Abstract
Detection of novelty is an essential component of recognition memory, which develops throughout cerebral maturation. To better understand the developmental aspects of this memory system, the novel object recognition task (NOR) was used with the immature rat and ontogenically profiled. It was hypothesized that object recognition would vary across development and be inferior to adult performance. The NOR design was made age-appropriate by downsizing the testing objects and arena. Weanling (P20-23), juvenile (P29-40) and adult (P50+) rats were tested after 0.25 hr, 1 hr, 24 hr and 48 hr delays. Weanlings exhibited novel object recognition at 0.25 and 1 hrs, while older animals showed a preference for the novel object out to 24 hrs. These findings are consistent with previous research performed in humans and monkeys, as well as to studies using the NOR after medial temporal lobe damage in adult rats.
Keywords: Object Recognition, Development, Hippocampus, Perirhinal Cortex
The purpose of the current work was to study performance on the novel object recognition task (NOR) across development. The NOR, as first laid out in methodological detail by Ennaceur and Delacour (1988), assesses a rat's ability to recognize a familiar object over a variable length of time; this ability has been coined recognition memory. The NOR task is particularly amenable to developmental work because it is free from response contingencies and requires no pre-training. It instead relies upon a rat's intrinsic exploratory drives to investigate novel stimuli. The NOR also lacks overt stress components, such as forced swimming or food deprivation. The young animal may be especially vulnerable to interference from such factors and, depending upon age, may be unable to learn task rules and contingencies (Bachevalier & Beauregard, 1993).
A second strength of the NOR is that simple non-rule-based tests of object recognition ability are well understood in humans (including preverbal infants) and monkeys. The maturation of visual recognition has been extensively characterized both behaviorally and neurophysiologically utilizing visual paired comparison (VPC) procedures (Alvardo & Bachevalier, 2000; Bachevalier & Vargha-Khadem, 2005). In the young primate the time spent visually fixating on a novel object is quantified and in rat models (which have even been published under the guise of ‘VPC’ [Wu, Zhang, Shao, Qin, Yang & Zhao, 2008]) the amount of interaction with the novel object is assessed. Thus the NOR and similar recognition memory tasks are amenable to cross-species generalization and could prove promising to the modeling of pediatric/adolescent disorders.
Recognition memory is comprised of both familiarity detection and recollection (Aggleton & Brown, 2006; Fortin, Wright & Eichenbaum, 2004). These functions are primarily localized within the medial temporal lobe (MTL) (Bachevalier et al., 2005; Brown & Aggleton, 2001), structures that undergo substantial postnatal development and reorganization in rats, monkeys and humans. Currently there is controversy over the role of the hippocampal complex vs. the perirhinal cortex in object recognition abilities (Dere, Huston & De Souza Silva, 2007; Murray, Bussey & Saksida, 2007). Better understanding of the ontogeny of recognition memory in conjunction with studies of neurodevelopment would shed light on the debated neurophysiology.
Given the extensive developmental work in primates, the relative dearth of maturational literature on the NOR in rats was surprising. Prior to the present study, only one paper had been published directly applying the NOR to the immature rat. In that study the authors reported a decrement in pre-weanlings (18 days old) on object recognition with retention intervals between 1 minute and 2 hours, while adults showed stable performance across this interval (Anderson, Barnes, Briggs, Ashton, Moody, Joynes & Riccio, 2004). However as a substantial number of the pre-weanlings were dropped from analyses because they did not perform the task, this begs the question as to whether the NOR can be used with young rats at all. No publications specifically characterizing the NOR in weanling or juvenile rats were found, although several studies have used the NOR as part of a panel of behavioral tests in adolescent/juvenile aged (post-natal day >25-42) rats to demonstrate neurocognitive deficits due to perinatal iron deficiency (Wu et al., 2008) or early disruptions of circadian light cycles (Toki, Morinobu, Imanaka, Yamamoto, Yamawaki & Homma, 2007). One recent study presented methodology for automating the NOR task in adolescent (P35) rats (Silvers, Harrod, Mactutus & Booze, 2007), but did not investigate younger ages. Developmental characterization of the NOR over a range of retention intervals is also lacking.
