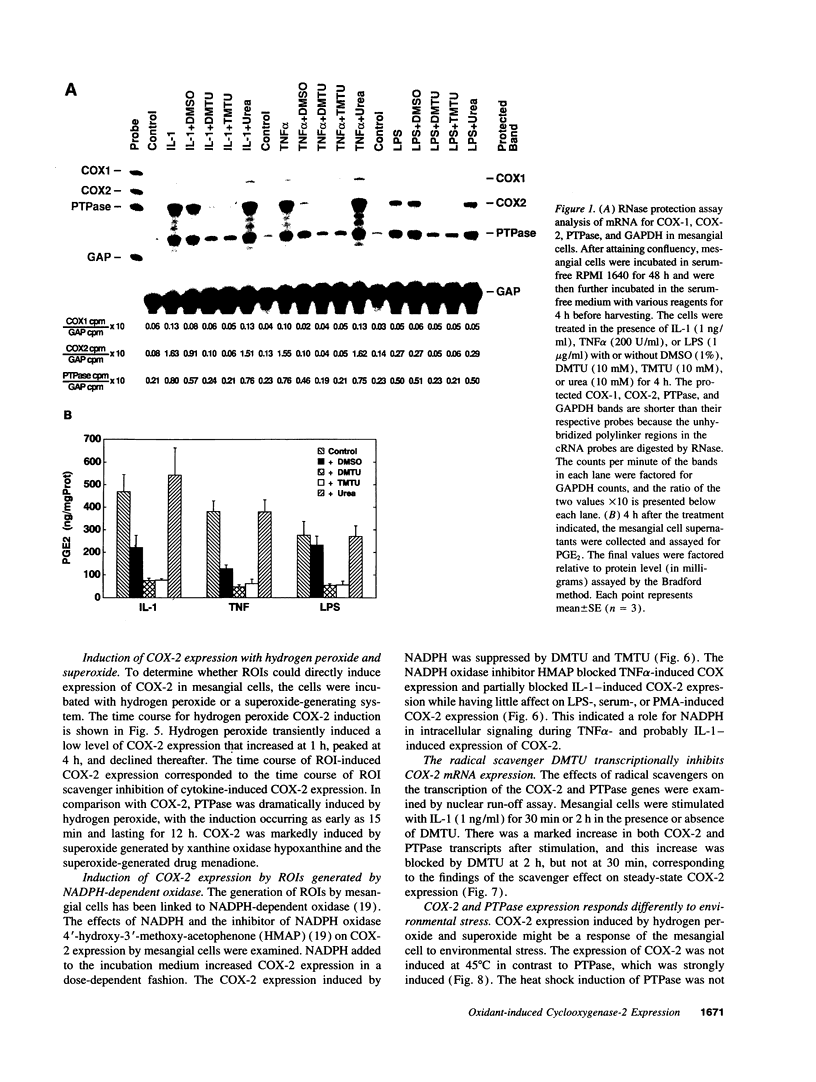
Abstract
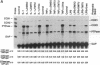
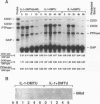
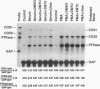
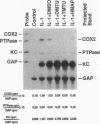
Reactive oxygen intermediates (ROIs) play an important role in inflammatory processes as mediators of injury and potentially in signal transduction leading to gene expression. Cyclooxygenase (COX) is a rate-limiting enzyme in prostanoid biosynthesis, and its recently cloned inducible form, COX-2, is induced by proinflammatory cytokines. This study linked ROIs to the signaling pathways that induce COX-2 expression. The hydroxyl radical scavengers DMSO (1%), as well as di- and tetramethylthiourea, inhibited IL-1-, TNF alpha-, and LPS-induced COX-2 expression in rat mesangial cells. The suppression of COX-2 mRNA expression correlated with the COX-2 protein level. In comparison with the prolonged induction of the inducible gene encoding protein-tyrosine phosphatase by hydrogen peroxide, the COX-2 gene was only transiently induced. Protein-tyrosine phosphatase is also induced by heat shock and chemical stress, whereas COX-2 is not. Superoxide was a more potent inducer for COX-2 than hydrogen peroxide. In addition, NADPH stimulated COX-2 expression, and an inhibitor of NADPH oxidase blocked COX-2 expression induced by TNF alpha. COX-2 and KC gene expression costimulated by IL-1 were inhibited differentially by the scavengers. These studies demonstrate that oxidant stress is a specific and important inducer of COX-2 gene expression. This induction may contribute to the deleterious amplification of prostanoids in inflammation and compound the direct effects of ROI production.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baud L., Fouqueray B., Philippe C., Ardaillou R. Reactive oxygen species as glomerular autacoids. J Am Soc Nephrol. 1992 Apr;2(10 Suppl):S132–S138. doi: 10.1681/ASN.V210s132. [DOI] [PubMed] [Google Scholar]
- Bautista A. P., Spitzer J. J. Superoxide anion generation by in situ perfused rat liver: effect of in vivo endotoxin. Am J Physiol. 1990 Dec;259(6 Pt 1):G907–G912. doi: 10.1152/ajpgi.1990.259.6.G907. [DOI] [PubMed] [Google Scholar]
- Charles C. H., Abler A. S., Lau L. F. cDNA sequence of a growth factor-inducible immediate early gene and characterization of its encoded protein. Oncogene. 1992 Jan;7(1):187–190. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crofford L. J., Wilder R. L., Ristimäki A. P., Sano H., Remmers E. F., Epps H. R., Hla T. Cyclooxygenase-1 and -2 expression in rheumatoid synovial tissues. Effects of interleukin-1 beta, phorbol ester, and corticosteroids. J Clin Invest. 1994 Mar;93(3):1095–1101. doi: 10.1172/JCI117060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForge L. E., Preston A. M., Takeuchi E., Kenney J., Boxer L. A., Remick D. G. Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem. 1993 Dec 5;268(34):25568–25576. [PubMed] [Google Scholar]
- Feng L., Sun W., Xia Y., Tang W. W., Chanmugam P., Soyoola E., Wilson C. B., Hwang D. Cloning two isoforms of rat cyclooxygenase: differential regulation of their expression. Arch Biochem Biophys. 1993 Dec;307(2):361–368. doi: 10.1006/abbi.1993.1601. [DOI] [PubMed] [Google Scholar]
- Feng L., Xia Y., Kreisberg J. I., Wilson C. B. Interleukin-1 alpha stimulates KC synthesis in rat mesangial cells: glucocorticoids inhibit KC induction by IL-1. Am J Physiol. 1994 May;266(5 Pt 2):F713–F722. doi: 10.1152/ajprenal.1994.266.5.F713. [DOI] [PubMed] [Google Scholar]
