Abstract
Background
In high-income countries viral load (VL) is routinely measured to detect failure of antiretroviral therapy (ART) and guide switching to second-line ART. VL monitoring is not generally available in resource-limited settings. We examined switching from non-nucleoside reverse transcriptase inhibitor (NNRTI)-based first-line regimens to protease inhibitor (PI)-based regimens in Africa, South America and Asia.
Design and methods
Multi-cohort study of 17 ART programmes. All sites monitored CD4 counts and had access to second-line ART, 10 sites monitored VL. We compared times to switching, CD4 counts at switching and obtained adjusted hazard ratios for switching (aHR) with 95% confidence intervals (CIs) from random-effects Weibull models.
Results
A total of 20,113 patients, including 6,369 (31.7%) patients from 10 programmes with access to VL monitoring were analysed; 576 patients (2.9%) switched. Low CD4 counts at ART initiation were associated with switching in all programmes. Median time to switching was 16.3 months (interquartile range [IQR] 10.1–26.6) in programmes with VL and 21.8 months (IQR 14.0–21.8) in programmes without VL monitoring (p<0.001). Median CD4 cell counts at switching were 161 cells/μl (IQR 77–265) and 102 cells/μl (44–181), respectively (p<0.001). Switching was more common in programmes with VL monitoring during months 7–18 after starting ART (aHR 1.38; 95% CI 0.97–1.98), similar during months 19–30 (aHR 0.97; 0.58–1.60) and less common during months 31–42 (aHR 0.29; 0.11–0.79).
Conclusions
In resource-limited settings switching to second-line regimens tends to occur earlier and at higher CD4 cell counts in ART programmes with VL monitoring compared to programmes without VL monitoring.
Keywords: Adolescent; Adult; Anti-HIV Agents; therapeutic use; Antiretroviral Therapy, Highly Active; adverse effects; methods; CD4 Lymphocyte Count; Developing Countries; Drug Monitoring; methods; Female; HIV Infections; drug therapy; immunology; virology; HIV Protease Inhibitors; therapeutic use; HIV-1; isolation & purification; Humans; Male; Medically Underserved Area; Middle Aged; Reverse Transcriptase Inhibitors; therapeutic use; Time Factors; Treatment Failure; Viral Load; Young Adult
Keywords: Antiretroviral therapy, switching to second-line regimens, resource-limited settings, CD4 cell count, viral load monitoring, cohort studies, collaborative research
Introduction
In industrialized countries the prognosis of HIV infection has improved considerably since highly active antiretroviral therapy (ART) was introduced from 1995 onwards [1, 2, 3]. In low-income countries with a high burden of HIV and AIDS, ART has become more widely available in recent years. The World Health Organisation (WHO) estimates that about 3 million people were receiving ART in low- and middle-income countries end of 2007, a 7.5-fold increase during the past four years [4]. With increasing exposure to ART, the risk of resistance and subsequent treatment failure has become more important, and switching of patients to alternative, second-line regimens is increasingly needed.
Whereas HIV-1 RNA concentration (viral load) is regularly assessed to diagnose treatment failure in high-income countries [5], viral load measurements are often not available, or only available at high cost, in resource-constrained settings. Costs of second-line drugs are also high [6]. The lack of viral load monitoring in resource-limited settings may lead to late switching of regimens, increase the risk of viral resistance and jeopardize long-term prognosis: second-line regimens are the last treatment option for many patients in these settings. Conversely, if treatment is switched unnecessarily, resources may be wasted and future treatment options reduced. Switching to second-line regimens is less common in lower-income settings compared to high-income countries [7, 8] but direct comparisons of switching rates between treatment programmes with and without access to viral load monitoring in resource-limited settings are lacking at present.
We studied rates of switching to second-line regimens, time to switching and determinants of switching in a collaborative network of treatment programmes in Africa, Asia and Latin America that includes both programmes that routinely monitor viral load, and programmes without access to viral load monitoring.
Methods
The ART-LINC collaboration
The Antiretroviral Therapy in Lower Income Countries (ART-LINC) collaboration of the International epidemiological Databases to Evaluate Aids (IeDEA) is a collaborative network of ART programmes, which has been described in detail elsewhere [9, 10]. Briefly, programmes from resource-constrained settings that collect data on patient characteristics and treatment outcomes were eligible for participation in ART-LINC. In all sites institutional review boards approved the participation in ART-LINC.
Inclusion criteria and definitions
We included all patients aged 16 years and older with known date of starting ART, who had not previously received ART, started ART with a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimen and had at least 6 months of follow-up. A switch to a second-line regimen was defined as a change from the initial NNRTI-based regimen to a protease inhibitor (PI)-based regimen after at least six months of follow-up, since WHO recommends switching due to treatment failure not before completion of at least 6 months of therapy [11]. In addition at least one NRTI had to be changed. A patient was considered lost to follow up if the time between the last visit and the closing date of the cohort was longer than one year.
