Abstract
High-dose immunosuppressive therapy followed by autologous hematopoietic stem cell transplantation (HSCT) is currently being evaluated for the control of severe autoimmune diseases. The addition of antithymocyte globulin (ATG) to high-dose chemoradiotherapy in the high-dose immunosuppressive therapy regimen and CD34 selection of the autologous graft may induce a higher degree of immunosuppression compared with conventional autologous HSCT for malignant diseases. Patients may be at higher risk of transplant-related complications secondary to the immunosuppressed state, including Epstein-Barr virus (EBV)–associated posttransplantation lymphoproliferative disorder (PTLD), but this is an unusual complication after autologous HSCT. Fifty-six patients (median age, 42 years; range, 23–61 years) with either multiple sclerosis (n = 26) or systemic sclerosis (n = 30) have been treated. The median follow-up has been 24 months (range, 2–60 months). Two patients (multiple sclerosis, n = 1; systemic sclerosis, n = 1) had significant reactivations of herpesvirus infections early after HSCT and then developed aggressive EBV-PTLD and died on days +53 and +64. Multiorgan clonal B-cell infiltrates that were EBV positive by molecular studies or immunohistology were identified at both autopsies. Both patients had positive screening skin tests for equine ATG (Atgam) and had been converted to rabbit ATG (Thymoglobulin) from the first dose. Of the other 54 patients, 2 of whom had partial courses of rabbit ATG because of a reaction to the intravenous infusion of equine ATG, only 1 patient had a significant clinical reactivation of a herpesvirus infection (herpes simplex virus 2) early after HSCT, and none developed EBV-PTLD. The T-cell count in the peripheral blood on day 28 was 0/μL in all 4 patients who received rabbit ATG; this was significantly less than in patients who received equine ATG (median, 174/μL; P = .001; Mann-Whitney ranked sum test). Although the numbers are limited, the time course and similarity of the 2 cases of EBV-PTLD and the effect on day 28 T-cell counts support a relationship between the development of EBV-PTLD and the administration of rabbit ATG. The differences between equine and rabbit ATG are not yet clearly defined, and they should not be considered interchangeable in this regimen without further study.
Keywords: EBV, Lymphoma, Hematopoietic stem cell transplantation, CD34 selection, Autoimmune diseases, Systemic sclerosis, Multiple sclerosis
INTRODUCTION
Pilot studies were performed of high-dose immuno-suppressive therapy (HDIT) followed by transplantation of autologous CD34-selected peripheral blood stem cells (PBSC) to obtain safety and preliminary efficacy data in patients with severe autoimmune diseases. The HDIT regimen included high-dose cyclophosphamide (Cy) and total-body irradiation (TBI). However, the regimen was modified from the conventional high-dose chemoradio-therapy regimens used for the treatment of malignancies to reduce the severity of regimen-related toxicities but yet maximize the degree of immunosuppression. The dose of TBI was of moderate intensity (800 cGy), the autologous PBSC graft was CD34 selected, and equine antithymocyte globulin (ATG) was administered before and after transplantation for in vivo T-cell depletion. If patients had positive skin tests or adverse infusional reactions to equine ATG (Atgam; Pharmacia, Peapack, NJ), rabbit ATG (Thymoglobulin; Sangstat, Lyon, France) was administered instead.
Lymphoproliferative disorders associated with the Epstein-Barr virus (EBV) occur with congenital or acquired immunodeficiency disorders but are most often seen in immunosuppressed patients after solid organ or allogeneic hematopoietic stem cell transplantation (HSCT). A lymphoproliferative disorder occurs when there is a failure to control proliferation of EBV-infected B cells because of impaired T-cell immunity. EBV-associated posttransplantation lymphoproliferative disorder (PTLD) is an infrequent complication after high-dose chemoradiotherapy and autologous HSCT for malignant diseases. In 1 single-center study, it was reported that there were 4 (0.7%) cases among 612 patients reviewed [1]. There have been other infrequent case reports of EBV-PTLD after autologous HSCT, but none to date have been reported after HDIT for patients with severe autoimmune diseases [2–8]. As a result of the modifications to the high-dose chemoradiotherapy, including lymphocyte depletion by CD34 selection and the addition of ATG before and after HSCT, patients may be profoundly immunosuppressed. We report here 2 cases of EBV-PTLD after autologous HSCT that were associated with an increased degree of immunosuppression after rabbit ATG was substituted for equine ATG in the HDIT regimen for patients with a positive skin test to the equine product.
METHODS
Patients
Fifty-six patients were enrolled on 2 multicenter phase II studies coordinated by the Fred Hutchinson Cancer Research Center (FHCRC) of HDIT and HSCT for multiple sclerosis (MS) and severe systemic sclerosis (SSc) from January 1997 to September 2002 [9,10]. The primary objective of the pilot studies was to assess the safety of the HDIT regimen and the transplantation of autologous CD34-selected stem cells. Secondary end points included disease response, safety of mobilization with granulocyte colony-stimulating factor (G-CSF), and immunologic recovery. Patients were enrolled at FHCRC (n = 35), the University of Michigan (n = 5), the University of Nebraska (n = 4), Loma Linda University (n = 3), Wayne State University (n = 3), Washington University (n = 2), the Texas Transplant Institute (n = 2), the University of Colorado (n = 1), and City of Hope National Medical Center (n = 1). The stem cell collection procedure and the HDIT regimen were initially the same for both protocols. Because of positive skin tests, 2 patients were treated with only rabbit ATG instead of equine ATG. Two other patients had infusion reactions to equine ATG initially and then were later switched to rabbit ATG. Protocols were reviewed and approved by the Institutional Review Office of the FHCRC (Seattle, WA). All patients consented to participate in the protocols, according to the institutional requirements.