Consequently, once we ascertained the capability of weanlings to perform the NOR utilizing an age-appropriate design, we profiled the task across development. Groups of weanling, juvenile and adult rats were tested while employing retention intervals of 15 minutes, 1 hour, 24 hours and 48 hours. Based upon the work by Anderson et al. (2004) it was hypothesized that an age-dependent difference in object recognition would arise after longer delays, with worse performance at younger ages.
Method
Animals
Three different age groups of male Sprague-Dawley rats were utilized corresponding to the period of weaning (post-natal day 20-23), the onset of sexual maturation (juvenile: P29-40) and young adulthood (P50+). The sample sizes by group per retention interval were: 0.25 hours (weanling: n = 26; juvenile: n = 17; adult: n = 13), 1 hour (n = 14, n = 13, n = 12), 24 hours (n = 14, n = 14, n = 17) and 48 hours (n = 10, n = 10, n = 13). Rats were not bred on site. Multiple litters of rat pups were obtained from a commercially available vendor (Charles River, Hollister, CA) where it is standard procedure to cross-foster pups with different dams; hence animals were littermates rather than necessarily siblings, providing genetic variation equivalent to that of older groups. Post-weanling rats were obtained as needed from the vendor. Animals were maintained in standard vivarium housing with food and water ad libitum. All procedures were conducted with approval from the University of California at Los Angeles Chancellor's Animal Research Committee and were in conformity with the NIH Guide for the Care and Use of Laboratory Animals.
Apparatus
After piloting the task with different parameters an age-appropriate NOR design was developed. That is, to increase weanling object interaction rates and decrease attrition it was found necessary to scale the object and arena sizes to the size of the animal within a given age group. The NOR testing chamber (Fig. 1A) was a white, Plexiglas® arena which could be partitioned into age-appropriate arenas (weanling: 32 × 52 × 9 cm, juvenile: 52 × 52 × 9 cm, adult: 70 × 70 × 9 cm) that maximized exploratory behavior while minimizing incidental contact with testing objects. The weanling arena accommodated rats that weighed at least 50g, rats were run in the juvenile arena beginning