- Fletcher B. S., Kujubu D. A., Perrin D. M., Herschman H. R. Structure of the mitogen-inducible TIS10 gene and demonstration that the TIS10-encoded protein is a functional prostaglandin G/H synthase. J Biol Chem. 1992 Mar 5;267(7):4338–4344. [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Hemler M. E., Cook H. W., Lands W. E. Prostaglandin biosynthesis can be triggered by lipid peroxides. Arch Biochem Biophys. 1979 Apr 1;193(2):340–345. doi: 10.1016/0003-9861(79)90038-9. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Graff G., Lands W. E. Accelerative autoactivation of prostaglandin biosynthesis by PGG2. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1325–1331. doi: 10.1016/0006-291x(78)91148-8. [DOI] [PubMed] [Google Scholar]
- Henkel T., Machleidt T., Alkalay I., Krönke M., Ben-Neriah Y., Baeuerle P. A. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993 Sep 9;365(6442):182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- Hla T., Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayalcin S. S., Sturbaum C. W., Wachsman J. T., Cha J. H., Powell D. W. Hydrogen peroxide stimulates rat colonic prostaglandin production and alters electrolyte transport. J Clin Invest. 1990 Jul;86(1):60–68. doi: 10.1172/JCI114715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse S. M., Emslie E. A. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature. 1992 Oct 15;359(6396):644–647. doi: 10.1038/359644a0. [DOI] [PubMed] [Google Scholar]
- Kreisberg J. I., Venkatachalam M. A. Vasoactive agents affect mesangial cell adhesion. Am J Physiol. 1986 Oct;251(4 Pt 1):C505–C511. doi: 10.1152/ajpcell.1986.251.4.C505. [DOI] [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Lanahan A., Williams J. B., Sanders L. K., Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992 Sep;12(9):3919–3929. doi: 10.1128/mcb.12.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Soyoola E., Chanmugam P., Hart S., Sun W., Zhong H., Liou S., Simmons D., Hwang D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992 Dec 25;267(36):25934–25938. [PubMed] [Google Scholar]
- Martin M., Neumann D., Hoff T., Resch K., DeWitt D. L., Goppelt-Struebe M. Interleukin-1-induced cyclooxygenase 2 expression is suppressed by cyclosporin A in rat mesangial cells. Kidney Int. 1994 Jan;45(1):150–158. doi: 10.1038/ki.1994.18. [DOI] [PubMed] [Google Scholar]
- Marui N., Offermann M. K., Swerlick R., Kunsch C., Rosen C. A., Ahmad M., Alexander R. W., Medford R. M. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 1993 Oct;92(4):1866–1874. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol. 1986 Nov 15;137(10):3295–3298. [PubMed] [Google Scholar]
- O'Banion M. K., Sadowski H. B., Winn V., Young D. A. A serum- and glucocorticoid-regulated 4-kilobase mRNA encodes a cyclooxygenase-related protein. J Biol Chem. 1991 Dec 5;266(34):23261–23267. [PubMed] [Google Scholar]
- Pilbeam C. C., Kawaguchi H., Hakeda Y., Voznesensky O., Alander C. B., Raisz L. G. Differential regulation of inducible and constitutive prostaglandin endoperoxide synthase in osteoblastic MC3T3-E1 cells. J Biol Chem. 1993 Dec 5;268(34):25643–25649. [PubMed] [Google Scholar]