Stage of disease was defined as less advanced (CDC stage A/B or WHO stage I/II) or advanced (CDC stage C or WHO stage III/IV). Routine viral load monitoring was defined as at least one viral load measurement between 3 and 9 months after starting ART in at least 50% of patients treated at that site. Measurements of CD4 and RNA values closest to the starting date of ART (within 6 months before up to one week after the date of starting ART) were taken as the baseline levels. For CD4 counts below 25 cells/μl and HIV viral loads above 100,000 copies/ml the time window was extended to one year before starting therapy. At switching the time window was 3 months before up to 2 weeks after switching.
In patients who switched to a second-line regimen we determined whether immunological or virological failure had occurred during the 3 months before switching and examined the reasons for switching recorded in the database. Following WHO criteria [11] we defined immunological failure as a decline in the CD4 cell count to the baseline value or below, a decline of at least 50% from the highest count on treatment or a persistent CD4 cell count below 100 cells/μl after six months of antiretroviral therapy. Virological failure was defined as a plasma HIV viral load value above 10,000 copies/ml [11].
Statistical analysis
Patient characteristics at start of first and second-line ART was compared between sites with and without viral load monitoring using chi-squared tests and Wilcoxon ranksum tests. We calculated times to switching and rates of switching per 100 person-years, and fitted random-effects Weibull models, comparing sites with and without routine viral load monitoring. We plotted Kaplan-Meier curves of the probability of switching to second-line ART therapy and obtained p values from log rank tests comparing programmes with and without virological monitoring. We measured time from 6 months after start of ART (baseline) to the time of switching, the time of the last follow-up visit, or three years after baseline, whichever came first. Hazards were not proportional over time (p=0.0012 from test of proportional hazards [12] and follow-up was therefore split into periods 7–18, 19–30 and 31–42 months after starting ART. Analyses were adjusted for sex, age, calendar year, CD4 cell count, clinical stage (advanced or less advanced) at baseline and the number of available antiretroviral drugs in the clinic (tertiles; ≤10, 11–13, ≥14 drugs). We examined predictors of switching separately for programmes with and without access to viral load monitoring and used tests of interaction to determine whether programmes differed.
We compared CD4 cell counts at the time of switching in sites with and without routine viral load monitoring and used linear regression analysis to examine whether differences persisted when adjusting for age, sex, clinical stage and CD4 cell count at baseline. The square-root of CD4 cell counts was used in these analyses. In sensitivity analyses we restricted data to sites from sub-Saharan Africa. All analyses were performed using Stata version 10 (Stata Corporation, College Station, Texas, USA). Results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs) or medians with interquartile range (IQR).
Results
Sites and study populations
Seventeen treatment programmes from 14 countries were included (Table 1). All sites carried out routine CD4 monitoring and had access to second-line therapy, 10 sites routinely monitored viral load. The database included 34,715 adult patients of whom 14,602 patients (42.1%) were excluded for the following reasons: 1,403 (4.0%) died before 6 months, 11,250 (32.4%) had less than 6 months of follow-up and 1,949 (5.6%) started with a non-NNRTI-based ART. A total of 20,113 patients, including 6,369 patients (31.7%) from programmes with routine viral load monitoring, and 16,591 (82.5%) patients from sub-Saharan Africa, were analysed. A viral load value at 6 months after ART initiation was available in 58% to 99% of patients at sites with routine viral load testing, and 0% to 7% at the other sites (Table 1).
Table 1.
