Abstract
Within the hippocampus and neocortex, GABA is considered excitatory in early development due to a relatively depolarized Cl- reversal potential. Although the depolarizing nature of synaptic GABAergic events has been well established, it is unknown whether cortical tonic currents mediated by extrasynaptically located GABAA receptors (GABAARs) are also excitatory. Here we examined the development of tonic currents in the neocortex and their effect on neuronal excitability. We found that mean tonic current, recorded from Layer 5 pyramidal cells of the mouse somatosensory cortex, is robust in newborns (P2-4) then decreases dramatically by the second postnatal week (P7-10 and P30-40). Pharmacological studies, in combination with Western blot analysis, show that neonatal tonic currents are partially mediated by the GABAAR α5, and likely the δ, subunit. In newborns, the charge due to tonic current accounts for nearly 100% of total GABA charge, a contribution that decreases to less than 50% in mature tissue. Current clamp recordings reveal that tonic current contributes to large fluctuations in the membrane potential that may disrupt its stability. Bath application of 5 μM GABA, to induce tonic currents, markedly decreased cell firing frequency in most recorded cells while increasing it in others. Gramicidin perforated patch recordings reveal heterogeneity in ECl recorded from P2-5 Layer 5 pyramidal cells. Taken together, these findings demonstrate that tonic currents activated by low GABA concentrations can dominate GABAergic transmission in newborn neocortical pyramidal cells and that tonic currents can exert heterogeneous effects on neuronal excitability.
Keywords: inhibition, extrasynaptic, development, neocortex
Introduction
GABA (γ-aminobutyric acid) is the primary inhibitory neurotransmitter in the central nervous system. As the brain matures, neurons undergo changes that affect both the function and kinetics of GABAergic transmission. These changes include a well characterized shift in the Cl- reversal potential (ECl) from a depolarized to a hyperpolarized membrane potential that can render GABA excitatory in early postnatal development then inhibitory in mature tissue (Ben-Ari et al., 1989; Rivera et al., 1999; Ben-Ari, 2002; Dzhala & Staley, 2003; Dzhala et al., 2005; Sipila et al., 2005; Blaesse et al., 2009).
GABAergic transmission is mediated by both fast “phasic” and persistent “tonic” currents. Phasic inhibition is involved in generation or regulation of network oscillations in the hippocampus (Cobb et al., 1995; Klausberger & Somogyi, 2008; Wulff et al., 2009), thalamus (Huntsman et al., 1999) and olfactory bulb (Laurent, 2002). These network oscillations have been implicated in diverse brain functions ranging from exploratory behavior and memory processing (Kahana, 2006) (Seager et al., 2002) to sleep (Steriade et al., 1993). In addition to synaptic inhibition, it is now well-known that low concentrations of GABA in the extracellular space activate high affinity, non-desensitizing perisynaptic and extrasynaptic GABAARs that mediate a tonic current (Semyanov et al., 2004). Activation of such receptors in the developing somatosensory cortex facilitates network activity that may contribute to cortical development (Hanganu et al., 2009; Yang et al., 2009). Whereas phasic currents supply temporally discrete inhibitory events, tonic activation can produce a persistent increase in input conductance. This can reduce a cell's ability to respond to synaptic inputs such that a given current input produces a voltage response of diminished magnitude and duration. Tonic inhibition can also reduce action potential firing probability (Semyanov et al., 2003; Farrant & Nusser, 2005).
Despite significant interest in the development of chloride-mediated transmission and the substantial contribution of tonic current to the total GABA current, only one previous study examined development of tonic GABA currents in the central nervous system (Brickley et al., 1996). Here we examined tonic current in the developing and mature neocortex, both of which exhibit oscillatory activities that are implicated in cortical development and movement, respectively (MacKay & Mendonca, 1995; Yang et al., 2009). First, we used voltage-clamp techniques to measure tonic current from Layer 5 pyramidal cells during two different stages of early postnatal murine development. Second, we used Western blot analysis and pharmacology to examine GABAAR subunits thought to mediate such currents in newborns. Third, we determined the relative contributions of phasic and tonic currents to the total GABA charge in pyramidal neurons from newborn and mature neocortex. Lastly, we used perforated-patch recordings to measure ECl and cell-attached recordings to study the effect of GABA on spontaneous cell firing.
Methods
Slice preparation
Acute brain slices were prepared from male or female CD-1 mice (Charles River, Hollister, CA) in three age groups: neonate (P2-4), juvenile (P7-10), and mature (P30-40). All procedures were in accordance with the NIH Guidelines for Use and Care of Laboratory Animals and approved by the University of California, San Francisco Institutional Animal Care and Use Committee. Mice were anesthetized with a ketamine and xylazine mixture and decapitated. The brain was rapidly removed and 300 μm slices were prepared with a vibratome (Leica VT1000S, Bannockburn, IL) in oxygenated high sucrose ACSF (in mM, 150 sucrose, 50 NaCl, 25 NaHCO3, 10 dextrose, 2.5 KCl, 1 Na2HPO4-H2O, 0.5 CaCl2, and 7 MgCl2) at 4°C. During incubation (40 min at 35°C) and recording, slices were perfused with a carbogen bubbled ACSF containing (in mM): 124 NaCl, 3KCl, 1.25 NaH2PO4-H2O, 2 MgSO4-7H2O, 26 NaHCO3, 10 dextrose, and 2 CaCl2. For recording, slices were maintained at 33-34°C with ACSF flowing at 9-10 ml/min.