Treatment and Supportive Care
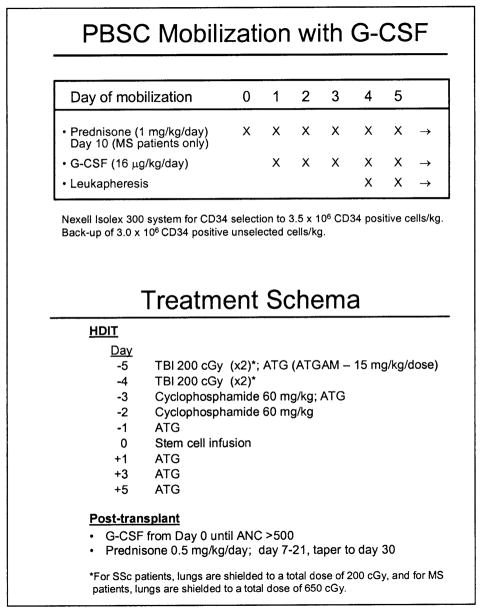
PBSC were mobilized in the outpatient department with subcutaneous G-CSF at 16 μg/kg/d (Figure 1). The first apheresis was done on day 4. G-CSF–mobilized PBSC products were CD34-selected by using an Isolex 300I (Baxter, Deerfield, IL) with targets of >3.5 × 106 CD34+ cells per kilogram [11]. Unmodified PBSC (>3.0 × 106 CD34+ cells per kilogram) were also stored for potential backup use. The autologous cell product was evaluated after CD34 selection for content of CD34+ cells and T cells (CD3+) by flow cytometry. Cells were cryopreserved by using standard techniques [12]. Because of a flare of MS during mobilization of the fourth patient, routine administration of prednisone was started with the fifth MS patient enrolled in that study. Prednisone 1 mg/kg/d was administered 1 day before the start of G-CSF and was continued for 10 days. Prednisone was not used during PBSC mobilization of SSc patients.
Figure 1.
Mobilization and treatment schema for patients with MS and SSc receiving autologous HSCT after HDIT. MS patients were given prednisone during mobilization to prevent flares.
HDIT included fractionated TBI delivered as 200 cGy fractions, 2 fractions per day, on day −5 and day −4, for a total dose of 800 cGy; Cy 60 mg/kg intravenously on days −3 and −2; and equine ATG (Atgam) 15 mg/kg/d intravenously on days −5, −3, −1, +1, +3, and +5. Patients who had a positive skin test or infusional reactions to equine ATG were switched to rabbit ATG (Thymoglobulin) at 2.5 mg/kg/d on the same schedule as the equine ATG. Day 0 was designated as the day of PBSC infusion. Methyl-prednisolone 1 mg/kg intravenously was given with each dose of ATG. TBI was administered from either opposing dual cobalt 60 sources or linear accelerators at dose rates of 7 to 15 cGy/min. TBI from the dual opposed cobalt 60 sources was administered without lung shielding, which was not required because of the patient positioning in the radiation field. If TBI was administered from a linear accelerator, lung shielding was used to limit whole-lung doses to approximately 650 cGy. After the eighth SSc patient, all patients in this study received lung shielding to ensure that the lungs received a total dose of no more than 200 cGy. This was accomplished with the use of a partial transmission block. G-CSF 5 μg/kg/d intravenously or subcutaneously was given from day 0 until the absolute neutrophil count was >500/μL for 3 days. In the MS study, prednisone 0.5 mg/kg/d was given as a single dose from day +7 to day +21 and then was tapered and completed at day +30 to prevent neurologic complications during engraftment. In the SSc study, as protection against lung toxicity and to standardize steroid therapy after HDIT, prednisone (0.5 mg/kg/d) was given from the start of conditioning until day +30 and tapered over 1 month. Patients were hospitalized from the start of conditioning therapy until there was neutrophil engraftment (absolute neutrophil count >500/μL) and resolution of major toxicities. Infection prophylaxis included trimethoprim-sulfamethoxazole for Pneumocystis carinii [13], fluconazole for candida [14], and acyclovir for herpes simplex virus and varicellazoster virus (1 year). Patients were monitored for cytomegalovirus (CMV) reactivation, and if positive, preemptive therapy with ganciclovir was started [15].
Diagnosis of EBV-PTLD
Biopsy specimens were evaluated morphologically and with immunocytochemistry stains by using CD20 and latent membrane protein 1 (LMP1). Clonality was assessed by κ and λ light chain restriction. Molecular studies were done for EBV by in situ hybridization for EBV-encoded RNA (EBER). Copy numbers of EBV DNA were assessed in patient serum by polymerase chain reaction as previously described [16].