Characteristics of antiretroviral treatment programmes included in analyses
| Site | Site (Country) | Start of programme | No. of patients (No. switching) | Rate of switching per 100 person-years (95% CI) | Routine HIV-1 viral load testing* | No. of patients with viral load (%) |
|---|---|---|---|---|---|---|
| CEPREF | Abidjan (Côte d’Ivoire) | 1999 | 1,941 (46) | 1.6 (1.2–2.2) | No | 139 (7%) |
| 1290 ANRS | Dakar (Senegal) | 1998 | 194 (0) | 0 (0–3.7) | Yes | 153 (79%) |
| AMPATH | Eldoret (Kenya) | 2002 | 3,229 (106) | 5.0 (4.1–6.0) | No | 0 |
| AMU | Kampala (Uganda) | 2002 | 84 (3) | 2.7 (0.9–8.2) | Yes | 83 (99%) |
| Lighthouse | Lilongwe (Malawi) | 2000 | 4,082 (9) | 0.2 (0.1–0.3) | No | 0 |
| Connaught | Harare (Zimbabwe) | 2002 | 707 (31) | 3.5 (2.5–5.0) | No | 0 |
| Gugulethu | Cape Town (South Africa) | 2002 | 1,243 (23) | 1.8 (1.2–2.6) | Yes | 1106 (89%) |
| Khayelitsha | Cape Town, (South Africa) | 2001 | 1,527 (45) | 2.4 (1.8–3.2) | Yes | 1444 (95%) |
| Themba Lethu | Johannesburg, (South Africa) | 1999 | 1,874 (97) | 6.5 (5.3–7.9) | Yes | 1127 (60%) |
| PHRU | Soweto (South Africa) | 2004 | 439 (2) | 0.8 (0.2–3.3) | Yes | 387 (88%) |
| Morocco | Casablanca (Morocco) | 1999 | 231 (8) | 2.5 (1.3–5.0) | Yes | 136 (59%) |
| MTCT-Plus | Several& | 2003 | 1,455 (22) | 1.7 (1.1–2.5) | No | 0 |
| PUMA | Buenos Aires (Argentina) | 2003 | 136 (6) | 5.2 (2.3–11.5) | Yes | 102 (75%) |
| SOBRHIV | Porto Alegre (Brazil) | 1996 | 349 (7) | 0.8 (0.4–1.7) | Yes | 289 (83%) |
| RIOHIV | Rio de Janeiro (Brazil) | 1996 | 292 (45) | 5.7 (4.3–7.6) | Yes | 183 (63%) |
| YRG Care | Chennai (India) | 1996 | 2,114 (122) | 3.2 (2.7–3.8) | No | 154 (7%) |
| HIV-NAT | Bangkok (Thailand) | 2003 | 216 (4) | 1.0 (0.4–2.6) | No | 0 |
| Total | 20,113 (576) | 2.4 (2.2–2.6) | 10/17 | 5303 (26%) |
See appendix for further details on participating sites
Network including sites in South Africa, Zambia, Kenya, Rwanda, Uganda, Ivory Coast, Thailand
Routine viral load monitoring was defined as at least one measurement between 3 and 9 months after starting ART, in at least 50% of patients
Table 2 shows patient characteristics separately for programmes with and without viral load monitoring. The characteristics of patients at start of ART were similar with respect to age, and sex, but median CD4 cell counts at the start of ART were lower in programmes with viral load monitoring compared to programmes without viral load monitoring (97 cells cells/μl versus 129 cells/μl, p<0.001). Both in sites with and without viral load monitoring the four most common initial regimens were lamivudine (3TC) with stavudine (d4T) or zidovudine (ZDV), combined with efavirenz (EFV) or nevirapine (NVP) (Table 2).
Table 2.
Patients characteristics at baseline and at switching to second-line antiretroviral regimen
| Variable | At baseline |
At start of second-line regimen |
||||
|---|---|---|---|---|---|---|
| Routine viral load monitoring (n=6,369) | No routine viral load monitoring (n=13,744) | P value | Routine viral load monitoring (n=236) | No routine viral load monitoring (n=340) | P value | |
| Women (%) | 4,101 (64.4) | 8,350 (60.8) | <0.001 | 145 (61.4) | 181 (53.2) | 0.05 |
| Median age [IQR] (years) | 34 [30–41] | 35 [30–41] | <0.001 | 35 [31–41] | 38 [33–44] | <0.001 |
| Clinical stage (%) | <0.001 | |||||
| Stage available | 5,831 (91.6) | 11,588 (84.3) | 0 | 0 | ||
| Less advanced | 2,591 (44.4) | 3,343 (28.9) | ||||
| Advanced * | 3,240 (55.6) | 8,245 (71.2) | - | |||
| CD4 cell count (cells/μl) | <0.001 | |||||
| CD4 count available | 5,462 (85.8) | 10,315 (75.1) | 141 (59.7) | 261 (76.8) | ||
| Median [IQR] | 97 [42–163] | 129 [60–195] | 161 [77–265] | 102 [44–181] | ||
| HIV-1 viral load (copies/ml) | ||||||
| Viral load available (%) | 3,750 (58.9) | 271 (2.0) | 143 (60.6) | 14 (4.1) | ||
| < 10,000 (%) | 511 (13.6) | 71 (49.6%) | ||||
| 10,000–100,000 (%) | 1,455 (38.8) | 36 (25.2%) | ||||
| > 100,000 (%) | 1,784 (47.6) | 36 (25.2%) | ||||
| Median log viral load [IQR] | 5.0 [4.4–5.4] | 4.0 [3.4–5.0] | ||||
| First-line regimens (%) | <0.001 | |||||
| 3TC d4T EFV | 3,495 (54.9) | 9,717 (70.7) | ||||
| 3TC ZDV EFV | 1,193 (18.7) | 1,757 (12.8) | ||||
| 3TC d4T NVP | 1,050 (16.5) | 1,254 (9.1) | ||||
| 3TC ZDV NVP | 358 (5.6) | 955 (7.0) | ||||
| Other | 273 (4.3) | 61 (0.4) | ||||
| Second-line regimens (%) | <0.001 | |||||
| ZDV ddI LPV | 114 (48.3) | 58 (17.1) | ||||
| 3TC ddI IDV RTV | 0 (0) | 60 (17.7) | ||||
| ABC ddI LPV | 3 (1.3) | 43 (12.7) | ||||
| d4T ddI LPV | 19 (8.1) | 1 (0.3) | ||||
| 3TC ZDV LPV | 18 (7.6) | 18 (5.3) | ||||
| 3TC ZDV IDV RTV | 6 (2.5) | 28 (8.2) | ||||
| Other | 76 (32.2) | 132 (38.8) | ||||
IQR are shown in square brackets and percentages in brackets. ABC, abacavir; ddI, didanosine; EFV, efavirenz; d4T, stavudine; IQR, interquartile range; LPV, lopinavir; NVP, nevirapine; RTV: boost of ritonavir; 3TC, lamivudine; ZDV, zidovudine.