Electrophysiology
All recordings were obtained from L5 pyramidal cells that were visually identified using infrared differential interference contrast (IR-DIC) optics on an Olympus BX-51WI microscope. Data were acquired using Clampex software (Molecular Devices, Sunnyvale, CA) at a gain of 5 and filtered at 1 kHz. For whole-cell recordings of GABA currents in the presence of exogenous GABA, patch electrodes (3-5 MΩ) were filled with (in mM): 40 K-Gluconate, 100 KCl, 2 NaCl, 10 HEPES, 4 EGTA, 4 MgATP, and 0.3 Na2GTP. For whole-cell recordings of GABA currents in the presence of GABA uptake blockers, patch electrodes (3-5 MΩ) were filled with (in mM): 140 CsCl, 1 MgCl2, 10 HEPES, 11 EGTA, 2 NaATP, 0.5 Na2GTP and 1.25 QX-314. For all voltage-clamp recordings, pyramidal neurons were held at -70 mV and bathed in 3 mM kynurenic acid (Tocris, Ellisville, MO) or 25 μM APV and 20 M DNQX (Sigma) to block glutamate receptors. To record tonic GABA currents in the presence of exogenous GABA, slices were bath perfused in 1 or 5 μM GABA (Sigma) for 5-10 min prior to recording followed by 100 μM gabazine (Sigma) to block GABAARs. The GABAAR α5 subunit antagonist L655,708 (200 nM) and δ subunit agonist THIP (1 μM, Sigma) were bath applied as indicated in Figure 2. To block GABA uptake, NO711 (20 μM, Sigma) and SNAP-5114 (100 μM, Sigma) were bath applied for at least 2 minutes prior to recording tonic current. During this 2 minute window, the holding current increased and stabilized. In current-clamp recordings, DC current was injected to hold cells at -60 to -70 mV and slices were bathed in 3 mM kynurenic acid. For all whole-cell recordings, the series resistance was measured after each recording and data were discarded if the resistance changed by more that 20% or if the series resistance was > 10 MΩ. For cell-attached recordings, patch electrodes were filled with 150 mM NaCl and were held at a command potential that resulted in zero holding current (Perkins, 2006). For gramicidin perforated-patch recordings, the tip of the patch electrode (0.7-2 MΩ) was filled with a gramicidin free internal solution containing (in mM): 150 KCl and 10 HEPES. The electrode was then backfilled with the same internal solution containing 50-100 μg/ml gramicidin. After establishing a GΩ seal, cells were held on average for 40 min -1.5 hrs until the series resistance stabilized at <50 MΩ. During this time and between recordings, cells were held at -70mV. For recordings, the holding potential was stepped in 10 mV increments from -80 to 0 mV. To measure the Cl- currents through GABAARs, 500 μM muscimol (Sigma) was applied via a picospritzer (8 psi, 3-12 ms pulse duration; Parker Hannifin, Cleveland, Ohio). For each cell, the recording was repeated at least twice to confirm repeatability and all responses were abolished when 100 μM gabazine was added to the bath perfusate.
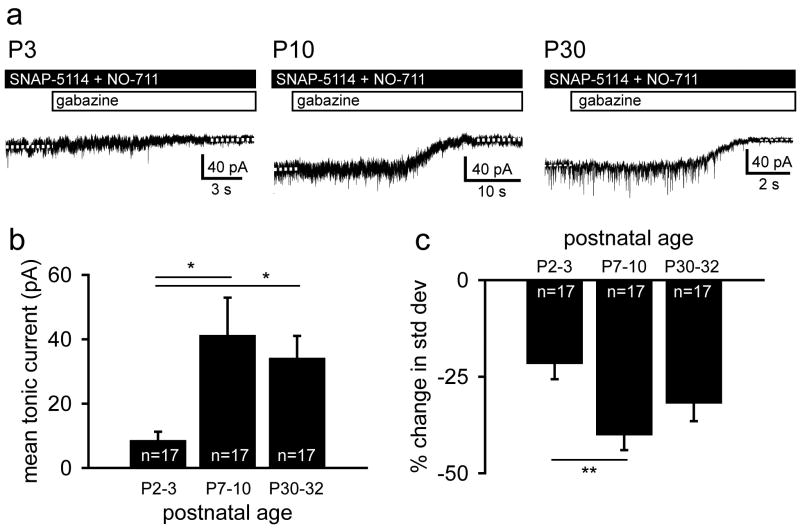
Figure 2.
Tonic GABA current increases with postnatal development when GABA uptake is blocked. A. Representative voltage clamp recordings of GABAAR mediated tonic current from L5 neocortical pyramidal cells in P3, P10 and P30 mice. Dashed white lines indicate the baseline holding current. B. Summary plot of mean tonic current recorded from neocortical pyramidal cells representing three postnatal age groups. Mean tonic current substantially increases from the first to the second week of postnatal life then remains stable one month after birth. C. Summary plot of the change in the standard deviation of the baseline holding current before and after GABAAR blockade. The percent decrease in standard deviation is greater in neonates than in juveniles. Data are shown as mean ± S.E.M. (one-way ANOVA, Tukey post hoc: **, p < 0.01).
Western Blots
The somatosensory cortices were dissected from P3 and P30 mice and homogenized in PBS. Protein was extracted with Cytosol Extraction Buffer containing protease inhibitors (Biovision, Inc., Mountain View, CA) and protein concentrations were determined using a Bradford Assay (Sigma). 12 μg of total protein was loaded into an SDS-PAGE gel, transferred to a nitrocellulose membrane (BioRad, Hercules, CA), and blocked in 5% milk. Membranes were incubated in primary antibodies for α4 (1:1000; Millipore #AB5457), δ (1:250; Millipore #AB9752) and Tuj1 (1:5000; Covance, Inc., Princeton, NJ; #PRB-435P). Tuj1 loading controls were used in all experiments. NIH Image J software was used to measure optical density and unpaired t-tests were used to determine statistical significance.
Data Analysis
To measure the amplitude of tonic current and the standard deviation of the holding current, we selected regions of the recording at which there were no synaptic currents and computed the mean holding current and standard deviation of the mean (pClamp, Molecular Devices). To measure the charge contributed by GABAergic IPSCs, Mini Analysis software (Synaptosoft, Inc., Decatur, GA) was used to determine IPSC frequency and to create an average IPSC trace per cell. The IPSC frequency and integrated area of the average trace were used to calculate the charge due to synaptic current. The charge contributed by tonic GABA current was calculated using the integrated area of the tonic current for each cell and the same time frame used in the IPSC analysis. The Cl- reversal potential was determined by plotting the peak muscimol currents vs. the holding potential, fitting a linear regression line, finding the potential at y=0 and correcting for the liquid junction potential. The liquid junction potential was calculated as -4 mV with reference to the bath using the pClamp liquid junction calculator (Molecular Devices).