at 100g and the adult arena was for rats 200g and more, so that the ratio of body weight to arena area was kept relatively constant between groups (pup=.03, juvenile=.04, adult=.04). The small, white-walled, enclosed enclave which housed the testing chamber was kept at a dim, ambient light level. Rats were always transported from the home cage in the testing room to the testing chamber in a plastic, standard vivarium cage covered by a white towel.
Figure 1.
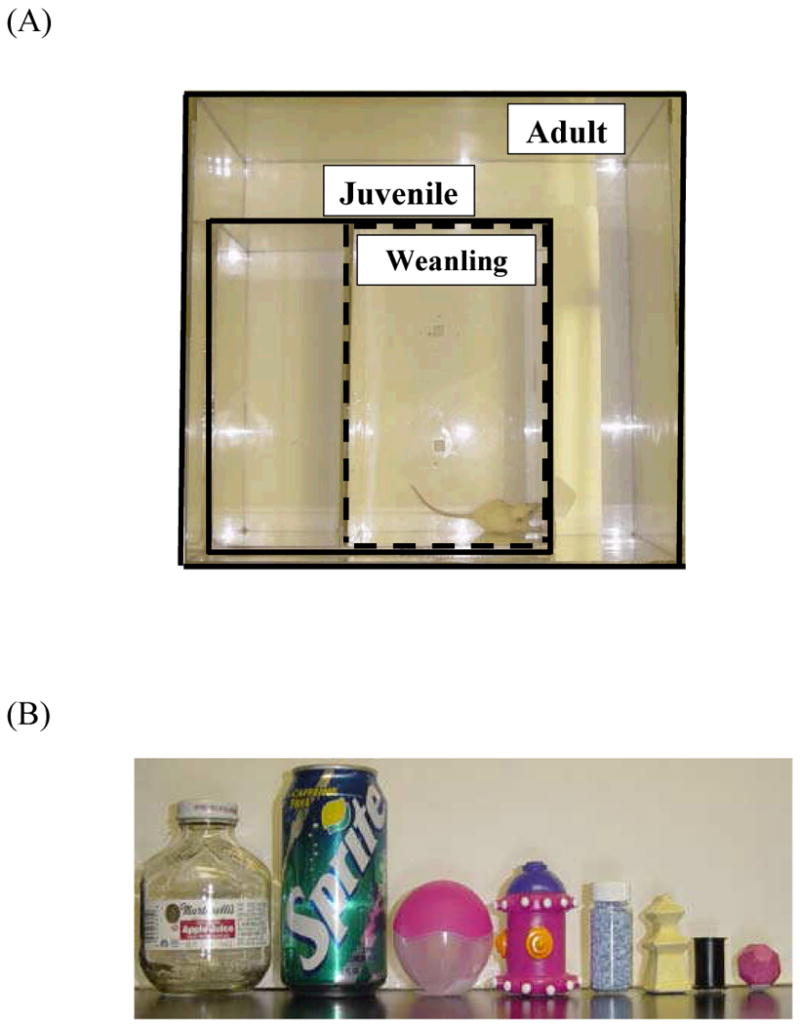
(A) NOR testing chamber. The chamber size depended on the age of the animal: weanling (32 × 52 × 9 cm), juvenile (52 × 52 × 9 cm) and adult (70 × 70 × 9 cm adult), with the weanling arena approximating the size of its home cage. (B) NOR testing object pairs, shown here decreasing in size from those used with adults to juveniles to weanlings.
The objects used for NOR testing (Fig. 1B) were easy to clean materials such as rubber, plastic, glazed ceramic and glass and consisted of a variety of household items and pet toys. Object sizes were age-appropriate, that is, no taller than twice the size of the rat, and it was important that objects not resemble living stimuli (i.e., no eye-spots or animal shapes). The arena and all objects were cleaned with 70% ethanol to remove odors between rats. Objects were secured to the testing chamber floor via Velcro® and positioned centrally near the back wall of the chamber. Black permanent marker was used to shade from the tip of the rat's nose to between the ears, which allowed the tracking system (SMART, San Diego Instruments) to measure the time spent interacting with the objects. Digitally, a circular zone was created around each object so that movement ≤ 5 cm from the object's center could be detected as object interaction by the tracking system (Fig. 2A,B).
Figure 2.
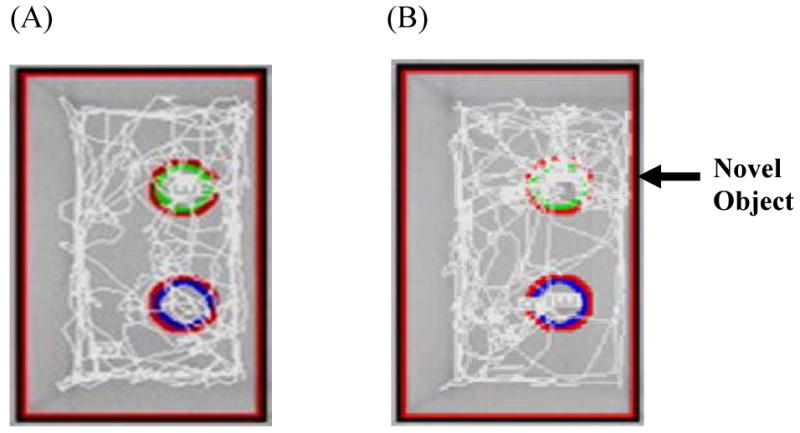
Computer generated tracks of weanling movement during the test trial. Testing objects were contained in each of the circular zones and entry into the zones was quantified. (A) The tracing on the left shows equal amounts of tracking within the two zones. This indicates a lack of an object preference. (B) The animal in the tracing on the right was tracked more frequently in the top (novel) zone than in the bottom zone, indicating a novel object preference.