US Centers for Disease Control and Prevention (CDC) stage C, or World Health Organization (WHO) stages III or IV.
Rates and time to switching
A total of 576 patients (2.9%) switched to a second-line regimen. The rate of switching overall was 2.4 per 100 person-years (95% CI 2.2–2.6). It was higher in programmes with than in programs without access to viral load monitoring: 3.2 (95% CI 2.8–3.6) compared to 2.0 (95% CI 1.8–2.3) per 100 person-years (p<0.001). Figure 1 shows the Kaplan-Meier curves of the probability of switching to a second-line regimen separately for programmes with and without viral load monitoring. Switching occurred earlier in programmes with access to viral load monitoring. Median time to switching was 16.3 months (IQR 10.1–26.6 months) in 236 patients from programmes with and 21.8 months (IQR 14.0–21.8 months) in 340 patients from programmes without viral load monitoring (p<0.001). The rate of switching tended to be higher in sites with virologic monitoring during months 7–18 after starting ART, similar during months 19–30 and lower than in programmes without viral load monitoring during months 31–42. The adjusted HRs for the three periods were 1.38 (0.97–1.98, p=0.07), 0.97 (0.58–1.60, p=0.90) and 0.29 (0.11–0.79, p=0.02), respectively. The results were similar when in addition adjusting for the first line regimen (3TC d4T NVP, 3TC d4T EFV, 3TC ZDV EFV, 3TC ZDV NVP, other).
Figure 1.
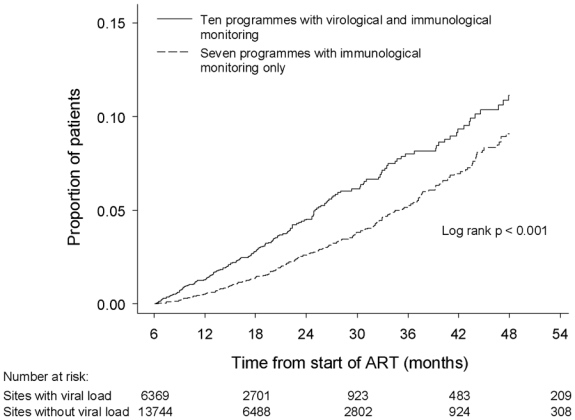
Kaplan-Meier curves of the probability of switching to second-line antiretroviral regimens in 10 treatment programmes with and 7 programmes without virological monitoring.
A majority of patients who switched (316 patients; 54.9%) changed both nucleoside reverse transcriptase inhibitors (NRTIs). The NRTI component of the new regimen consisted of zidovudine/didanosine (190 patients; 33.0%), abacavir/didanosine (65; 11.3%), other drugs combined with didanosine (102; 17.7%), other drugs combined with abacavir (12; 2.1%), combinations with tenofovir (65; 11.3%) or other combinations (142; 24.7%). Common PIs in the second-line regimen were lopinavir boosted with ritonavir (340 patients; 59.0%), indinavir boosted with ritonavir (140; 24.3%) and nelfinavir (57; 9.9%).
CD4 cell count at the time of switching
The median CD4 cell count at the time of switching was 102 cells/μl (IQR 44–181 cells/μl) in 261 patients from sites without viral load monitoring compared to 161 cells/μl (77–265 cells/μl) in 141 patients from sites with routine viral load monitoring (p<0.001). The remaining patients did not have a CD4 count at the time of switching. Differences persisted in the multivariable regression analyses (p=0.001).