To calculate statistical significance, we used unpaired t-tests (Microsoft Excel, Microsoft), one-way ANOVAs (SPSS, IBM, Chicago, IL) and two-way ANOVAs (GraphPad Prism, GraphPad Software, Inc., La Jolla, CA) depending on which test was appropriate for the data set. The statistical test used is always noted in the text.
Results
GABAAR mediated tonic current decreases dramatically with postnatal development
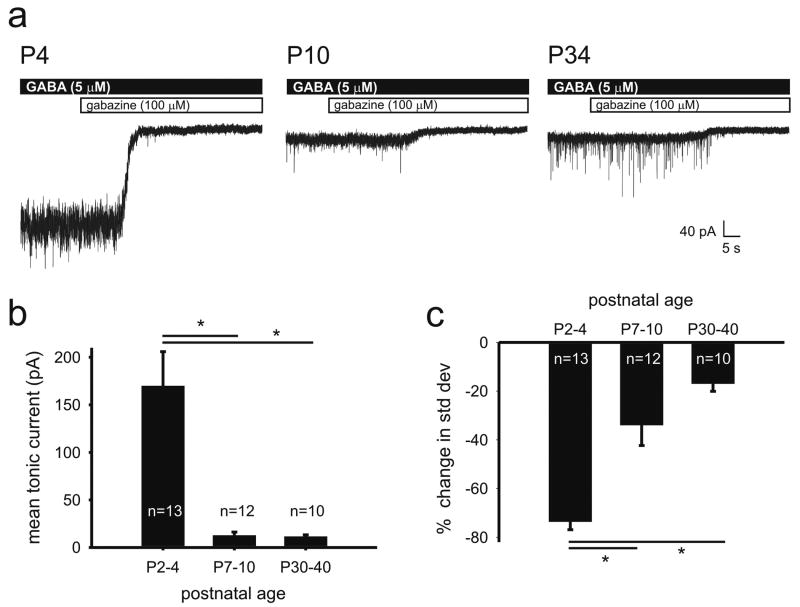
To examine developmental changes in tonic current, we made whole-cell voltage-clamp recordings from L5 neocortical pyramidal cells at three postnatal ages: neonatal (P2-4), juvenile (P7-10), and mature (P30-40). To measure tonic current, neocortical slices were bath perfused with normal ACSF containing 5 μM GABA then ACSF containing the GABAAR antagonist gabazine (100 μM). The difference in baseline holding current before and after GABAAR blockade was used as a measure of the GABAAR mediated tonic current (Bright et al., 2007; Baraban et al., 2009; Pavlov et al., 2009). Shown in Figure 1A are the experimental paradigm and representative traces of tonic currents recorded at each of the three ages. As reported previously in the somatosensory cortex (Kobayashi et al., 2008), phasic current frequency, seen as sharp inward currents in the pre-gabazine region of the traces, increased with age. Interestingly, mean tonic current dramatically and rapidly decreased with development from a peak of 170 ± 36 pA (n = 13) in the first week of postnatal life (P2-4) to only 12 ± 4 pA (n = 12) just a few days later (P7-10). The mean tonic current then remained stable at 12 ± 2 pA (n = 10) a month after birth (P30-40). The nearly 93% decrease in mean tonic current in neonates compared to juveniles and mature mice was statistically significant (One-way ANOVA, Tukey posthoc, p < 0.01); no difference between the tonic current recorded from juvenile and mature cells was noted (One-way ANOVA, Tukey posthoc, p > 0.05). We also measured percent change in standard deviation of the baseline current before and after GABAAR blockade at each of the age groups. Consistent with the substantial tonic current in neonatal neurons, standard deviation of the baseline current decreased most dramatically in neonates following GABAAR blockade (Figure 1C: 74 ± 3%, n = 13). The change in standard deviation was also statistically different between neonates versus those than in juveniles (22 ± 5%, n = 12) and mature mice (17 ± 3%, n = 10) (ANOVA, Tukey posthoc, p< 0.01). However, there was no difference in the percent change in standard deviation between juveniles and mature mice (ANOVA).
Figure 1.
Tonic GABA current decreases with postnatal development when recorded in the presence of exogenous GABA. A. Representative voltage clamp recordings of GABAAR mediated tonic current from L5 neocortical pyramidal cells in P4, P10 and P34 mice in the presence of 5 μM GABA. B. Summary plot of mean tonic current recorded from neocortical pyramidal cells representing three postnatal age groups. Mean tonic current substantially decreases from the first to the second week of postnatal life then remains stable one month after birth. C. Summary plot of the change in the standard deviation of the baseline holding current before and after GABAAR blockade. The percent decrease in standard deviation is greater in neonates than juveniles and mature mice. Data are shown as mean ± S.E.M. (one-way ANOVA, Tukey post hoc: *, p < 0.01).
When GABA uptake is blocked tonic current increases with development
To measure tonic current in the presence of endogenous GABA, we blocked the GABA transporters expressed in neocortex (GAT1 and GAT2/3) (Keros & Hablitz, 2005) via bath application of NO-711 (20 μM) and SNAP-5114 (100 μM), respectively (Figure 2A). In this condition, the mean tonic current in P2-3 pyramidal cells was 9 ± 3 pA (n = 17) then increased to 41 ± 12 pA (n = 17) in juveniles (One-way ANOVA, Tukey posthoc, p < 0.05). One month after birth (P30-32), the tonic current had stabilized at this elevated value (34 ± 7 pA; n = 17; One-way ANOVA, Tukey posthoc, p < 0.05) (Figure 2B). For all three age groups, the standard deviation of the holding current before and after GABAAR blockade decreased (P2-3: 22 ± 4%; P7-10: 40 ± 4%; P30-32: 32 ± 5%; paired t-test p<0.001). The percent change in the standard deviation was significantly greater for juveniles relative to neonates (ANOVA, Tukey posthoc, p< 0.01) (Figure 2C).