Immune Recovery
Lymphocyte subsets were enumerated by using 3-color flow cytometry as described [17–20]. Briefly, blood mononuclear cells (MNCs) were stained with mouse monoclonal antibodies conjugated to fluorochromes (fluorescein isothiocyanate, phycoerythrin, and phycoerythrin plus cyanin 5). Flow cytometry data were acquired by using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and were analyzed with Winlist software (Verity, Topsham, ME). T cells were defined as CD3+ MNCs. B cells were defined as MNCs expressing CD19 or CD20 and not brightly expressing CD3, CD10, CD13, CD14, CD16, CD34, or CD56. Natural killer (NK) cells were defined as MNCs expressing CD16 or CD56 and not expressing CD3 or CD14. Absolute counts (per unit volume) were calculated as percentages (determined by flow cytometry) multiplied by absolute lymphocyte plus monocyte counts (determined by the clinical hematology laboratory) and divided by 100.
Statistics
Outcomes are reported as of November 2002 and are based on the last follow-up of each patient. Medians and ranges are reported unless otherwise specified. A Mann-Whitney ranked sum test was used for comparing peripheral blood CD3+ T-cell numbers at day 28 in patients who received rabbit ATG with those that received equine ATG.
RESULTS
Patient Characteristics
Of the 56 patients enrolled in the study, 35 patients were female (Table 1). Thirty patients had SSc, and 26 patients had MS. The median age of the patient population was 42 years (range, 23–61 years). The median duration of the disease was 45 months (range, 4–277 months) before HDIT. The median follow-up for all patients was 24 months (range, 2–60 months). All patients were scheduled to receive Atgam as part of the HDIT. However, because of positive skin tests to equine ATG, 2 patients were treated with only rabbit ATG (total dose, 12.5 and 15 mg/kg, respectively). Two other patients who had infusion reactions with equine ATG were then switched to rabbit ATG (total dose, 10 mg/kg each). Patients who received rabbit ATG did not receive more pretrans-plant immunosuppressive therapy compared with the patients who did not receive rabbit ATG. One patient with a positive skin test to equine ATG was treated with Cy and TBI only.
Table 1.
Patient Characteristics (n = 56)
| Variable | Data |
|---|---|
| Age, y, median (range) | 42 (23–61) |
| Sex (male/female) | 21/35 |
| Disease | |
| Multiple sclerosis | 26 |
| Systemic sclerosis | 30 |
| Follow-up, mo, median, (range) | 24 (3–60) |
| Type of antithymocyte globulin | |
| Equine | 51 |
| Rabbit | 2 |
| Both | 2 |
| Neither | 1 |
PBSC Collections and Engraftment
The median number of aphereses required for collection of the required number of CD34+ cells for HSCT and backup was 3 (range, 2–8; Table 2). One patient with SSc failed mobilization with G-CSF and required remobilization with the combination of Cy and G-CSF. The median number of CD34+ cells infused on day 0 was 4.1 (range, 2.5–8.2) × 106/kg. The median purity of the CD34-selected cell product was 88% (54%–99%). The median number of B cells (30 evaluable patients) and T cells (41 evaluable patients) in the autologous graft after CD34 selection was 18.7 (range, 1.91–14.60) × 104 CD19/20+ cells per kilogram and 3.2 (range, 0.1–16.8) × 104 CD3+ cells per kilogram, respectively. Three of the 4 patients who received rabbit ATG were evaluated for the T-cell content of the autologous grafts after CD34 selection, and all had values greater than the median for the overall group. The median time to neutrophil (>500/μL) and platelet (>20,000/μL with transfusion support) engraftment was 9 days (range, 6–13 days) and 9 days (range, 5–25 days), respectively. One patient with SSc did not recover platelet counts at 6 months after autologous HSCT; this was related to the development of a consumptive thrombocytopenia.
Table 2.
Characteristics of the Grafts and Engraftment
| Variable | Median | Range | Evaluable Patients (n) |
|---|---|---|---|
| Mobilization and grafts | |||
| Number of aphereses* | 3 | 2–8 | 56 |
| CD34+ cells × 106/kg | 4.12 | 2.46–8.24 | 56 |
| Purity (%) | 88 | 75–99 | 56 |
| T cells × 104/kg | 3.20 | 0–25.20 | 41 |
| B cells × 104/kg | 18.7 | 1.91–14.60 | 30 |
| Engraftment | |||
| Days to ANC > 500/μL | 9 | 6–13 | 56 |
| Days to platelets > 20 000/μL | 9 | 5–25 | 56† |
ANC indicates absolute neutrophil count.
Backup autografts were also collected.
One patient did not have recovery of platelet counts after HSCT and was considered not evaluable for determining the range.
EBV-PTLD after HDIT and Autologous Stem Cell Transplantation
Two of the 56 patients developed reactivation of herpesvirus infections and then were diagnosed with EBV-PTLD at day 50 and 61 after autologous HSCT. These 2 patients received only rabbit ATG at a total dose of 12.5 and 15 mg/kg. The 2 patients who received equine ATG initially and then rabbit ATG did not develop any significant opportunistic infections or EBV-PTLD.
Case 1
A 28-year-old woman had been well until she was diagnosed with diffuse SSc at 27 months before HDIT. The disease process involved the skin, gastrointestinal tract, joints, and lungs. She had a history of hypertension requiring treatment with nifedipine, captopril, and labetalol. Recurrence of a pericardial effusion led to the opening of a pericardial window. Renal function was normal. The patient had progressive lung disease with a decrease in CO2-diffusing capacity of the lungs from 83% to 66% over the previous 6 months. The serologic studies for the antinuclear antibody were positive at a titer of 1:5120, and those for Scl-70 were negative. Other medications at baseline before transplantation were prednisone and methotrexate.