Predictors and reasons of switching
The baseline CD4 cell count, but not clinical stage, was associated with switching both in sites with and without viral load monitoring: the lower the CD4 cell count, the higher the probability of switching to a second-line regimen (Table 3). Switching was more frequent in earlier calendar periods.
Table 3.
Predictors of switching to second-line antiretroviral therapy
| Variable | Sites with routine viral load monitoring (n=5058) |
Sites without routine viral load monitoring (n=8699) |
P for interaction* |
||
|---|---|---|---|---|---|
| Adjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | ||
| Age (years) | 0.55 | 0.25 | 0.23 | ||
| 16–29 | 1 | 1 | |||
| 30–39 | 0.93 (0.62–1.39) | 0.93 (0.62–1.39) | |||
| 40–49 | 0.69 (0.41–1.17) | 1.33 (0.84–2.12) | |||
| ≥ 50 | 0.97 (0.47–1.98) | 1.46 (0.73–2.91) | |||
| Sex | 0.91 | 0.11 | 0.34 | ||
| Male | 1 | 1 | |||
| Female | 0.98 (0.68–1.41) | 1.36 (0.94–1.98) | |||
| Time period of starting ART | 0.001 | 0.06 | 0.53 | ||
| < 2002 | 1 | 1 | |||
| 2002–2004 | 0.40 (0.25–0.65) | 0.58 (0.32–1.03) | |||
| 2005–2006 | 0.55 (0.29–1.06) | 0.88 (0.45–1.72) | |||
| Baseline CD4 (cells/μl) | 0.03 | <0.001 | 0.87 | ||
| < 25 | 4.81 (0.64–35.94) | 6.21 (1.47–26.12) | |||
| 25–49 | 3.59 (0.47–27.35) | 5.12 (1.19–21.97) | |||
| 50–99 | 3.13 (0.42–23.59) | 4.01 (0.95–16.82) | |||
| 100–199 | 2.35 (0.32–17.54) | 2.55 (0.61–10.64) | |||
| 200–349 | 2.03 (0.26–15.92) | 1.50 (0.34–6.69) | |||
| ≥ 350 | 1 | 1 | |||
| Clinical stage | 1.00 | 0.91 | 0.67 | ||
| Less advanced | 1 | 1 | |||
| Advanced | 1.00 (0.55–1.82) | 1.02 (0.67–1.57) | |||
| Number of antiretroviral drugs available in programme | 0.26 | 0.58 | 0.45 | ||
| ≤ 10 | 1 | 1 | |||
| 11–13 | 1.96 (0.36–10.82) | 1.90 (0.47–7.73) | |||
| ≥ 14 | 3.56 (0.78–16.34) | 1.04 (0.22–5.05) | |||
Test for interaction between sites with and without routine viral load monitoring
Reasons for switching were available for 241 (42%) of the 576 patients who switched. Reasons were treatment failure (179 patients; 74%), toxicity (25; 10%) and other reasons (37; 15%). At the time of switching 399 (69.3%) of 576 patients had a CD4 count, 153 (26.6%) had a viral load measurement and 118 (20.5%) patients had both. Of 399 patients with available measurements, 263 patients (66%) had immunological failure, virological failure or both.
Results were similar when restricting analyses to sites from sub-Saharan Africa. For example, the median time to switching was 15.2 months (IQR 9.8–24.9 months) in 170 patients from programmes with viral load monitoring and 18.0 months (IQR 12.3–24.2 months) in 212 patients from programmes without viral load monitoring (p=0.04). The median CD4 cell count at the time of switching was 113 cells/μl (IQR 44–186 cells/μl) in 149 patients from sites without viral load monitoring compared to 168 cells/μl (85–236 cells/μl) in 97 patients from sites with routine viral load monitoring (p=0.007). Again, this difference persisted in multivariable regression (p=0.003).
Discussion
Many ART programmes in resource-limited settings do not have access to viral load testing to monitor treatment response, identify treatment failure and inform decisions on when to switch to a second-line regimen, but rely on clinical examinations and, where available, on CD4 cell counts. In this study of ART programmes from sub-Saharan Africa, Asia and Latin America all sites had access to CD4 cell counts and some had access to viral load monitoring. We could thus study patterns of switching to second-line regimens in sites with and without viral load monitoring. We found that patients tended to switch earlier and at higher CD4 cell counts in programmes with, compared to programmes without, access to viral load monitoring. Low CD4 cell counts at the start of ART predicted switching both in programmes with and without viral load monitoring, and fewer patients switched in more recent calendar periods.