Tonic current is mediated by both the GABAAR α5 and δ subunits
Given that tonic GABAergic current in the hippocampus is mediated by GABAAR α5 and δ subunits (Caraiscos et al., 2004; Glykys et al., 2008), we next examined whether these subunits play a similar role in neonatal pyramidal cells of somatosensory cortex. To isolate tonic current, neocortical slices were bath perfused with normal ACSF containing 5 μM GABA then ACSF supplemented with the α5 subunit antagonist L655,708 (200 nM) followed by gabazine. As shown in the representative trace and summary data in Figure 3A and B, respectively, blockade of the α5 subunit decreases mean tonic current from 92 ± 24 to 63 ± 15 pA (n = 15, p < 0.01). To determine whether the δ subunit contributes to tonic current in these cells, we bath perfused slices with ACSF containing 5 μM GABA then applied THIP (1 μM), or gaboxadol, which in juvenile and adult rodent tissue acts as a δ subunit agonist (Cope et al., 2005; Maguire et al., 2005; Drasbek & Jensen, 2006). In one cell, THIP application increased the holding current by 172 % (from -199 to -371 pA). Surprisingly, we found that in the remaining 10 out of 11 cells, THIP application decreased the holding current from -140 ± 29 to -91 ± 16 pA (n = 10, p<0.01) (Figure 3C). We used this unexpected finding to determine the amount of tonic current in these cells likely mediated by δ subunit containing GABAARs. Overall, α5 and δ subunit mediated currents account for 26 ± 5% and 31 ± 4%, respectively, of the mean tonic current recorded in neonatal L5 neocortical pyramidal cells. As in Figures 1 and 2, we measured the standard deviation of the holding current before and after bath application of L655,708 or, separately, THIP. We found that blocking the α5 subunit containing GABAARs did not significantly affect the standard deviation of the holding current (from 7.7 ± 0.8 to 7.7 ± 0.5 pA, n = 15). However, THIP significantly decreased the standard deviation by 15 ± 3% from 13 ± 2 to 11 ± 1 pA (n = 10, p < 0.05)(Figure 3D).
Figure 3.
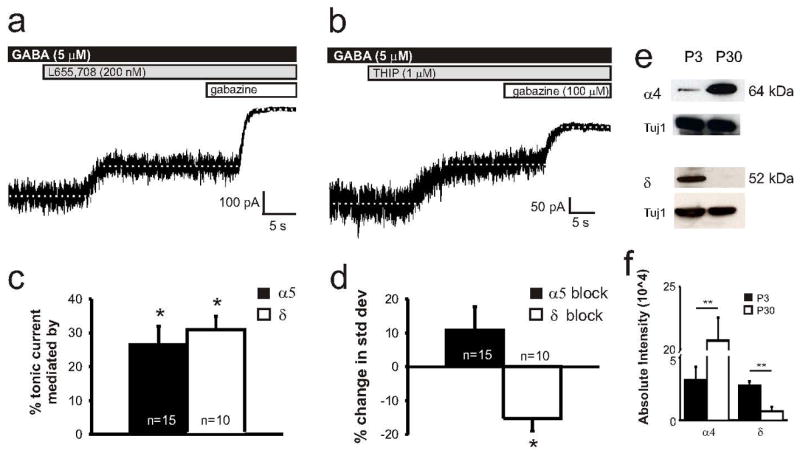
Tonic currents are mediated by the GABAAR α5 and δ subunits in neonates. A. Representative tonic current recording from a P3 mouse shows a partial reduction in tonic current following GABAAR α5 subunit blockade (200 nM L655, 708). B. Representative tonic current recording from a P3 mouse shows a partial reduction in tonic current following bath application of THIP (1 μM). C. Summary plot of percent change in the tonic current following bath application of the α5 subunit antagonist and THIP. The α5 and δ subunits mediate 26 ± 5% and 31 ± 4%, respectively, of the mean tonic current. D. Summary plot of percent change in standard deviation of the baseline holding current before and after bath application of α5 subunit antagonist and THIP. THIP decreased the standard deviation relative to control whereas α5 subunit blockade had no effect. Data are shown as mean ± S.E.M. Paired t-test: *, p < 0.01. E. Representative western blots of the GABAAR α4 and δ subunits accompanied by the Tuj1 loading controls for each experiment. F. GABAAR α4 subunit expression is upregulated in mature neocortex, whereas δ subunit expression decreases with development (*, p<0.01, **, p<0.001; unpaired t-test)
Our electrophysiological findings suggest that tonic current in neonatal neocortical pyramidal cells is, at least in part, mediated by the GABAAR δ subunit. Therefore, we conducted Western blots to examine subunit expression. We surgically isolated somatosensory cortex from neonatal and mature tissue and examined expression of GABAARs using commercially available antibodies to the α4 and δ subunits (Figure 3E). Western blot analysis showed that GABAAR α4 subunit expression increased from low to high levels with development (P3: 31508 ± 10270; P30: 207370 ± 18439; n = 4, unpaired t-test, p < 0.001)(Fig. 3F). However, δ subunit expression was high in neonatal tissue but nearly below the level of detection in mature neocortex (P3: 26879 ± 3516; P30: 7376 ± 3077; n = 6, unpaired t-test, p < 0.01). Representative Western blots accompanied by Tuj1 loading controls and a summary data plot are shown in Figure 3E and F, respectively
Tonic GABAergic currents can dominate inhibition early in postnatal development
Given the large tonic current recorded from neonatal pyramidal cells following bath application of GABA, we next measured the relative contributions of phasic and tonic currents to total GABAergic current in neonatal and mature tissue. Because 5 μM GABA increased the standard deviation of the baseline holding current to a level where IPSCs could not reliably be distinguished from the variability in holding current, for these experiments we reduced bath applied GABA concentration to 1 μM. GABA application induced a mean tonic current of 10 ± 3 pA in neonates (n = 8, p < 0.01) but only 0.2 ± 1 pA in neocortical slices from mature mice (n = 14, p = 0.7) (Figure 4A and B). GABAAR blockade reduced the standard deviation of the recording by 37 ± 5 % in neonates (n = 8, p < 0.001) and but had no effect in mature mice (0.7 ± 5 %; n = 14, p = 0.7). Spontaneous IPSC frequencies in neonatal and mature tissue were 0.4 ± 0.07 Hz and 16 ± 2 Hz, respectively. These values were used to calculate the charge of phasic, tonic and total GABA current. The total charge is significantly greater immediately after birth (970 ± 290 pC, n = 8) than 1 month into postnatal life (57 ± 25 pC, n = 13; two-way ANOVA, Tukey, p < 0.0001) (Figure 4C). Further, the percent of total charge contributed by tonic current is substantially greater in newborns than in mature mice (newborns: 99.8 ± 0.7 %, n = 8; mature: 41.2 ± 11.5 %, n = 13; paired t-test, p < 0.001)(Figure 4D).