The Novel Object Recognition Task (NOR)
The NOR procedure was based upon the original Ennanceur et al. (1988) procedure. It consisted of a habituation phase followed by a testing phase.
During the habituation phase each rat was allowed to freely explore the empty arena over three days and on the fourth day the testing phase begun. Habituation consisted of three ten minute sessions administered one per day and weaning of the weanling groups was always conducted after NOR testing.
The testing phase consisted of a (1) familiarization trial followed by a (2) test trial. During the familiarization trial a single rat was placed in the arena containing two identical objects and released against the center of the opposite wall with its back to the objects. This was done to prevent coercion to explore the objects. Object interaction was defined as entrance into the object-containing zone resulting in direct or nearly direct object contact with the nose or whiskers. It was observed that the weanling rats did not always begin exploring the chamber immediately, as their older counterparts did. To ensure equivalent object interaction rates between less explorative weanling rats and older animals, weanlings were allowed 5 minutes to explore the chamber and older animals were given 3 minutes, and total object interaction times were quantified. All rats were returned to their home cages between habituation and test trials.
The test trial was administered in the aforementioned way except that one sample object from the familiarization trial and one novel object were presented; object pairs were similar in form and color yet discernibly different (Fig. 1B). The zone in which the novel object was placed was randomized, as was the object that served as the novel object. It was assumed that an animal that recognized the sample object after the delay would spend greater than 50% of its total object interaction time with the novel object (Fig. 2B). An animal unable to recognize the sample object would therefore split its interaction time 50:50 between the two objects (Fig. 2A). To make the consideration of performance at testing more equivalent across age groups, the test trial was evaluated after 3 minutes of chamber exploration, even in weanling groups (see results for further discussion).
The test trials were administered after delays of 0.25 hour (15 minutes), 1 hour, 24 hours or 48 hours post-familiarization. It should be noted that in an attempt to conserve the number of animal used a portion of the rats were retested up to three times as rats matured to fill out juvenile and adult groups, utilizing new object pairs. Due to the nature of the task, and based upon the original Ennaceur et al. (1998) data, practice effects were not anticipated and indeed retesting did not produce any statistically significant effects on total amounts of object interaction or novel object interaction during the test trial.
Merely sitting on the edge of or within a zone or quick incidental passage through a zone was not counted as object interaction. The experimenter carefully watched the trial or if warranted reviewed the NOR run via backup video footage for such tracking errors, which were subtracted from the interaction time totals. Individual animals demonstrating insufficient task performance were excluded from later specific statistical analyses for the following reasons: (1) non-exploration, which was defined as total objection interaction for ≤ 2 seconds or (2) technical malfunctions during data collection.
Statistics
An alpha level of ≤ 0.05 was used for all analyses. To evaluate within group performance the percentage of total object interaction time spent with the novel object (known henceforth as %novel) was compared to 50% utilizing one-sample T-Tests. ANOVA was utilized for between-group analyses of memory performance, with delay and age group as fixed factors and %novel as the variate. Univariate ANOVA was also utilized in the analysis of total object interaction amounts during the habituation trial, with delay and age group as fixed factors and the mean total time of object interaction as the variate. To ensure that one object of a pair was not inherently more desirable than the other independent sample T-tests were carried out after every habituation trial comparing the total amount of interaction with one object to that of the other object. When necessary, significant differences between levels of a factor were detected post-hoc with a Bonferroni adjusted α of ≤ 0.05.