After consenting to participate in the study and undergoing baseline evaluation, the patient underwent mobilization and HDIT according to the protocol as previously outlined. Because there was a positive skin test to the equine ATG, rabbit ATG (2.5 mg/kg per dose) was administered instead for 5 doses. The day +5 dose of rabbit ATG was not given. The patient received 4.2 × 106 CD34-selected cells per kilogram on day 0, and additional unselected PBSCs were cryo-preserved. The patient engrafted uneventfully on day +10 and was discharged from the hospital. On day +38, the patient was readmitted to the hospital with severe stomatitis secondary to a herpes simplex (type 1) infection that was resistant to acyclovir. Foscarnet and hyperalimentation were started. On day +48, diffuse bilateral interstitial infiltrates developed in the lung that were associated with respiratory failure. The patient had an endotracheal tube placed and was mechanically ventilated. Bronchoscopy with bronchoalveolar lavage was nondiagnostic. On day +61, EBV-PTLD was suspected on the basis of the development of hepatosplenomegaly and B-cell lymphocytosis, which was lambda light chain predominant. The backup autologous graft (unselected cryopreserved peripheral blood cells) was reinfused to restore T-cell immunity. This was followed by the administration of rituximab 375 mg/m2. The lymphocyte count decreased, but the patient’s clinical condition worsened, and she died on day +64. Core needle biopsies were performed of the lung, liver, spleen, and bone marrow as a limited autopsy. A diffuse large B-cell infiltrate was observed in all the tissues sampled. The infiltrate was CD20 and lambda light chain predominant, and EBV studies, including immunocytochemistry stains for EBV-LMP1, were positive.
Case 2
A 57-year-old woman was diagnosed with MS 5 years before transplantation after presenting with sensory and motor neurological dysfunction. Magnetic resonance imaging of the brain showed 5 white matter lesions in the periventricular region and corona radiata bilaterally. Oligoclonal bands were present in the cerebrospinal fluid. The patient was started on β-interferon. Secondary progressive disease had developed by 3 years before transplantation. β-Interferon was continued, and glatiramer acetate was started. The patient’s disease continued to progress, and methotrexate was added to the treatment regimen 1 year before transplantation. Before transplantation, the patient had an expanded disability status scale score of 6.0 that had increased by 1.0 point from the previous year. Eligibility for the protocol was confirmed with 2 separate assessments by a study and an independent neurologist.
After consenting to the study, the patient underwent PBSC collection, and HDIT was administered according to the protocol as previously outlined (Figure 1). There was a positive skin test to equine ATG, so rabbit ATG (2.5 mg/kg per dose) was administered for 6 doses. The patient received 3.4 × 106 CD34-selected cells per kilogram on day 0. Neutrophil engraftment occurred on day +10. In the second week after the transplantation, the patient became CMV antigenemia positive and developed pneumonitis and gastroenteritis. The washings from a bronchoalveolar lavage at bronchoscopy were positive for CMV. Treatment was initiated with ganciclovir, cidofovir, and CMV immunoglobulin. There was gradual improvement in the patient’s clinical condition and a decrease in the pulmonary infiltrates. On day +42, the serum copy number of EBV DNA per microgram of DNA was 76. On day +49, the patient developed increasing dyspnea, with an increase in pulmonary infiltrates and the development of cervical lymphadenopathy. The serum copy number of EBV DNA per microgram of DNA was 13,000. On day +50, a biopsy of the cervical lymph node was performed, which showed a diffuse large B-cell lymphoma. Flow studies on a cell suspension from the lymph node were positive for CD20, CD19, HLA-DR, and κ light chain. Immunocytochemistry studies for EBV-LMP1 and EBER in situ hybridization were both positive. Treatment was started with rituximab 375 mg/m2 and Cy 1 g/m2. Despite treatment and mechanical ventilation, there was progressive respiratory failure, and the patient died on day +53.
At autopsy, there was widespread dissemination of large B-cell lymphoma throughout multiple organs, including lung, liver, gastrointestinal tract, adrenal glands, kidney, and bladder. Rare cells in the lung were positive by immunocytochemistry for CMV antigen.
Peripheral Blood T Cells
The absolute CD3+ T-cell counts in the peripheral blood of 44 of the 56 study patients at 1 month after transplantation were determined. The CD3+ T-cell count was 0/μL in all 4 patients who received rabbit ATG in whole or in part during HDIT (Table 2). The median CD3+ T-cell count was 174/μL (range, 16–4371/μL) in the 40 patients who did not receive rabbit ATG. This was significantly different from the patients who received rabbit ATG (P < .001). In the 2 patients who received rabbit ATG and survived to day 80, the CD3+ T-cell counts had recovered to the median count observed on day 80 in the equine ATG group. B- and NK-cell counts in the 4 patients who received rabbit ATG were comparable to those in the patients in the equine ATG group (Table 3).
Table 3.