Figure 4.
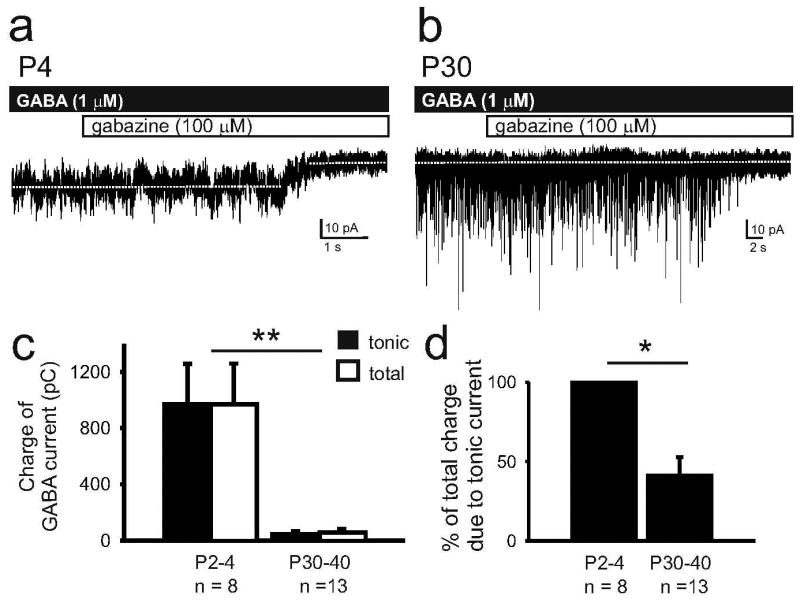
Tonic currents can contribute the majority of charge carried by GABA currents in neonates. A. Representative recording from a P4 pyramidal cell showing both the magnitude of tonic current induced by 1 μM GABA and the low IPSC frequency. B. Representative recording from a P30 pyramidal cell is characterized by an undetectable tonic current and high IPSC frequency. C. Summary data of the charge carried by GABA currents during bath application of 1 μM GABA. Due to the substantial contribution of tonic currents, the total charge of GABA current is greater in newborn than in mature neocortex (**, p< 0.0001, two-way ANOVA, Tukey). D. Histogram of the percent of total charge due to tonic current shows that tonic current carries 99.8 ± 0.7 %of the GABA charge in neonates but only 41.2 ± 11.5 % in mature tissue. (*, p<0.001, unpaired t-test).
Tonic GABA currents increase the membrane potential variability and reduces spontaneous action potential firing in neonates
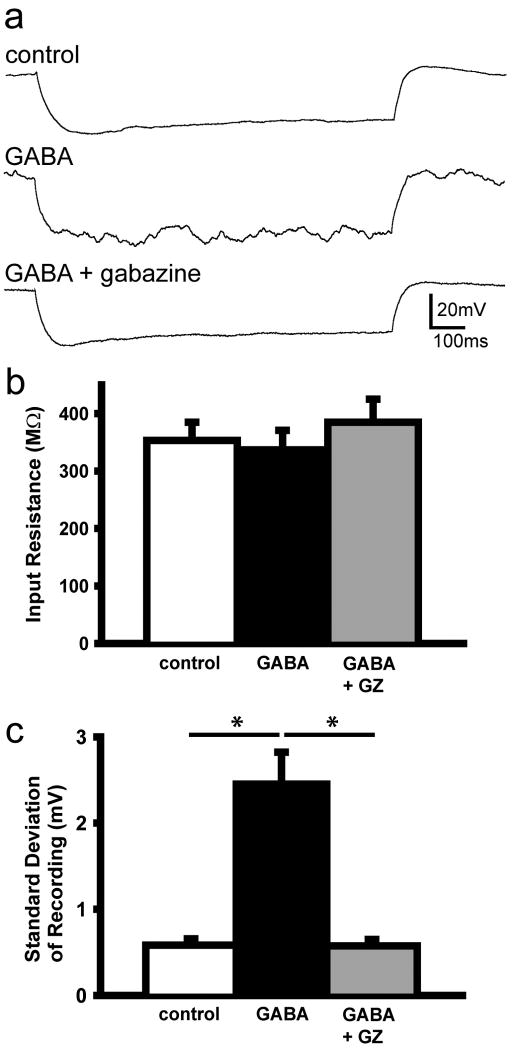
To examine the effect of tonic GABA currents on intrinsic membrane properties, we recorded input resistance and standard deviation of the membrane potential in ACSF only (control), 5 μM GABA (GABA) and 5 μM GABA and 100 μM gabazine (GABA +GZ) bathed neocortical slices (Figure 5). GABA or GABA plus gabazine had no effect on the input resistance (control: 353 ± 32 MΩ, GABA: 337 ± 34 MΩ, and GABA +GZ: 385 ± 40 MΩ; n = 10)(Figure 5B). Despite the stability of the input resistance, GABA application increased standard deviation of the membrane potential by 334 + 50% (control: 0.6 ± 0.07 mV, GABA: 2.5 ± 0.4 mv, GABA + GZ: 0.6 ± 0.08 mV, n = 10; ANOVA, Tukey, p < 0.001)(Figure 5C), an effect that completely reversed following GABAAR blockade.