Results
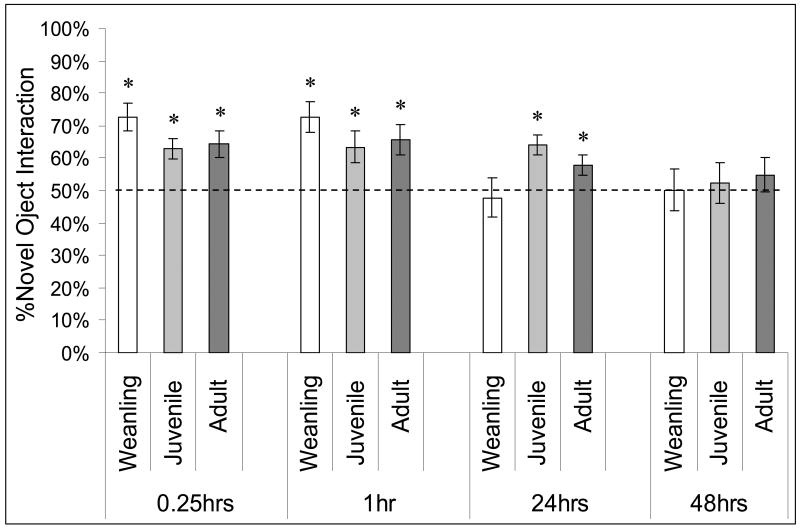
After the 0.25 hour delay all three age groups spent significantly greater than 50% of their object interaction time with the novel object, adult: 64%, t(12) = 3.37, p < 0.01; juvenile: 62%, t(16) = 3.71, p < 0.01; weanling: 73%, t(19) = 5.22, p < 0.01 (Fig. 3). The across group analysis found no significant mean differences by age. One weanling was excluded from the analyses due to technical problems and another five (out of 26) were excluded due to non-exploration.
Figure 3.
The percentage of total interaction time (i.e., with the object pair) spent with the novel object (%Novel) versus a 50% criterion. The performance of each age group after each retention interval is shown (* indicates p<0.05). A weanling deficit was found at 24 hours as compared to older animals, while adults and juveniles showed loss of object preference at the 48 hour retention interval.
Results from the 1 and 0.25 hour delays mirrored each other, adult: 66%, t(11) = 3.26, p < 0.01; juvenile: 63%, t(11) = 2.76, p = 0.02; weanling: 73%, t(13) = 4.85, p < 0.01, and again there were no significant effects of age. One juvenile animal was excluded from memory analyses for being non-explorative.
As shown in Figure 3, after a 24 hour delay adult and juvenile groups spent significantly greater than 50% of their object interaction time with the novel object, adult: 58%, t(15) = 2.58, p = 0.02; juvenile: 64%, t(13) = 4.41, p < 0.01, while the weanling group did not (48%). One non-explorative adult was excluded from the analysis. Based upon NOR performances from this and the previous two delays, these data demonstrated no difference between juvenile and adult recognition abilities. Subsequently, an across groups analysis comparing older animals (juvenile/adult) to weanlings yielded a significant mean difference on the memory measure at this retention interval in favor of the older group, %novel : 61% vs. 48%, F(1, 42) = 6.17, p = 0.02.
All age groups showed absence of object recognition by 48 hours post-familiarization (%novel: adult = 55%, juvenile = 52%, weanling = 50%) (Fig.3). One non-exploratory weanling was excluded, as was a single adult due to technical problems.
As an aside and in further support of the age-dependent difference, rather than using a normalized memory measure (%novel) ANCOVAs were also run at each delay to correct novel object preference rates for individual variation in general object interaction. The raw amount of novel object interaction served as the variate, the total raw amount of object interaction with both objects served as the covariate and age group was the fixed factor. Again, significant group differences were detected only after the 24 hour delay.
Importantly, despite providing weanlings with 5 minutes of exploration time (rather than 3 minutes) there were no significant group differences in the total amount of object interaction during the familiarization trial (adult = 30s ± 16s, juvenile = 26s ± 15s, weanling = 30s ± 25s). Because weanlings exhibited object recognition abilities equivalents to that of older animals at both the 0.25 and 1 hour delays after 3 minutes of exploration time during the test trial (as opposed to 5 minutes), the lack of object recognition after the 24 and 48 hour delays is not due to a lack of weanling object interaction at testing. Lastly, no confounding object preferences were found.