EBV-PTLD after HDIT*
| Variable | Patients |
||||
|---|---|---|---|---|---|
| 1† | 2† | 3† | 4† | 5–56 (n = 52) | |
| Total rabbit ATG dose (mg/kg) | 12.5 | 15.0 | 10 | 10 | 0 |
| Total equine ATG dose (mg/kg) | 0 | 0 | 30 | 30 | 90 (0–90)‡ |
| EBV-PTLD | Yes | Yes | No | No | None |
| Disease from other opportunistic infection in first 60 days | HSV1 | CMV | No | No | 1 (HSV2) |
| Flow cytometry studies (day 28) | (n = 40) | ||||
| CD3+ cells/μL§ | 0 | 0 | 0 | 0 | 174 (16–4371) |
| B cells/μL§ | 47.3 | 0.4 | 1.0 | 8.1 | 2 (0–53.8) |
| NK cells/μL§ | 109 | 108 | 510 | 1401 | 301 (51–1401) |
HSV indicates herpes simplex virus.
CD3+ T cells on day 28 after autologous HSCT were significantly fewer in patients who received rabbit ATG (P = .001; Mann-Whitney ranked sum test).
Patients who received rabbit ATG with or without equine ATG.
Fifty patients had 90 mg/kg of equine ATG.
Day 28 counts in peripheral blood; median (range).
DISCUSSION
More than 90% of humans become infected with EBV. After infection, EBV persists within the body in resting memory B cells. The EBV genome encodes nearly 100 viral proteins during viral replication, whereas only 10 are expressed in latently infected B cells in vitro. Latently infected B cells in the blood of healthy carriers express only EBER, LMP2, and, in some studies, EBV nuclear antigen 1 [21]. In B cells from EBV-associated lymphoproliferative diseases, all the latency genes are expressed, including LMP1. LMP1 is an oncogene, and expression of this protein in transgenic mice results in B-cell lymphoma [22,23]. Infection of B cells in vitro by EBV results in latent infection and immortalization of the cells. In vivo, the cellular immune response (NK cells and CD4+ and CD8+ cytotoxic T cells) controls proliferating EBV-infected B cells during primary infection and during reactivation. In patients with impaired T-cell immunity from conditions such as congenital immunodeficiencies and acquired immunodeficiency syndrome or after solid organ transplantation or HSCT, the T cells may be unable to control the proliferation of EBV-infected cells.
EBV-PTLD may initially manifest as a limited or extensive polyclonal or monoclonal disease. If EBV-PTLD is extensive and monoclonal at diagnosis, it may have a rapidly progressive course with a fatal outcome, as in the 2 patients described in this study. EBV-PTLD has an overall incidence of 1.0% after allogeneic HSCT and may be as high as 18%–25% in certain subsets of patients but is only rarely described after autologous HSCT [1]. Risk factors associated with the development of EBV-PTLD after allogeneic HSCT are those that are known to be associated with inducing a higher degree of immunosuppression, including T-cell depletion of the stem cell grafts or intensification of immunosuppression with T cell–specific agents to control graft-versus-host disease [24–26]. Seventeen cases of EBV-PTLD after autologous HSCT have been identified in the literature [1–4, 6–8, 27, 28] (Table 4). Nine of these cases (mostly the earlier case reports) were after marrow transplantation, and 8 were after transplantation with PBSC. Twelve of the cases had some type of graft manipulation, including CD34 selection (n = 6), T-cell depletion (n = 3), or purging with an alkylating agent (n = 2). One patient with non-Hodgkin lymphoma had a graft that was B-cell depleted. All of the cases of EBV-PTLD except 2 had onset before day 100 after transplantation. Seven of the 17 cases remitted with treatment. None of the reported cases were treated for autoimmune diseases or received ATG in combination with the high-dose chemoradiotherapy. Although an interpretation of the case reports is limited by the selected nature of the reporting, the risk factors associated with the development of EBV-PTLD after autologous HSCT may relate to the underlying immune status at the time of the stem cell harvest secondary to more intensive pretransplantation chemotherapy or T-cell depletion (including CD34 selection) of the graft.
Table 4.
EBV-PTLD after Autologous HSCT: Case Reports
| Study | Year of Publication | No. Patients | Disease | Source of Stem Cells | Graft Manipulation | Time of EBV-PTLD Onset after HSCT | Outcome |
|---|---|---|---|---|---|---|---|
| Anderson et al. [27] | 1990 | 2 | 1 T-NHL1 T-NHL |
Marrow | T-depl. | 31, 42 d | Fatal, resolved (died later from PCP) |
| Chao et al. [3] | 1993 | 1 | NHL | Marrow | B-depl. | 47 d | Fatal |
| Shepherd et al. [7] | 1995 | 1 | CML | Marrow | Ex vivo culture (× 10 d) | 33 d | Resolved |
| Briz et al. [4] | 1997 | 1 | T-ALL | Marrow | T-depl. | 60 d | Fatal |
| Hauke et al. [2] | 1998 | 2 | 1 HD 1 NHL |
Marrow | None | 38, 87 d | Fatal (×2) |
| Peniket et al. [6] | 1998 | 1 | MM | PBSC | CD34 selected | 63 d | Fatal |
| McCaul et al. [1] | 1999 | 3 | 2 MM | Marrow | 4-HC | <80 d | Fatal (×3) |
| (4)* | 1 NHL | Marrow | Mafosfamide | <80 d | |||
| 1 CML | PBSC | None | 31 mo | ||||
| Powell et al. [8] | 2002 | 5 | Neuroblastoma | PBSC | CD34 selected | Median, 3 (range, 1–5) mo | Fatal (×1), resolved (×4) |
| Heath et al. [28] | 2002 | 1 | Retinoblastoma | PBSC | None | 21 d | Resolved |
4-HC indicates 4-hydroperoxycyclophosphamide; B-depl, B-cell depletion; CML, chronic myelogenous leukemia; EBV-PTLD, Epstein-Barr virus-associated posttransplantation lymphoproliferative disorder; HD, Hodgkin disease; HSCT, hematopoietic stem cell transplantation; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; PBSC, peripheral blood stem cells; PCP, Pneumocystis carinii pneumonia; T-ALL, T-cell acute lymphocytic leukemia; T-depl., T-cell depletion; T-NHL, T-cell non-Hodgkin lymphoma.