Our study included over 20,000 patients who started ART and almost 600 patients who switched to second-line ART. The decision to measure, or not to measure, viral load in an individual patient will often be related to prognosis and the probability of switching to a second line regimen. By comparing sites with and without a policy of monitoring viral load, rather than comparing patients with and without available viral load measurements, such confounding by the indication to test was avoided. The definition we used for second-line ART is in accordance with WHO recommendations [11] and was used in a previous analysis of the Médecins Sans Frontières (MSF) programmes in Africa, Asia, Latin America and Eastern Europe [8]. Our study includes patients who were treated in 17 programmes from 14 countries, and results should therefore be applicable to many other patients on ART in resource-limited countries. Of note, results were robust when restricting analyses to programmes from sub-Saharan Africa. However, we stress that the sites participating in the ART-LINC collaboration are not necessarily representative of all sites providing ART in these countries: they represent a sample of programmes with electronic medical record systems [13] and access to CD4 counts and second-line regimens.
A substantial number of patients did not have a CD4 cell count recorded at the time of switching, and this could have introduced bias in the comparison of CD4 cell counts at switching in sites with and without viral load monitoring. This is unlikely, however: the baseline CD4 counts were similar in patients with missing counts in sites with and without viral load monitoring (data not shown). We did not examine clinical failure: not all sites systematically collect data on opportunistic infections and diagnostic capabilities and criteria vary between sites. Also, we had no information on adherence or drug-resistance, and data about reasons for switching were only available for some patients. In a programme of a faith-based organization in three countries in sub-Saharan Africa, immunological failure was the most common reason for switching, followed by virological failure. Clinical failures were rare [14].
We only considered switching of regimens, as recommended by WHO in case of treatment failure, and not substitutions of single drugs or other changes. A recent study comparing Switzerland with the Khayelitsha and Gugulethu township programmes in South Africa showed that changes to first-line regimens of any type occurred twice as often in Switzerland than in South Africa [7]. The difference was, however, explained by a higher rate of changes due to toxicity or patient wishes in Switzerland, while changes due to treatment failure were infrequent in both settings [7]. In the present study, most of the patients with information on the reason for switching changed regimens because of treatment failure, and results were similar when analyses were adjusted for differences in the first line regimens used in programmes with and without viral load monitoring.
Both in programmes with and without access to viral load monitoring the rates of switching were substantially higher than in the MSF programmes [8]: the MSF programmes do not have access to viral load monitoring and the rate was 0.5 per 100 person-years. Rates were, however, lower than in the programme in three African countries, which includes routine viral load monitoring in all sites: the rate of switching was 4.9 per 100 person-years [14]. A multi-country survey by WHO found highly variable rates of switching to second-line regimens [15]. It seems unlikely that this variability is explained by differences in primary resistance to NRTIs or NNRTIs. At present viral resistance is rare in most resource-limited settings, although important levels of resistance have been reported from Nigeria and North India [16]. The availability of viral load monitoring and generally differences in clinical practice are more likely explanations: practice varies across sites participating in the collaboration, even within the same country. For example, in township programmes in Cape Town, therapy is switched after two consecutive viral loads above 5,000 copies/ml in Khayelitsha whereas in Gugulethu the threshold is 1,000 copies/ml.
A low CD4 cell count when starting ART was the most important predictor of switching to a second-line regimen, in line with a previous study [14]. Starting ART earlier might thus not only reduce the high mortality during the initial months of ART [7, 10, 17] but also help preserve first-line regimens. The rate of switching was lower in more recent calendar years compared to the early years of the ART scale-up, again confirming previous findings [14]. Of note, this was not explained by the increase in CD4 counts at the start of ART, which was observed in more recent years [10]: the effect was evident in analyses adjusted for baseline CD4 count, and might reflect a change in practice associated with the substantial increase in patients starting ART since the early 2000s.
There is debate on the feasibility and cost-effectiveness of viral load monitoring in the context of scaling up of ART in resource-limited settings [18, 19, 20, 20–22]. WHO stipulates that viral load monitoring is desirable, but not essential, for a public health approach to ART [23]. According to a recent modelling study, routine viral load monitoring has only limited benefit on survival and cost-effectiveness is poor [20]. An analysis of mortality in the first year after starting ART showed similar survival in sites with and without viral load monitoring [17], and preliminary results of a randomized trial led to similar conclusions [24]. The results from empirical research are reassuring for sites without access to routine viral load monitoring but they are based on short-term follow-up and the effect of viral load monitoring on long-term outcomes is unclear at present. The higher CD4 cell counts at the time of switching in sites with, compared to sites without access to viral load monitoring indicate that treatment failure is detected earlier in programmes using viral load monitoring, and this might translate into better clinical outcomes in the long-term.
