Abstract
The transcription factor CHF1/Hey2 has been implicated in a variety of cardiovascular developmental abnormalities including ventricular septal defect, deformed valves and cardiomyopathy. To date, its role in coronary vascular development remains unknown. We have found that KO mice developed coronary vascular abnormalities accompanied by a thin compact ventricular myocardium but grossly normal epicardial and subepicardial layers. The coronary vascular anomalies included dysmorphic large vessels and abnormal vascular structures at E15.5 and reduced recruitment of vascular smooth muscle cells into the coronary arteries at E18.5. In E18.5 KO hearts, the abnormal coronary veins demonstrated reduced expression of markers for vein identity. Whole mount PECAM staining of the E18.5 KO hearts indicated that EphB4 negative vein networks were increased in the surface layers of the myocardium compared to those of the controls. CHF1/Hey2 was not expressed in the epicardium in vivo, and cultured epicardium-derived cells isolated from E12.5 wild type mice showed no CHF1/Hey2 expression. KO mice with a myocardially expressed CHF1/Hey2 transgene partially rescued the vascular phenotypes. Quantitative RT-PCR analysis demonstrated that PDGF and Angiopoietin / Tie2 signaling pathways are altered in E12.5 KO hearts. Taken together, global CHF1/Hey2 deficiency caused impaired vascular formation, the reduced recruitment of vascular smooth muscle cells into coronary arteries and abnormally remodeled vein networks. These findings suggest that CHF1/Hey2 regulates the later steps of coronary vascular development in both a myocardial-dependent, non-cell autonomous fashion and likely a vascular cell- specific effect as well.
Keywords: coronary vascular development, transcription factor, knockout mouse
Introduction
Coronary vessel development is essential for providing the developing myocardium with a blood supply. The epicardium, comprising the outermost cell layer of the postlooped heart, is derived from proepicardium located on the dorsal wall of foregut endoderm. After epithelial-mesenchymal transformation (EMT), the epicardial cells migrate into the myocardial layer and give rise to the vascular smooth muscle cells (VSMCs), endothelial cells (ECs) and fibroblasts of the coronary arteries (Manner, 2006; Ratajska et al., 2008). In line with recent studies, the epicardium is found to regulate the development of the myocardium. The ablation of the epicardium by surgical removal (Manner et al., 2005) or with genetic modifications (Wu et al., 1999; Jenkins et al., 2005; Zamora et al., 2007; Huang et al., 2008; Mahtab et al., 2008) leads to impaired coronary artery development together with a thin wall of myocardium due to the failure of myocardial growth.
We and others have previously identified a family of cardiovascular restricted, bHLH transcription factors related to hairy, a drosophila bHLH gene involved in Notch signaling and patterning of the peripheral nervous system (Kokubo et al., 1999; Leimeister et al., 1999; Nakagawa et al., 1999; Chin et al., 2000; Zhong et al., 2000; Iso et al., 2001). This family has received various names, including Hey, Hesr, HRT, CHF, gridlock and HERP. CHF1/Hey2 has been identified in the ventricle and vasculature during embryonic stages and in the vasculature after birth. The importance of CHF1/Hey2 in the vascular system was demonstrated by gridlock, the ortholog of CHF1/Hey2 in zebrafish (Zhong et al., 2000). Gridlock plays an important role in development of the aorta and in arterial versus venous cell fate decisions (Zhong et al., 2000; Zhong et al., 2001). In mice, there are three subtypes of CHF/Hey genes; CHF1/Hey2, CHF2/Hey1 and HeyL. CHF2/Hey1 knockout mice showed no apparent cardiovascular phenotype, although CHF1/Hey2 and CHF2/Hey1 double KO mice died after embryonic day (E) 9.5 with a global lack of vascular remodeling and massive hemorrhage (Fischer et al., 2004). Large arteries in both Notch1 knockout and CHF1/Hey2 and CHF2/Hey1 double KO mice failed to express arterial endothelial markers indicating that a Notch -CHF/Hey pathway is essential in arterial cell fate decisions (Fischer et al., 2004). However, due to embryonic lethality, a further analysis at later stages has not been done. We have created CHF1/Hey2 KO mice for determination of the function of CHF1/Hey2 in the cardiovascular system. Our results have shown that the myocardial wall is thinned, beginning at E13.5, and the ventricular myocardium ectopically expresses a variety of genes normally restricted to the developing atria and trabeculae on a C57BL/6 background (Koibuchi and Chin, 2007), while only subtle anatomical defects in major systemic arteries were reported (Sakata et al., 2006). To the best of our knowledge, however, the role of CHF1/Hey2 in coronary vascular development has not been investigated.