One case had been previously reported.
As after allogeneic HSCT, use of a T cell–depleted autologous graft after high-dose chemoradiotherapy may result in a more immunosuppressed state and a higher incidence of transplant-related complications [29]. Although the experience was small, there seemed to be a higher risk of Pneumocystis carinii pneumonia and CMV reactivation after transplantation with T cell-depleted or CD34-selected autologous grafts [27]. The B-cell origin of the EBV-PTLD when it occurs after allogeneic HSCT is normally donor derived and, therefore, is arising from the graft. Because EBV-PTLD after autologous HSCT has occurred after CD34 selection (3-log B-cell depletion) and B-cell depletion of the graft, the malignant B-cell population may derive from residual B cells that have survived the high-dose chemoradiotherapy [3,4,6,8].
The standard form of ATG used in the HDIT regimen used in this report was equine ATG. If patients had a positive skin test or had an adverse reaction during the infusion of equine ATG, rabbit ATG was used instead. Rabbit ATG is a very effective immunosuppressive agent and has been safely used at doses of 10.5–21 mg/kg for the treatment of rejection after kidney transplantation (most patients received rabbit ATG at the dose of 15 mg/kg) [30]. The use of rabbit ATG in that study was associated with a significantly greater degree of T-cell depletion, as well as a more prolonged depletion of T cells than equine ATG. There are reports in the literature of rabbit ATG being associated with the development of EBV-PTLD after allogeneic HSCT when it was used for the treatment of graft-versus-host disease [31]. In this report, only patients treated with rabbit ATG had significant complications secondary to reactivated herpes infection, EBV-PTLD, and absent circulating T cells at day 30. This is likely related to the more potent immunosuppressive effects of this biologic agent.
EBV-specific cellular immunity is important for the prevention of EBV reactivation and EBV-PTLD after HSCT [32]. This is similar to studies in which it was demonstrated that the recovery of a CMV-specific HLA class 1–restricted CD8+ cytotoxic T lymphocyte response was critical for protection from severe CMV disease [33–35]. Recovery of the EBV cellular immune response after high-dose chemoradiotherapy and autologous HSCT for non-Hodgkin lymphoma occurred between 8 and 12 weeks after autologous transplantation with a PBSC graft [36]. This may be significantly delayed with CD34 selection of the graft and in vivo T-cell depletion with ATG. Reconstitution of the EBV cellular immune response by infusion of unselected donor lymphocytes or donor-derived EBV-specific cytotoxic T lymphocytes is effective for the prevention of EBV reactivation or the treatment of EBV-PTLD [37–40]. However, the use of donor-derived lymphocytes would not have a role for patients after autologous transplantation. The use of B cell–specific antibodies as prophylaxis or as preemptive therapy after EBV reactivation is likely to be a more appropriate therapy for patients after autologous transplantation [41,42]. However, neither the infusion of backup autologous PBSC in 1 patient nor the administration of rituximab to both patients prevented the fatal outcome in this study.
Profound immunosuppression can be induced in patients with severe autoimmune diseases after HDIT with ATG and transplantation with autologous CD34-selected hematopoietic stem cells. Rabbit ATG at a total dose of 10.0 to 15.0 mg/kg was more immunosuppressive than equine ATG at a total dose of 90 mg/kg and was associated with the development of EBV-PTLD. Even if EBV-PTLD could be prevented, the severity of the immunosuppression induced by this dose of rabbit ATG (in the context of chemoradiotherapy plus CD34-selected autologous grafts) would likely increase the risk of developing other severe opportunistic infections. Our studies were not designed to identify a safe dose or schedule of rabbit ATG, so subsequent patients on the study did not receive any ATG if they had a positive skin test or an intolerable infusion reaction to equine ATG. Caution is required in any attempts to further intensify the immunosuppressive effects of HDIT with other agents specific for T cells, especially if grafts are T-cell depleted or CD34 selected. Routine monitoring for EBV reactivation and preemptive therapy with rituximab if high levels of EBV DNA are detected may be required for some HDIT regimens. Until the immunosuppressive differences between equine and rabbit ATG are further defined, these biologic agents should not be considered interchangeable in HDIT regimens.
Acknowledgments
This work was supported in part by grants HL36444 from the National Heart, Lung and Blood Institute; AI-05419 from the National Institute of Allergy and Infectious Diseases; CA15704 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services; H133B980017 from the U.S. Department of Education’s National Institute on Disability and Rehabilitation Research; MD1-RR00042 from the University of Michigan General Clinical Research Center; and VIF.097 from the University of Michigan Venture Investment Fund. The authors wish to thank Kate Ryan (formerly at FHCRC), Gretchen Henstorf, and all the study coordinators and data technicians at the collaborating sites. We thank Helen Crawford, Bonnie Larson, Sue Carbonneau, and Connie Chan for their excellent support in preparing the manuscript.