In conclusion, we found that in programmes with access to viral load monitoring patients tended to switch earlier and at higher CD4 cell counts than in sites without viral load monitoring. Future studies should examine what the consequences of earlier or later switching are for clinical outcomes. For example, the effect of switching based on virological failure in patients monitored virologically with switching based on immunological and clinical criteria could be compared in a randomised trial. Alternatively, this comparison could be mimicked in longitudinal, observational studies using causal modelling [25, 26]. Finally, further research is required to determine the optimal frequency of determining CD4 cell counts and of measuring viral load to maximize cost-effectiveness and optimize patient outcomes in different settings in lower income countries.
Acknowledgments
The ART-LINC collaboration of the International epidemiological Databases to Evaluate AIDS (IeDEA) is funded by the US National Institutes of Health (Office of AIDS Research and National Institute of Allergy and Infectious Diseases) and the French Agence Nationale de Recherches sur le Sida et les hépatites virales (ANRS). We are grateful to Mina Hosseinipour for helpful comments on a previous draft of this paper.
Footnotes
Contributors
O Keiser and M Egger conceived and coordinated the analyses and wrote the first draft of the paper; all authors contributed to the final text. O Keiser did the statistical analyses. All investigators assisted in implementation, fieldwork, or data collection at study sites. F Dabis, M Egger, and M Schechter conceived the ART-LINC Collaboration.
Writing committee
Olivia Keiser, Hannock Tweya, Andrew Boulle, Paula Braitstein, Mauro Schechter, Martin W.G. Brinkhof, François Dabis, Suely Tuboi, Eduardo Sprinz, Mar Pujades-Rodriguez, Alexandra Calmy, Nagalingeswaran Kumarasamy, Denis Nash, Andreas Jahn, Patrick MacPhail, Ruedi Lüthy, Robin Wood, Matthias Egger
Conflict of interest statement
The authors declare that they have no conflict of interest.
The ART-LINC of IeDEA Central Coordinating Team
Eric Balestre, Martin Brinkhof, François Dabis (principal investigator), Matthias Egger (principal investigator), Claire Graber, Beatrice Fatzer, Olivia Keiser, Charlotte Lewden, Mar Pujades, Mauro Schechter (principal investigator).
Collaborating centres
Centre de Prise en Charge de Recherches et de Formation (CEPREF); ANRS 1290 (Dakar, Senegal); Academic Model for the Prevention and Treatment of HIV/AIDS (AMPATH), Moi University College of Health Sciences/University of Indiana (Eldoret, Kenya); Adherence Monitoring Uganda (AMU) cohort; Kamuzu Central Hospital/Lighthouse Trust (Lilongwe, Malawi); Connaught Clinic (Harare, Zimbabwe); Gugulethu ART Programme, (Cape Town, South Africa); Khayelitsha ART Programme, (Cape Town, South Africa); Themba Lethu/WITS (Johannesburg, South Africa);; Perinatal HIV Research Unit (Soweto, South Africa); Morocco Antiretroviral Treatment Cohort, Centre Hospitalier Universitaire (Casablanca, Morocco); MTCT-Plus Initiative, International Center for AIDS Care and Treatment Programs, Mailman School of Public Health, Columbia University, New York, USA; Prospective Evaluation in the Use and Monitoring of Antiretrovirals in Argentina (PUMA), Buenos Aires, Argentina; South Brazil HIV Cohort (SOBRHIV), Hospital de Clinicas (Porto Alegre, Brazil); Rio de Janeiro HIV Cohort, Hospital Universitario Clementino Fraga Filho (Rio de Janeiro, Brazil); Y R Gaitonde Centre for AIDS Research and Education (YRG) Care Cohort (Chennai, India); HIV-NAT, Thai Red Cross AIDS Research Centre (Bangkok, Thailand).