Figure 5.
GABA increases membrane potential variability. A. Representative traces of current clamp recordings in control, following bath application of GABA (GABA) and GABA and gabazine (GABA +GZ). B and C. GABA does not affect mean input resistance but increases the standard deviation of the membrane potential (*, p < 0.001; one-way ANOVA, Tukey).
To determine the effect of GABA on cellular excitability, we recorded spontaneous firing frequency in the cell-attached voltage-clamp configuration (Figure 6). Bath application of GABA (5 μM) elicited heterogenous effects on excitability. In 15 out of 24 cells, GABA decreased firing frequency by 98.2 ± 0.9 % (One-way ANOVA, Tukey, p < 0.01), a pronounced inhibitory effect that reversed following application of GABA and 100 μM gabazine (One-way ANOVA, Tukey, p < 0.05) (Figure 6B). Figure 6A shows raw traces from two different L5 pyramidal cells in which GABA reduced cell firing frequency. In the remaining 9 out of 24 cells (Figure 6D), GABA initially increased firing frequency by 348 ± 101 % (One-way ANOVA, Tukey, p < 0.01). In this group of 9 cells, the firing frequency decreased (-88.6 ± 6.7 %, One-way ANOVA, Tukey, p < 0.001; Supplemental Figure 1) in 45 ± 6 sec following the switch to bath perfusate containing GABA. Cell firing recovered following GABAAR blockade (One-way ANOVA, Tukey, p < 0.01) in 24 ± 6 sec following the switch to bath perfusate containing GABA and the GABAAR antagonist. The above durations include the time needed to prime the tubing supplying solution to the recording chamber.
Figure 6.
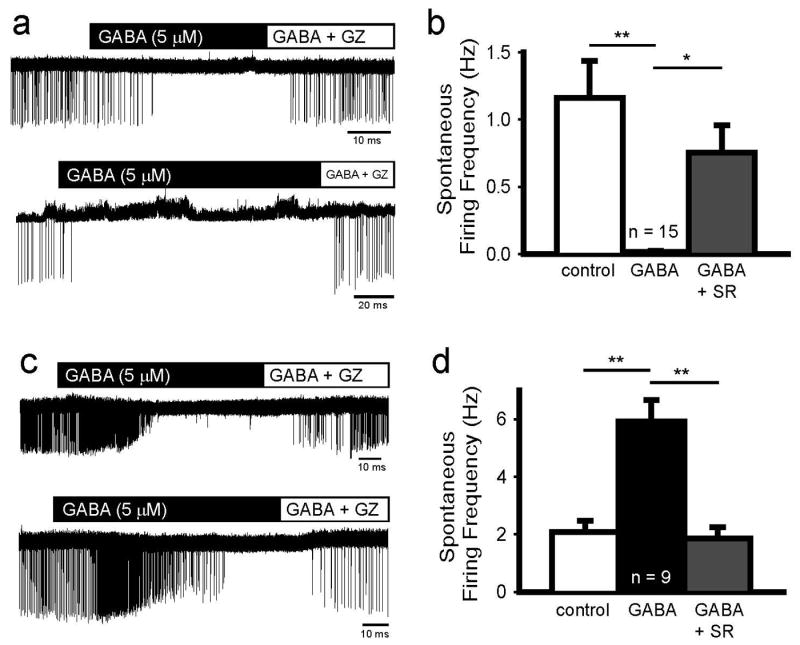
GABA decreases or transiently increases spontaneous firing frequency in newborn cells. A. Raw traces from two P3 cells show that GABA abolishes spontaneous firing in a reversible manner. B. Summary data demonstrates that GABA reduces firing frequency in 62.5% of recorded cells (*, p < 0.05; **, p < 0.01; one way ANOVA, Tukey). C. Raw traces from P2 (top) and P3 (bottom) cells show that GABA initially stimulates spontaneous firing, and prolonged application diminishes firing. These data are summarized in D (**, p < 0.01; one-way ANOVA, Tukey).
That GABA transiently increased cell firing frequency in some cells while reducing it in most is consistent with the variability in ECl measured from Layer V pyramidal cells in P2-4 mice. Using gramicidin perforated-patch recordings, we found that the ECl ranges from -29.9 to -67.0 mV (Figure 7E) and that the mean ECl is -56.0 ± 4.5 mV. Figure 7A and C show raw recordings from two P3 cells in which the ECl were -57.5 and -25.9 mV (Figure 7B and D), respectively, or -61.5 and -29.9 mV following junction potential correction
Figure 7.