References
- 1.McCaul KG, Nevill TJ, Toze CL, et al. Post-transplant lymphoproliferative disorder (PTLD) after autologous stem cell transplantation (SCT): a single center experience. Blood. 1999;94(suppl 1):378b. (abstr. 4920) [Google Scholar]
- 2.Hauke RJ, Greiner TC, Smir BN, et al. Epstein-Barr virus-associated lymphoproliferative disorder after autologous bone marrow transplantation: report of two cases. Bone Marrow Transplant. 1998;21:1271–1274. doi: 10.1038/sj.bmt.1701258. [DOI] [PubMed] [Google Scholar]
- 3.Chao NJ, Berry GJ, Advani R, Horning SJ, Weiss LM, Blume KG. Epstein-Barr virus-associated lymphoproliferative disorder following autologous bone marrow transplantation for non-Hodgkin’s lymphoma. Transplantation. 1993;55:1425–1428. [PubMed] [Google Scholar]
- 4.Briz M, Fores R, Regidor C, et al. Epstein-Barr virus associated B-cell lymphoma after autologous bone marrow transplantation for T-cell acute lymphoblastic leukaemia. Br J Haematol. 1997;98:485–487. doi: 10.1046/j.1365-2141.1997.2153034.x. [DOI] [PubMed] [Google Scholar]
- 5.Young L, Alfieri C, Hennessy K, et al. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989;321:1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]
- 6.Peniket AJ, Perry AR, Williams CD, et al. A case of EBV-associated lymphoproliferative disease following high-dose therapy and CD34-purified autologous peripheral blood progenitor cell transplantation. Bone Marrow Transplant. 1998;22:307–309. doi: 10.1038/sj.bmt.1701335. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd JD, Gascoyne RD, Barnett MJ, Coghlan JD, Phillips GL. Polyclonal Epstein-Barr virus-associated lymphoproliferative disorder following autografting for chronic myeloid leukemia. Bone Marrow Transplant. 1995;15:639–641. [PubMed] [Google Scholar]
- 8.Powell J, Callahan C, Aplenc R, Bunin NJ, Grupp SA. An unexpectedly high incidence of Epstein-Barr virus lymphopro-liferative disease after CD34-selected autologous peripheral blood stem cell transplant in children with neuroblastoma. Blood. 2002;100(part 1):173a. doi: 10.1038/sj.bmt.1704402. (abstr. 649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McSweeney PA, Nash RA, Sullivan KM, et al. High-dose immunosuppressive therapy for severe systemic sclerosis: initial outcomes. Blood. 2002;100:1602–1610. [PMC free article] [PubMed] [Google Scholar]
- 10.Nash RA, Bowen JD, McSweeney PA, et al. Treatment of severe multiple sclerosis (MS) with high-dose immunosuppressive therapy (HDIT) and autologous stem cell transplantation (SCT): 2 year follow-up. Blood. 2002;100(part 1):864a. (abstr. 3408) [Google Scholar]
- 11.Rowley SD, Loken M, Radich J, et al. Isolation of CD34+ cells from blood stem cell components using the Baxter Isolex system. Bone Marrow Transplant. 1998;21:1253–1262. doi: 10.1038/sj.bmt.1701257. [DOI] [PubMed] [Google Scholar]
- 12.Rowley SD. Processing and storage. In: Snyder EL, Haley NR, editors. Hematopoietic Progenitor Cells: A Primer for Medical Professionals. Bethesda, MD: American Association of Blood Banks; 2000. pp. 71–105. [Google Scholar]
- 13.Sullivan KM, Meyers JD, Flournoy N, Storb R, Thomas ED. Early and late interstitial pneumonia following human bone marrow transplantation. Int J Cell Cloning. 1986;4:107–121. doi: 10.1002/stem.5530040712. [DOI] [PubMed] [Google Scholar]
- 14.Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole for fungal infections after marrow transplant—a prospective, randomized, double-blind study. J Infect Dis. 1995;171:1545–1552. doi: 10.1093/infdis/171.6.1545. [DOI] [PubMed] [Google Scholar]
- 15.Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063–4071. [PubMed] [Google Scholar]
- 16.Limaye AP, Huang M-L, Atienza EE, Ferrenberg JM, Corey L. Detection of Epstein-Barr virus DNA in sera from transplant recipients with lymphoproliferative disorders. J Clin Microbiol. 1999;37:1113–1116. doi: 10.1128/jcm.37.4.1113-1116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storek J, Ferrara S, Ku N, Giorgi JV, Champlin RE, Saxon A. B cell reconstitution after human bone marrow transplantation: recapitulation of ontogeny? Bone Marrow Transplant. 1993;12:387–398. [PubMed] [Google Scholar]
- 18.Storek J, Witherspoon RP, Storb R. T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant. 1995;16:413–425. [PubMed] [Google Scholar]
- 19.Storek J, Dawson MA, Storer B, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97:3380–3389. doi: 10.1182/blood.v97.11.3380. [DOI] [PubMed] [Google Scholar]
- 20.Storek J, Joseph A, Espino G, et al. Immunity of patients surviving 20 to 30 years after allogeneic or syngeneic bone marrow transplantation. Blood. 2001;98:3505–3512. doi: 10.1182/blood.v98.13.3505. [plenary paper] [DOI] [PubMed] [Google Scholar]