Discussion
The novel object recognition task (NOR) as described by Ennaceur and Delacour (1988) examines a rodent's ability to recognize a previously explored object over time. In the current work the NOR was successfully used for the first time with weanling rats and characterized across development and retention time. As hypothesized, an age-dependent difference in long-term memory ability was detected. In the face of the general principle of ‘infantile amnesia’ [24] these results may not appear particularly new, however the current work contributes to knowledge of both the application and ontogeny of the NOR, which may be useful to developmental scientists. The NOR is particularly amenable to developmental work because it relies upon that rodent's innate preference for exploring novel over familiar stimuli and the task is unhindered by potentially confounding contingency rules or stress components. Secondly, the NOR lends itself well to cross-species generalization (Alvarado et al., 2000; Bachevalier et al., 1993). Although there is a lack of ontogenic NOR literature incorporating the rodent, there has been extensive characterization of object recognition in monkey and human infants via the employment of the visual paired comparison task (VPC), a task that has similarities to the rodent NOR.
In the present work it was found that adult (post-natal day 50+) and juvenile (P29-40) rats exhibited comparable NOR performances. While weanling (P20-23) rats were able to exhibit robust object recognition across shorter delays, they exhibited inferior long-term memory retention. All age groups we able to recognize the object after shorter intervals of 0.25 and 1 hour and performance after the two delays was similar. A difference was detected after the longer interval of 24 hours; adults and juveniles exhibited comparable, significant object recognition/preference while weanlings did not. Lastly, all groups failed to show a novel object preference after a 48 hour retention interval. The four intervals therefore spanned the length of NOR retention ability (at least under the experimental parameters used here) and suggested that weanling animals were able to perform the task and exhibited object recognition ability but nonetheless showed a retention deficit.
Our results support and extend those of Anderson et al. (2004), who found that pre-weanlings (18-day old rats, weaning generally takes place around 21 days of age) were better able to recognize a familiar object after a one minute retention interval than after a two hour interval, while adult performance remained stable across both delays. Although the authors did well to suggest ontogenic differences in recognition memory there were important caveats in the work. First, there was only a trend toward a difference in performance between the two age groups and second, nearly half of the substantial pre-weanling sample (n = 49) was excluded from data analysis due to a lack of exploration and object interaction (Anderson et al., 2004). Such a high exclusion rate was confounding because subsequent analyses did not provide a complete picture of the whole age-group's memory performance. By scaling the size of the chamber and toys to be age-appropriate, we were able to obtain analyzable performance on 92.5% of all trials.
Because animals were allowed to explore the testing chamber and interact with objects freely for a set amount of time rather than until a designated amount of object interaction had accumulated, the question arose as to whether task performance related to the amount of object interaction and whether interaction rates varied by age. Despite providing weanlings with an increased amount of exploration time weanlings nonetheless showed comparable amounts of object interaction to older groups and did not demonstrate prolonged memory retention. These results suggest that less explorative weanling rats may require more time for exploration to acquire comparable levels of object interaction and memory performance (at shorter delays) to older animals.
A precise explanation for the age-dependent decrement in long-term memory found is beyond the scope of this paper; however, our finding is consistent with the visual recognition memory literature across species. These findings correspond to the general observation of ‘infantile amnesia’ on learning and memory tasks in which young rats forget more quickly than older rats, thus reflecting a difference in short-term and long-term memory abilities (Rudy & Morledge, 1994). There is also evidence that rats simply do not show adult levels of habituation to novel stimuli and environments until P25-30 (Feigley, Parsons, Hamilton & Spear, 1972; File, 1978), which would be older than our weanling group. Furthermore, recognition memory ability is the earliest primate learning and memory function to appear and yet primate infants are not proficient on the VPC at longer delays until they are several months old (Alvarado et al., 2000).
