclopentenone (10).
Table 2.
Representative ring expansions of cycloadducts.
| entry | cycloadduct | conditionsa | product/yield (isolated) | dienophilic equivalent |
|---|---|---|---|---|
| 1 | A |
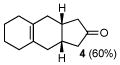 4 |
10 |
|
| 2 |
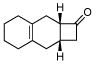 3f |
B |
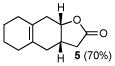 5 |
11 |
| 3 | C |
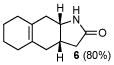 6 |
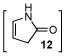 12 |
|
| 4 | A |
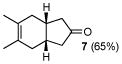 7 |
||
| 5 |
 3h |
B |
 8 |
|
| 6 | C |
 9 |
||
Key. A: Trimethylsulfoxonium iodide, NaH, DMF, then LiI, THF; B: CF3CH2OH, H2O2.; C: O-Mesitylenesulfonyl-hydroxylamine, CH2Cl2, 0°C, then Al2O3, PhH/MeOH(3:1).
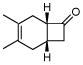
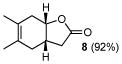
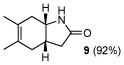
Correspondingly, 3f was converted to lactone 5 in 70% yield (entry 2). In this case, oxygen insertion had occurred, as anticipated, at the more substituted C–C bond. It is of note that lactone 5 corresponds to the equivalent of a Diels-Alder reaction between 2f and butenolide 11. Once again, butenolide 11 is not a useful dienophile in chemical synthesis. Finally, reaction of 3f with O-mesitylenesulfonyl-hydroxylamine (MSH) in CH2Cl2 followed by Al2O3-promoted rearrangement (C)12, afforded lactam 6 in 80% yield as a single isomer (entry 3). Lactam 6 may be seen as the equivalent of Diels-Alder reaction between 2f and 1,5-dihydropyrrol-2-one (12), which is, in reality, a non-functional dienophile. Similarly, exposure of 3h to the conditions described afforded cyclopentanone 7, lactone 8, and lactam 9 (entries 4–6).
These studies represent a potentially valuable approach to the assembly of complex substructure types by combining Diels-Alder reactions of the now available, and highly reactive 1 with a range of dienes to provide cycloadducts of the type 3, which serve as substrates for appropriate ring expansions.13
In summary, as a consequence of improved methods for its synthesis and management, the parent cyclobutenone (1) has become available as a reagent for chemical synthesis. In thermally driven Diels-Alder cycloadditions, it is far more reactive than Δ2 cyclopentenone, which is notably more reactive than Δ2 cyclohexenone. For instance, 1 reacts with cyclopentadiene at room temperature with high endo selectivity. Diels-Alder reactions can be readily conducted with other, less reactive diene types (remarkably, with furanoid dienes, high exo selectivity pertains). Ring expansion reactions following Diels-Alder cycloadditions can be used to generate structure types which correspond to cycloadducts of hypothetical but, in reality, nonfunctional dienophiles. In this way, the logic of the Diels-Alder reaction is extendable to reaching hitherto inaccessible structural types.
Supplementary Material
Acknowledgments
Support was provided by the NIH (HL25848 to S.J.D.). We thank Aaron Sattler and Wesley Sattler (Parkin group, Columbia University) for help with X-ray experiments (NSF, CHE-0619638); Mr. Zhang Wang, Drs. Jianglong Zhu and William F. Berkowitz for helpful discussions, and Dr. Fay W. Ng and Ms. Rebecca M. Wilson for editorial assistance and consultations.
Footnotes
Supporting Information Available Experimental procedures, copies of spectral data, and characterization data are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Corey EJ. Angew Chem, Int Ed. 2009;48:2100. doi: 10.1002/anie.200805374. [DOI] [PubMed] [Google Scholar]; (b) Corey EJ. Angew Chem, Int Ed. 2002;41:1650. doi: 10.1002/1521-3773(20020517)41:10<1650::aid-anie1650>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]; (c) Bear BR, Sparks SM, Shea KJ. Angew Chem, Int Ed. 2001;40:820. [PubMed] [Google Scholar]; (d) Williams RM, Stocking EM. Angew Chem, Int Ed. 2003;42:3078. doi: 10.1002/anie.200200534. [DOI] [PubMed] [Google Scholar]; (e) Jørgensen KA. Angew Chem, Int Ed. 2000;39:3558. [Google Scholar]
- 2.(a) Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew Chem, Int Ed. 2002;41:1668. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; (b) Danishefsky S. Acc Chem Res. 1981;14:400. [Google Scholar]
- 3.Karthikeyan M, Kamakshi R, Sridar V, Reddy BSR. Synth Commun. 2003;33:4199. [Google Scholar]
- 4.(a) Bienfait B, Coppe-Motte G, Merényi R, Viehe HG. Tetrahedron. 1991;47:8167. [Google Scholar]; (b) Kelly TR, McNutt RW. Tetrahedron Lett. 1975;16:285. [Google Scholar]
- 5.Sieja JB. J Am Chem Soc. 1971;93:2481. [Google Scholar]
- 6.3-oxo-cyclobutanecarboxylic acid is commercially available or can be synthesized on large scale: Pigou PE, Schiesser CH. J Org Chem. 1988;53:3841.
- 7.See Supporting Information for details: (a) synthesis. (b) spectral documentation. (c) crystallography.
- 8.Dols PPMA, Kluder AJH, Zwanenburg B. Tetrahedron. 1994;50:8515. [Google Scholar]
- 9.Computational studies into the amenability of unsubstituted cycloalkenones to Diels-Alder cycloaddition are in progress.
- 10.Computational studies explaining these results will be disclosed in due course. Fringuelli F, Pizzo F, Taticchi A, Halls TDJ, Wenkert E. J Org Chem. 1982;47:5056.Karthikeyan M, Kamakshi R, Sridar V, Reddy BSR. Synth Commun. 2003;33:4199.
- 11.Maulide N, Markó IE. Org Lett. 2007;9:3757. doi: 10.1021/ol7015753. [DOI] [PubMed] [Google Scholar]
- 12.Greene AE, Deprès JP, Nagano H, Crabbé P. Tetrahedron Lett. 1977;18:2365. [Google Scholar]
-
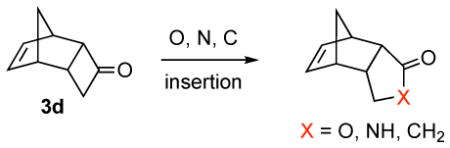
13.It should be noted that ring expansion reactions of unique compound 3d are not regiospecific, and in fact, occur in the sense shown. This finding is in line with the precedent of Miller and co-workers: Miller RD, Dolce DL, Merritt VY. J Org Chem. 1976;41:1221.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