- 21.Kerr BM, Lear AL, Rowe M, et al. Three transcriptionally distinct forms of Epstein-Barr virus latency in somatic cell hybrids: cell phenotype dependence of virus promoter usage. Virology. 1992;187:189–201. doi: 10.1016/0042-6822(92)90307-b. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 23.Kulwichit W, Edwards RH, Davenport EM, Baskar JF, Godfrey V, Raab-Traub N. Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc Natl Acad Sci U S A. 1998;95:11963–11968. doi: 10.1073/pnas.95.20.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin PJ, Hansen JA, Anasetti C, et al. Treatment of acute graft-versus-host disease with anti-CD3 monoclonal antibodies. Am J Kidney Dis. 1988;11:149–152. doi: 10.1016/s0272-6386(88)80201-4. [DOI] [PubMed] [Google Scholar]
- 25.Zutter MM, Martin PJ, Sale GE, et al. Epstein-Barr virus lymphoproliferation after bone marrow transplantation. Blood. 1988;72:520–529. [PubMed] [Google Scholar]
- 26.McCaul KG, Nevill TJ, Barnett MJ, et al. Treatment of steroid-resistant acute graft-versus-host disease with rabbit antithymocyte globulin. J Hematother Stem Cell Res. 2000;9:367–374. doi: 10.1089/15258160050079470. [DOI] [PubMed] [Google Scholar]
- 27.Anderson KC, Soiffer R, DeLage R, et al. T-cell-depleted autologous bone marrow transplantation therapy: analysis of immune deficiency and late complications. Blood. 1990;76:235–244. [PubMed] [Google Scholar]
- 28.Heath JA, Broxson EHJ, Dole MG, et al. Epstein-Barr virus-associated lymphoma in a child undergoing an autologous stem cell rescue. J Pediatr Hematol Oncol. 2002;24:160–163. doi: 10.1097/00043426-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Holmberg LA, Boeckh M, Hooper H, et al. Increased incidence of cytomegalovirus disease after autologous CD34-selected peripheral blood stem cell transplantation. Blood. 1999;94:4029–4035. [PubMed] [Google Scholar]
- 30.Gaber AO, First MR, Tesi RJ, et al. Results of the double-blind, randomized, multicenter, phase III clinical trial of Thymoglobulin versus Atgam in the treatment of acute graft rejection episodes after renal transplantation. Transplantation. 1998;66:29–37. doi: 10.1097/00007890-199807150-00005. [DOI] [PubMed] [Google Scholar]
- 31.Hoshino Y, Kimura H, Tanaka N, et al. Prospective monitoring of the Epstein-Barr virus DNA by a real-time quantitative polymerase chain reaction after allogenic stem cell transplantation. Br J Haematol. 2001;115:105–111. doi: 10.1046/j.1365-2141.2001.03087.x. [DOI] [PubMed] [Google Scholar]
- 32.Lucas KG, Small TN, Heller G, Dupont B, O’Reilly RJ. The development of cellular immunity to Epstein-Barr virus after allogeneic bone marrow transplantation. Blood. 1996;87:2594–2603. [PubMed] [Google Scholar]
- 33.Quinnan GV, Kirmani N, Rook AH, et al. Cytotoxic T cells in cytomegalovirus infection. N Engl J Med. 1982;307:7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- 34.Li C-R, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83:1971–1979. [PubMed] [Google Scholar]
- 35.Reusser P, Riddell SR, Meyers JD, Greenberg PD. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. [PubMed] [Google Scholar]
- 36.Nolte A, Buhmann R, Emmerich B, Schendel D, Hallek M. Reconstitution of the cellular immune response after autologous peripheral blood stem cell transplantation in patients with non-Hodgkin’s lymphoma. Br J Haematol. 2000;108:415–423. doi: 10.1046/j.1365-2141.2000.01841.x. [DOI] [PubMed] [Google Scholar]
- 37.Rooney CM, Smith CA, Ng CY, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 38.Papadopoulos EB, Ladanyi M, Emanuel D, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330:1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 39.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 40.O’Reilly RJ, Small TN, Papadopoulos E, Lucas K, Lacerda J, Koulova L. Biology and adoptive cell therapy of Epstein-Barr virus-associated lymphoproliferative disorders in recipients of marrow allografts. Immunol Rev. 1997;157:195–216. doi: 10.1111/j.1600-065x.1997.tb00983.x. [review] [DOI] [PubMed] [Google Scholar]
- 41.Benkerrou M, Jais JP, Leblond V, et al. Anti-B-cell monoclonal antibody treatment of severe posttransplant B-lymphoproliferative disorder: prognostic factors and long-term outcome. Blood. 1998;92:3137–3147. [PubMed] [Google Scholar]
- 42.Kuehnle I, Huls MH, Liu Z, et al. CD20 monoclonal antibody (rituximab) for therapy of Epstein-Barr virus lymphoma after hemopoietic stem-cell transplantation. Blood. 2000;95:1502–1505. [PubMed] [Google Scholar]