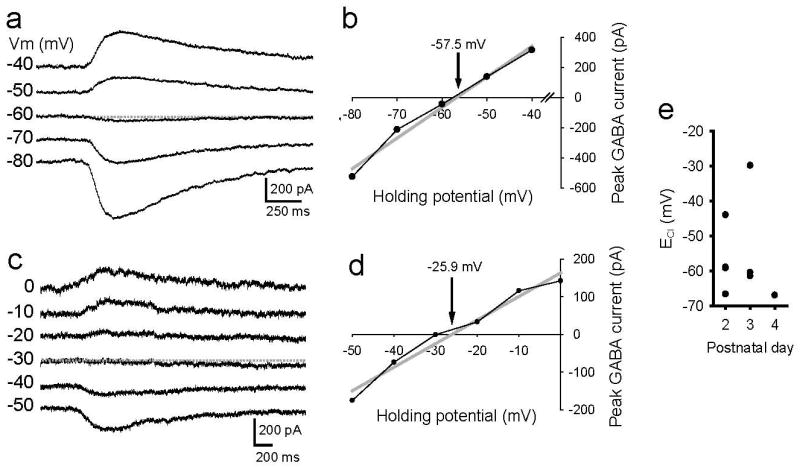
ECl in newborn L5 pyramidal cells is heterogeneous. A. Gramicidin perforated patch recording from a P3 cell shows that the muscimol induced responses reverse between -50 and -60 mV. B. Linear regression line was fit to the data points to determine the ECl which in this cell is -57.5 mV (61.5 mV with junction potential correction). C. In this P3 cell, responses reversed at ∼-30 mV and (D) the regression line crossed the x-axis at -25.9 mV (29.9 with junction potential correction). E. Scatter plot of ECl vs postnatal day shows a range of ECl from -29.9 to -66.7 mV.
Discussion
Our results demonstrate that newborn pyramidal cells are capable of robust tonic currents and that this capability decreases dramatically by the second week of postnatal life. Further, tonic current does not have a homogenous effect on cell excitability in that GABA can transiently boost spontaneous cell firing frequency in some cells while consistently inhibiting it many others. This diverse, but primarily inhibitory, effect on excitability in the neonatal cortex is consistent with the variability in the measured ECl.
Development of GABA uptake, synaptic release and GABAAR expression
Tonic currents were measured in the presence of GABA uptake blockers to determine whether the dramatic reduction in tonic current observed with development is at least partially due to an increase in GABA uptake. When GABA uptake is blocked we found that tonic currents increase with development, suggesting that GABA transporter function increases as the neocortex matures. In conjunction with an increase in GABA transporter blockade, the developmental increase in synaptic release likely elevates the endogenous GABA concentration, and as a result the mean tonic current. (Brickley et al., 2001; Glykys & Mody, 2007). In newborn neocortex, minimal GABA transport and the observed low sIPSC frequency may contribute to the small tonic currents in the presence of transporter blockers.
Although increases in transporter function and synaptic GABA release likely underlie part of the developmental reduction in tonic current, it is possible that neonatal neocortical cells are also more sensitive to extracellular GABA. This interpretation is supported by the higher density of GABAAR δ subunit expression in neonatal versus mature neocortex (Figure 2). If the newborn neocortex is characterized by reduced GABA uptake and increased GABAAR expression, it is both less capable of maintaining a low GABA concentration and more sensitive to increases in GABA, respectively. Thus, under conditions in which ambient GABA is elevated, tonic current may play a significant role in regulating neuronal excitability. It may be possible to increase the extracellular GABA concentration by activating endogenous mechanisms such as the non-vesicular release observed in cerebellar granule cells (Rossi et al., 2003) or by exogenous methods such as pharmacological, genetic or cell transplantation approaches.
Relevance of studying tonic currents in newborns
In this study, we primarily examined tonic current activated by exogenously applied GABA and the effect of such currents on neuronal excitability. Although the extracellular GABA concentration in vivo is unknown, particularly whether it is high enough to maintain the tonic currents we observed, we were interested more in the robust capacity of newborn neocortex to conduct such currents. In addition, given that studies of GABA in the developing brain have focused on phasic currents and their depolarizing nature, we explored whether tonic currents excite or inhibit cells by measuring their effect on spontaneous firing frequency.
Heterogenous effects of GABA in newborn brain
Although GABA is widely considered excitatory in newborn rodents, the majority of studies examining the depolarizing nature of GABA in neonatal brain tissue have centered on the hippocampus. Within the neonatal hippocampus, the ECl is depolarized relative to the resting membrane potential and GABA induces giant depolarizing potentials (Ben-Ari et al., 1989; Sipila et al., 2005) or bursts of epileptiform discharge (Dzhala & Staley, 2003). Also within the newborn rodent hippocampus, blockade of NKCC1 with bumetanide can reduce induced seizure activity (Dzhala et al., 2005). However, GABA is not uniformly excitatory in the neonatal brain. For example, in brain regions such as Layer V of the neocortex (Yamada et al., 2004), the brainstem (Ritter & Zhang, 2000) and spinal cord (Li et al., 2002; Jean-Xavier et al., 2007), GABA can by inhibitory during the first week of postnatal life, in some cases due to an already hyperpolarized ECl. Further, it appears that the heterogeneity in ECl may extend to different layers of the newborn neocortex. That is, cells within the most superficial and immature layer of the neocortex (cortical plate) exhibit more depolarized ECl (∼ -40 mV) than the more mature neurons found in deeper layers (Layer II/III and V: ∼ -70 mV) (Yamada et al., 2004). Our finding that the ECl of P2-4 Layer 5 neocortical pyramidal cells varies from +30 to -67 mV coupled with data that GABA application increases spontaneous firing in many cells while reducing it in others demonstrates that GABA exerts varied effects on cellular excitability. However, to assert that the diversity of GABA's function can solely be attributed to differences in ECl would be an oversimplification. Instead, GABA's effect on neuronal excitability is dependent on a number of factors. With regard to tonic activation of GABAARs, the focus of the present work, these factors include the degree to which GABA increases the input conductance, the concentration of extracellular GABA, how quickly GABA is cleared (Khalilov et al., 1999), and the ECl of the cell. Given that tonic currents result in a persistent increase in input conductance that reduces the magnitude, duration and spatial spread of voltage responses to a given current input, they may, despite a depolarized ECl, reduce action potential firing probability and thereby neuronal excitability.
Supplementary Material
Supplemental Figure 1: Prolonged GABA application decreased spontaneous firing frequency in all newborn cells. Summary plot showing a reduction in mean spontaneous firing frequency following GABA application that is reversed by subsequent GABAAR blockade (control: 1.5 ± 0.3 Hz; GABA: 0.2 ± 0.1 Hz; GABA + GZ: 1.2 ± 0.3; n=24; **, p < 0.01; ***, p < 0.001; one-way ANOVA, Tukey). Representative raw traces showing a reduction in spontaneous firing frequency following prolonged GABA exposure are show in Figure 5A and C. Data are shown as mean ± S.E.M.