- Pritchard K. A., Jr, O'Banion M. K., Miano J. M., Vlasic N., Bhatia U. G., Young D. A., Stemerman M. B. Induction of cyclooxygenase-2 in rat vascular smooth muscle cells in vitro and in vivo. J Biol Chem. 1994 Mar 18;269(11):8504–8509. [PubMed] [Google Scholar]
- Radeke H. H., Cross A. R., Hancock J. T., Jones O. T., Nakamura M., Kaever V., Resch K. Functional expression of NADPH oxidase components (alpha- and beta-subunits of cytochrome b558 and 45-kDa flavoprotein) by intrinsic human glomerular mesangial cells. J Biol Chem. 1991 Nov 5;266(31):21025–21029. [PubMed] [Google Scholar]
- Radeke H. H., Meier B., Topley N., Flöge J., Habermehl G. G., Resch K. Interleukin 1-alpha and tumor necrosis factor-alpha induce oxygen radical production in mesangial cells. Kidney Int. 1990 Feb;37(2):767–775. doi: 10.1038/ki.1990.44. [DOI] [PubMed] [Google Scholar]
- Satriano J. A., Shuldiner M., Hora K., Xing Y., Shan Z., Schlondorff D. Oxygen radicals as second messengers for expression of the monocyte chemoattractant protein, JE/MCP-1, and the monocyte colony-stimulating factor, CSF-1, in response to tumor necrosis factor-alpha and immunoglobulin G. Evidence for involvement of reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidase. J Clin Invest. 1993 Sep;92(3):1564–1571. doi: 10.1172/JCI116737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk H., Klein M., Erdbrügger W., Dröge W., Schulze-Osthoff K. Distinct effects of thioredoxin and antioxidants on the activation of transcription factors NF-kappa B and AP-1. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1672–1676. doi: 10.1073/pnas.91.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieven G. L., Kirihara J. M., Myers D. E., Ledbetter J. A., Uckun F. M. Reactive oxygen intermediates activate NF-kappa B in a tyrosine kinase-dependent mechanism and in combination with vanadate activate the p56lck and p59fyn tyrosine kinases in human lymphocytes. Blood. 1993 Aug 15;82(4):1212–1220. [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois J., Levy L. O., Simmons D. L., Richards J. S. Characterization and hormonal regulation of the promoter of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Identification of functional and protein-binding regions. J Biol Chem. 1993 Jun 5;268(16):12199–12206. [PubMed] [Google Scholar]
- Toledano M. B., Leonard W. J. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winrow V. R., Winyard P. G., Morris C. J., Blake D. R. Free radicals in inflammation: second messengers and mediators of tissue destruction. Br Med Bull. 1993 Jul;49(3):506–522. doi: 10.1093/oxfordjournals.bmb.a072627. [DOI] [PubMed] [Google Scholar]
- Xia Y., Feng L., Yoshimura T., Wilson C. B. LPS-induced MCP-1, IL-1 beta, and TNF-alpha mRNA expression in isolated erythrocyte-perfused rat kidney. Am J Physiol. 1993 May;264(5 Pt 2):F774–F780. doi: 10.1152/ajprenal.1993.264.5.F774. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Kashiwabara Y., Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994 Feb 18;269(7):4705–4708. [PubMed] [Google Scholar]
- Xie W. L., Chipman J. G., Robertson D. L., Erikson R. L., Simmons D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Merrill J. R., Bradshaw W. S., Simmons D. L. Structural determination and promoter analysis of the chicken mitogen-inducible prostaglandin G/H synthase gene and genetic mapping of the murine homolog. Arch Biochem Biophys. 1993 Jan;300(1):247–252. doi: 10.1006/abbi.1993.1034. [DOI] [PubMed] [Google Scholar]