The age-dependent discrepancy may be rooted within the medial temporal lobe (MTL), the area of the brain responsible for recognition memory. The MTL (characterized by the interconnected structures of the hippocampus/dentate complex, peri- and entorhinal cortices and the parahippocampal gyrus) undergoes a protracted period of postnatal development in humans, monkeys and rodents, with its different structures maturing along different time continuums and the learning and memory functions subserved emerging differently in time as well (Alvarado et al., 2000). The hippocampus was originally thought to be the seat of recognition memory and some authors have reported hippocampal related long-term NOR deficits in the face of intact short-term performance (Baker & Kim, 2002; Bruel-Jungermann, Laroche & Rampon, 2005; Clark, Zola & Squire, 2000; Hammond, Tull & Stackman, 2004; Vnek & Rothblat, 1996). However more recent publications have argued against this point (Mumby, 2001; Mumby, Gaskin, Glenn, Schramek & Lehmann, 2002; Murray et al., 2007; Winters, Forwood, Cowell, Saksida & Bussey, 2004) and have instead placed importance primarily on the perirhinal cortex (Ainge, Heron-Maxwell, Theofilas, Wright, de Hoz & Wood, 2006; Bussey, Muir & Aggleton, 1999; Mumby & Pinel, 1994); Winter et al., 2004).
Neurodevelopmentally, the emergence in rats of hippocampus-dependent behaviors coincides with the end of neurogenesis and synaptogenesis in the dentate of the hippocampal complex at around the time of weaning. Indeed proficient NOR performance emerged sometime between weaning and post-natal day 29 (beginning of the operationally defined juvenile period). Little is known about the development of the perirhinal cortex, although in primates its maturation has been shown to lag behind the entorhinal cortex (Alvarado et al., 2000). Nevertheless, MTL structures are highly integrated and perhaps the perirhinal cortex is sufficient for the processing and recognition of object attributes after short retention intervals but the hippocampus is involved in long-term object recognition.
It has been suggested that MTL damage is similar to MTL immaturity and therefore uncovering the neurophysiological underpinnings of recognition memory across rat development will be important to related models of pediatric disease and dysfunction. Traumatic brain injury (TBI) is the number one cause of death and disability in children and adolescents (Weiner & Weinberg, 2000), many of whom exhibit recognition memory deficits (Levin, Eisenberg & Wigg, 1982; Levin, High & Ewing-Cobbs, 1988). A lack of decrement in procedural or implicit memory (a known non-MTL mediated memory functions) after pediatric TBI has also been observed, lending support to the idea that MTL learning and memory are especially vulnerable to insult in children (Ward, Shum, Wallace & Boon, 2002). Future NOR characterization studies should incorporate pre-weanlings with an age-appropriate design as well as investigate how the development of the hippocampal complex and perirhinal cortex are correlated with performance.
Acknowledgments
This research was supported by grants NS027544, NS02197, NS057420, the Child Neurology Foundation/Winokur Family Foundation and the UCLA Brain Injury Research Center. We would like to express our appreciation to Luigi DiStefano for his initial contribution to the starting of this work.
Contributor Information
David A. Hovda, Email: dhovda@mednet.ucla.edu.
Christopher C. Giza, Email: cgiza@mednet.ucla.edu.