Acknowledgments
We thank M.T. Dinday for technical support, S.W. Chege for help with Western blots and M.A. Howard for advice regarding the gramicidin perforated patch recording technique. This work was supported by NIH Ruth L. Kirschstein National Research Service Award 5F32NS061497-02 (to J.Y.S) and NIH R01 grants NS048528-05 and NS040272-07 (to S.C.B.).
References
- Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, Garcia-Verdugo JM, Rubenstein JL, Alvarez-Buylla A. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci U S A. 2009;106:15472–15477. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497(Pt 3):753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Bright DP, Aller MI, Brickley SG. Synaptic release generates a tonic GABA(A) receptor-mediated conductance that modulates burst precision in thalamic relay neurons. J Neurosci. 2007;27:2560–2569. doi: 10.1523/JNEUROSCI.5100-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex. 2006;16:1134–1141. doi: 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Excitatory actions of endogenously released GABA contribute to initiation of ictal epileptiform activity in the developing hippocampus. J Neurosci. 2003;23:1840–1846. doi: 10.1523/JNEUROSCI.23-05-01840.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol. 2007;582:1163–1178. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Okabe A, Lessmann V, Luhmann HJ. Cellular mechanisms of subplate-driven and cholinergic input-dependent network activity in the neonatal rat somatosensory cortex. Cereb Cortex. 2009;19:89–105. doi: 10.1093/cercor/bhn061. [DOI] [PubMed] [Google Scholar]
- Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- Jean-Xavier C, Mentis GZ, O'Donovan MJ, Cattaert D, Vinay L. Dual personality of GABA/glycine-mediated depolarizations in immature spinal cord. Proc Natl Acad Sci U S A. 2007;104:11477–11482. doi: 10.1073/pnas.0704832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ. The cognitive correlates of human brain oscillations. J Neurosci. 2006;26:1669–1672. doi: 10.1523/JNEUROSCI.3737-05c.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keros S, Hablitz JJ. Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. J Neurophysiol. 2005;94:2073–2085. doi: 10.1152/jn.00520.2005. [DOI] [PubMed] [Google Scholar]
- Khalilov I, Dzhala V, Ben-Ari Y, Khazipov R. Dual role of GABA in the neonatal rat hippocampus. Dev Neurosci. 1999;21:310–319. doi: 10.1159/000017380. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Hamada T, Kogo M, Yanagawa Y, Obata K, Kang Y. Developmental profile of GABAA-mediated synaptic transmission in pyramidal cells of the somatosensory cortex. Eur J Neurosci. 2008;28:849–861. doi: 10.1111/j.1460-9568.2008.06401.x. [DOI] [PubMed] [Google Scholar]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 2002;3:884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- Li H, Tornberg J, Kaila K, Airaksinen MS, Rivera C. Patterns of cation-chloride cotransporter expression during embryonic rodent CNS development. Eur J Neurosci. 2002;16:2358–2370. doi: 10.1046/j.1460-9568.2002.02419.x. [DOI] [PubMed] [Google Scholar]
- MacKay WA, Mendonca AJ. Field potential oscillatory bursts in parietal cortex before and during reach. Brain Res. 1995;704:167–174. doi: 10.1016/0006-8993(95)01109-9. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Pavlov I, Savtchenko LP, Kullmann DM, Semyanov A, Walker MC. Outwardly rectifying tonically active GABAA receptors in pyramidal cells modulate neuronal offset, not gain. J Neurosci. 2009;29:15341–15350. doi: 10.1523/JNEUROSCI.2747-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KL. Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J Neurosci Methods. 2006;154:1–18. doi: 10.1016/j.jneumeth.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter B, Zhang W. Early postnatal maturation of GABAA-mediated inhibition in the brainstem respiratory rhythm-generating network of the mouse. Eur J Neurosci. 2000;12:2975–2984. doi: 10.1046/j.1460-9568.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M, Attwell D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J Physiol. 2003;548:97–110. doi: 10.1113/jphysiol.2002.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seager MA, Johnson LD, Chabot ES, Asaka Y, Berry SD. Oscillatory brain states and learning: Impact of hippocampal theta-contingent training. Proc Natl Acad Sci U S A. 2002;99:1616–1620. doi: 10.1073/pnas.032662099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sipila ST, Huttu K, Soltesz I, Voipio J, Kaila K. Depolarizing GABA acts on intrinsically bursting pyramidal neurons to drive giant depolarizing potentials in the immature hippocampus. J Neurosci. 2005;25:5280–5289. doi: 10.1523/JNEUROSCI.0378-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Wulff P, Ponomarenko AA, Bartos M, Korotkova TM, Fuchs EC, Bahner F, Both M, Tort AB, Kopell NJ, Wisden W, Monyer H. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2009;106:3561–3566. doi: 10.1073/pnas.0813176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Okabe A, Toyoda H, Kilb W, Luhmann HJ, Fukuda A. Cl- uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J Physiol. 2004;557:829–841. doi: 10.1113/jphysiol.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JW, Hanganu-Opatz IL, Sun JJ, Luhmann HJ. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J Neurosci. 2009;29:9011–9025. doi: 10.1523/JNEUROSCI.5646-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Prolonged GABA application decreased spontaneous firing frequency in all newborn cells. Summary plot showing a reduction in mean spontaneous firing frequency following GABA application that is reversed by subsequent GABAAR blockade (control: 1.5 ± 0.3 Hz; GABA: 0.2 ± 0.1 Hz; GABA + GZ: 1.2 ± 0.3; n=24; **, p < 0.01; ***, p < 0.001; one-way ANOVA, Tukey). Representative raw traces showing a reduction in spontaneous firing frequency following prolonged GABA exposure are show in Figure 5A and C. Data are shown as mean ± S.E.M.