In this study, we found that these knockout mice also have deformed intramural coronary artery branches, an immature dysmorphic microvasculature and reduced vein identity in the thinned left ventricles. These findings are consistent with a role for CHF1/Hey2 in regulating coronary vascular maturation.
Results
Impaired myocardial vascular formation concomitantly with defective expansion of compact myocardium
To analyze vascular phenotypes in the myocardial layer of CHF1/Hey2 knockout mice, we first did histological analysis of E15.5 hearts (KO n=7, WT n=5). As we previously reported, the compact myocardium of CHF1/Hey2 knockout mice failed to expand at this stage resulting in a thinned myocardium layer. Throughout the inspection of serial sections stained with hematoxylin and eosin, we observed dysmorphic larger blood vessels when compared with hearts of wild-type mice (Figure 1). The cardiac vasculature was notable by the presence of primitive nucleated erythrocytes within the luminal space (Figure 1).
Figure 1.
Altered large blood vessels in left ventricles of CHF1/Hey2 knockout mice. E15.5 embryos of either CHF1/Hey2 knockout mice (n = 7) (b) or wild-type control (n = 5) (a) were stained with hematoxylin and eosin by standard methods. The cardiac vasculature was notable by the presence of primitive nucleated erythrocytes within the luminal space. Scale bars = 100 μm.
To directly address if CHF1/Hey2 deletion causes defects in the myocardial vasculature as well as myocardial maturation, we applied antibody staining of PECAM and also labeling of isolectin B4, markers for endothelial cells, to E13.5-E18.5 CHF1/Hey2 knockout hearts. At E15.5, microvascular formation was subtly altered in CHF1/Hey2 knockout mice (n=7) compared to that of wild-type mice (n=5) (Figure 2). At later stages, the findings became more obvious. While well-developed coronary arteries and veins and a well-ordered punctate pattern of the microvasculature were seen in the myocardium of E18.5 wild-type mice (n=7), a disorganized microvasculature was detected in that of CHF1/Hey2 knockout mice (n=8), although the capillary density seemed to be indistinguishable (Figure 3a, b). In whole-mount PECAM staining, the number of nodules detected around the ventricular groove was significantly increased in CHF1/Hey2 knockout mice at early stages (WT: 19.7 ± 2.4 vs KO: 39.3 ± 2.4; p<0.05) (Figure 4d, e). These nodules have been hypothesized to represent abnormally formed aggregations of endothelial cells that result from impaired development of the fine and continuous coronary plexus (Smart et al., 2007; Huang et al., 2008). Detection of these nodules in the hearts of WT mice suggest that they are normal developmental intermediates that appear prior to the maturation of the coronary plexus. Their presence in greater numbers in the KO hearts is consistent with a perturbation in the developmental process, however, resulting in a hyperplastic coronary venous system observed on the dorsal surface of E18.5 hearts of CHF1/Hey2 knockout mice (KO n=6, WT n=5). This abnormal venous system is characterized by increased vascular arborization covering the entire ventricle (Figure 4). While we cannot be absolutely certain that the PECAM positive vessels are veins, whole mount PECAM staining is known to preferentially detect superficial cardiac veins in late stage embryos, due to the low penetration of the antibody into the heart tissue. For example, an earlier study showed PECAM staining for E18.5 hearts detected primarily veins and was able to detect arteries only with computer enhancement (Zamora et al., 2007). In fact, we tried 3D reconstruction for our PECAM stained E18.5 hearts with optical projection tomography, but were unsuccessful because less than 1/10 depth of the myocardium was stained (unpublished data). We believe that the dysmorphic vasculature as shown in Fig.2 and Fig.3 results from abnormal myocardial signaling to the developing vascular cells, with the increase in surface vasculature resulting as a secondary effect.
Figure 2.
Wall thinning and less differentiated myocardial vasculature in E15.5 left ventricles of CHF1/Hey2 knockout mice. PECAM immunostaining (a-d) and isolectin B4 labeling(e, f) were performed to detect endothelial cells of the myocardial vasculature. The control hearts (a, c; n = 5) generally form fine vessels (c, inset, e, arrows) and some larger vessels with stable lumens (c, inset, e, arrowheads) that give rise to the coronary vasculature. In contrast, mutant hearts (b, d; n = 7) are improperly remodeled and only form collapsed fine vessels (d, inset, f, arrows) and dysmorphic larger vessels (d, inset, f, arrowheads, inset) when compared to those in wild-type. Cos; primordium of costal cartilage, IVS; interventricular septum, MV; mitral valve, Peri; parietal pericardial wall. Scale bars = 1 mm in a, b; 200 μm in c-f.
Figure 3.