References
- Aggleton JP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends Cognitive Sciences. 2006;10:455–63. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Ainge JA, Heron-Maxwell C, Theofilas P, Wright P, de Hoz L, Wood ER. The role of the hippocampus in object recognition in rats: Examination of the influence of task parameters and lesion size. Behavioural Brain Research. 2006;167:183–195. doi: 10.1016/j.bbr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Bachevalier J. Revisiting the maturation of medial temporal lobe memory functions in primates. Memory. 2000;7:244–56. doi: 10.1101/lm.35100. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Barnes GW, Briggs JF, Ashton KM, Moody EW, Joynes RL, Riccio DC. Effects of ontogeny on performance of rats in a novel object-recognition task. Psychological Reports. 2004;94:437–43. doi: 10.2466/pr0.94.2.437-443. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Beauregard M. Maturation of medial temporal lobe memory functions in rodents, monkeys, and humans. Hippocampus. 1993;3:191–202. [PubMed] [Google Scholar]
- Bachevalier J, Vargha-Khadem F. The primate hippocampus: ontogeny, early insult and memory. Current Opinion in Neurobiology. 2005;15:168–74. doi: 10.1016/j.conb.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Memory. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. The European Journal of Neuroscience. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Aggleton JP. Functionally dissociating aspects of event memory: the effects of combined perirhinal and postperirhinal cotex lesions on object and place memory in the rat. The Journal of Neuroscience. 1999;19:495–502. doi: 10.1523/JNEUROSCI.19-01-00495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. The Journal of Neuroscience. 2000;20:8853–60. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rats. Neuroscience and Biobehavioral Reviews. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1:Behavioral Data. Behavioural Brain Research. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Feigley DA, Parsons PJ, Hamilton LW, Spear NE. Development of habituation to novel environments in the rat. Journal of Comparative and Physiological Psychology. 1972;79:443–452. doi: 10.1037/h0032842. [DOI] [PubMed] [Google Scholar]
- File SE. The ontogeny of exploration in the rat: Habituation and effects of handling. Developmental Psychobiology. 1978;11:321–28. doi: 10.1002/dev.420110405. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–91. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay–dependent involvement of the hippocampus in object recognition memory. Neurobiology of Learning and Memory. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Levin KS, Eisenberg HM, Wigg NR. Memory and intellectual ability after head injury in children and adolescence. Neurosurgery. 1982;11:668–673. doi: 10.1227/00006123-198211000-00009. [DOI] [PubMed] [Google Scholar]
- Levin KS, High WM, Jr, Ewing-Cobbs L. Memory functioning during the first year after closed head injury in children and adolescents. Neurosurgery. 1988;22:1043–1052. doi: 10.1227/00006123-198806010-00012. [DOI] [PubMed] [Google Scholar]
- Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behavioural Brain Research. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places and contexts. Learning & Memory. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Pinel JP. Rhinal cortex lesions and object recognition in rats. Behavioral Neuroscience. 1994;108:11–8. doi: 10.1037//0735-7044.108.1.11. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. Visual Perception and Memory: A new view of medial temporal lobe function in primates and rodents. Annual Review of Neuroscience. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal memory systems. Behavioral Neuroscience. 1994;108:227–34. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Harrod SB, Mactutus CF, Booze RM. Automation of the novel object recognition task for use in adolescent rats. Journal of Neuroscience Methods. 2007;1:99–103. doi: 10.1016/j.jneumeth.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki S, Morinobu S, Imanaka A, Yamamoto S, Yamawaki S, Honma K. Importance of early lighting conditions in maternal care by dam as well as anxiety and memory later in life of offspring. The European Journal of Neuroscience. 2007;25(3):815–829. doi: 10.1111/j.1460-9568.2007.05288.x. [DOI] [PubMed] [Google Scholar]
- Vnek N, Rothblat LA. The hippocampus and long-term object recognition memory in the rat. The Journal of Neuroscience. 1996;8:2780–87. doi: 10.1523/JNEUROSCI.16-08-02780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward H, Shum D, Wallace G, Boon J. Pediatric traumatic brain injury and procedural memory. Journal of Clinical and Experimental Neuropsychology. 2002;24(4):458–470. doi: 10.1076/jcen.24.4.458.1032. [DOI] [PubMed] [Google Scholar]
- Weiner, Weinberg . Head Injury in the Pediatric Age Group. In: Cooper, Golfinos, editors. Head Injury. San Francisco: McGraw-Hill Medical Publishing Division; 2000. [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. The Journal of Neurosc ience. 2004;24(26):5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zhang L, Shao J, Qin Y, Yang R, Zhao Z. Effect of perinatal iron deficiency on myelination and associated behaviors in rat pups. Behavioural Brain Research. 2008;188(2):263–270. doi: 10.1016/j.bbr.2007.11.003. [DOI] [PubMed] [Google Scholar]