Reference List
- 1.Egger M, Hirschel B, Francioli P, Sudre P, Wirz M, Flepp M, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. BMJ. 1997;315:1194–1199. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Vella S, Benfield TL, Chiesi A, Miller VT, Gargalianos P, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. Lancet. 1998;352:1725–1730. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. World Health Organization. Progress report 2008. Geneva: WHO; 2008. [(accessed February 2009)]. Towards universal access. Scaling up priority HIV/AIDS interventions in the health sector. Available at http://www.who.int/hiv/mediacenter/universal_access_progres_report_en.pdf. [Google Scholar]
- 5.Hammer SM, Eron JJ, Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300(5):555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 6.Report on WHO/UNAIDS Meeting on Forecasting ARV needs up to 2010 7–8 November 2005, Geneva. Geneva: WHO; 2006. pp. 1–35. [Google Scholar]
- 7.Keiser O, Orrell C, Egger M, Wood R, Brinkhof MW, Furrer H, et al. Public-health and individual approaches to antiretroviral therapy: township South Africa and Switzerland compared. PLoS Med. 2008;5(7):e148. doi: 10.1371/journal.pmed.0050148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pujades-Rodriguez M, O’Brien D, Humblet P, Calmy A. Second-line antiretroviral therapy in resource-limited settings: the experience of Medecins Sans Frontieres. AIDS. 2008;22(11):1305–1312. doi: 10.1097/QAD.0b013e3282fa75b9. [DOI] [PubMed] [Google Scholar]
- 9.Dabis F, Balestre E, Braitstein P, Miotti P, Brinkhof WGM, Schneider M, et al. Antiretroviral Therapy in Lower Income Countries (ART-LINC): International collaboration of treatment cohorts. Int J Epidemiol. 2005;34:979–986. doi: 10.1093/ije/dyi164. [DOI] [PubMed] [Google Scholar]
- 10.Keiser O, Anastos K, Schechter M, Balestre E, Myer L, Boulle A, et al. Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13(7):870–879. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Recommendations for a public health approach. Geneva: WHO; 2006. [(accessed Feb 2009)]. Antiretroviral therapy for HIV Infection in adults and adolescents in resource-limited settings: towards universal access. Available at http://www.who.int/hiv/mediacenter/universal_access_progres_report_en.pdf. [Google Scholar]
- 12.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weight residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 13.Forster M, Bailey C, Brinkhof MW, Graber C, Boulle A, Spohr M, et al. Electronic medical record systems, data quality and loss to follow-up: survey of antiretroviral therapy programmes in resource-limited settings. Bull World Health Organ. 2008;86(12):939–947. doi: 10.2471/BLT.07.049908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palombi L, Marazzi MC, Guidotti G, Germano P, Buonomo E, Scarcella P, et al. Incidence and Predictors of Death, Retention, and Switch to Second-Line Regimens in Antiretroviral-Treated Patients in Sub-Saharan African Sites with Comprehensive Monitoring Availability. Clin Infect Dis. 2008 doi: 10.1086/593312. [DOI] [PubMed] [Google Scholar]
- 15.Renaud-Thery F, Nguimfack BD, Vitoria M, Lee E, Graaff P, Samb B, et al. Use of antiretroviral therapy in resource-limited countries in 2006: distribution and uptake of first- and second-line regimens. AIDS. 2007;21 (Suppl 4):S89–S95. doi: 10.1097/01.aids.0000279711.54922.f0. [DOI] [PubMed] [Google Scholar]
- 16.Gupta RK, Pillay D. HIV resistance and the developing world. Int J Antimicrob Agents. 2007;29(5):510–517. doi: 10.1016/j.ijantimicag.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 18.Bagchi S, Kempf MC, Westfall AO, Maherya A, Willig J, Saag MS. Can routine clinical markers be used longitudinally to monitor antiretroviral therapy success in resource-limited settings? Clin Infect Dis. 2007;44(1):135–138. doi: 10.1086/510072. [DOI] [PubMed] [Google Scholar]
- 19.Calmy A, Ford N, Hirschel B, Reynolds SJ, Lynen L, Goemaere E, et al. HIV viral load monitoring in resource-limited regions: optional or necessary? Clin Infect Dis. 2007;44(1):128–134. doi: 10.1086/510073. [DOI] [PubMed] [Google Scholar]
- 20.Phillips AN, Pillay D, Miners AH, Bennett DE, Gilks CF, Lundgren JD. Outcomes from monitoring of patients on antiretroviral therapy in resource-limited settings with viral load, CD4 cell count, or clinical observation alone: a computer simulation model. Lancet. 2008;371(9622):1443–1451. doi: 10.1016/S0140-6736(08)60624-8. [DOI] [PubMed] [Google Scholar]
- 21.Schooley RT. Viral load testing in resource-limited settings. Clin Infect Dis. 2007;44(1):139–140. doi: 10.1086/510090. [DOI] [PubMed] [Google Scholar]
- 22.Walensky RP, Freedberg KA, Weinstein MC. Monitoring of antiretroviral therapy in low-resource settings. Lancet. 2008;372(9635):288. doi: 10.1016/S0140-6736(08)61102-2. [DOI] [PubMed] [Google Scholar]
- 23.Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368(9534):505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 24.Couthino R, Mermin J, Ekwaru J, Were W, Bunnell R, Kaharuza F, et al. Utility of Routine Viral Load, CD4 Cell Count, and Clinical Monitoring among HIV-infected Adults in Uganda: A Randomized Trial [Abstract]. 15th Conference on Retroviruses and Opportunistic Infections; Boston. February 3–6 2008; 2008. Abstract 125. [Google Scholar]
- 25.Hernan MA, McAdams M, McGrath N, Lanoy E, Costagliola D. Observation plans in longitudinal studies with time-varying treatments. Stat Methods Med Res. 2009;18(1):27–52. doi: 10.1177/0962280208092345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernan MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett WC, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]