Impaired coronary artery maturation and disorganized myocardial vasculature in left ventricles of CHF1/Hey2 knockout mice. PECAM (a, b) and αSMA (c, d) immunostaining were performed for E18.5 embryonic hearts. The control left ventricles (a, c; n=7) generally form well-developed coronary arteries with a distinct circumferential αSMA positive smooth muscle layer (arrows). In contrast, mutant ventricles (n = 8) showed dysmorphic vessels with a partially disrupted smooth muscle layer (d, arrows, inset) within a disorganized myocardial vasculature (b, arrowheads). Scale bars = 200 μm.
Figure 4.
Abnormal embryonic coronary vasculature in CHF1/Hey2 knockout embryonic hearts. Whole-mount PECAM staining of CHF1/Hey2 knockout mice (d (n = 3), e (n = 3) and f (n = 6)) and wild-type control (a (n = 3), b (n = 3) and c (n = 5)) hearts. (a, d) Dorsal view of E12.5 hearts. (b and e) Dorsal view of E15.5 hearts. (c and f) Ventral view of E18.5 hearts. Note the hyperplastic coronary veins in E18.5 hearts of CHF1/Hey2 knockout mice (f).
Impaired recruitment of vascular smooth muscle into coronary arteries of CHF1/Hey2 knockout mice
We next investigated whether there was a reduced incidence of smooth muscle cell recruitment to nascent vessels in CHF1/Hey2 knockout hearts. In control hearts (n=7), smooth muscle cells, detected by immunostaining for αSMA, were present in a continuous cell layer surrounding coronary arteries (Figure 3c, arrows and inset), whereas in the coronary arteries of CHF1/Hey2 knockout hearts (n=8) the smooth muscle layer was discontinuous (Figure 3d, arrows and inset).
Loss of coronary venous endothelial differentiation in CHF1/Hey2 knockout mice
To determine whether arterial and vein identity in the coronary vasculature is altered in CHF1/Hey2 knockout mice, Ephrin B2 and EphB4 expression were analyzed in E18.5 left ventricles (KO n=5, WT n=4). However, we could not determine whether arterial identity was downregulated in CHF1/Hey2 knockout mice because EphrinB2 is expressed not only in endothelium but also in vascular smooth muscle cells of the arteries (Gale et al., 2001) and the intensity of the expression did not seem reliable due to the poor quality of our antibody. On the other hand, expression of EphB4 in coronary veins was diminished in knockout hearts (Figure 5, b, arrows), but was normal in knockout endocardium. In contrast, EphB4 was expressed strongly in coronary veins (Figure 5, a, arrows) as well as endocardium of the control left ventricles.
Figure 5.
Venous identity is reduced in hearts of CHF1/Hey2 knockout mice. EphB4 (a, b) immunostaining were performed for E18.5 embryonic hearts of CHF1/Hey2 knockout mice (n = 5) (b;) and the control (a; n=4). Coronary veins (a, b) were indicated by arrows. LV; left ventricles, Lg; lung, Ao; aorta, Tr; trachea, SVC; superior vena cava. Scale bars = 200 μm.
Non-cell autonomous effects of CHF1/Hey2 on coronary development
Epicardium-derived cells (EPDCs) give rise to coronary vasculature as well as cardiac fibroblasts and to a lesser extent, myocardium. To examine whether CHF1/Hey2 is expressed in epicardium or EPDCs, we performed in situ hybridization and qRT-PCR. In situ hybridization showed that the epicardium did not demonstrate any CHF1/Hey2 signal at E12.5 and E15.5 (Figure 6a and data not shown), while expression in myocardium of wild-type hearts is robust. We also examined the expression levels in EPDCs isolated from E12.5 ventricles of CHF1/Hey2 knockout mice. Expression of CHF1/Hey2 was not detected in EPDCs evaluated by quantitative realtime PCR (Fig. 6b) and RT-PCR (not shown). We next examined whether CHF1/Hey2 regulates the epicardial-mesenchymal transformation of the epicardial cells. A subpopulation of the epicardial cells undergoes EMT and invades the subepicardial space to subsequently give rise to coronary vessels. The subepicardial matrix is a transient structure observed in the mouse hearts between E10.5 and E11.5 that separates the primitive epicardium from the myocardium. There was no difference in epicardium and subepicardial spaces of E11.5 and E13.5 left ventricles between CHF1/Hey2 knockout mice and wild-type mice (Figure 6c-f). At E12.5, WT1, a marker of epicardium, in CHF1/Hey2 knockout hearts was detected in the epicardial layer at a level comparable to that of wild-type hearts (Figure 6g, h).
Figure 6.
CHF1/Hey2 is not expressed in epicardium or EPDCs and does not affect early epicardial development. (a) In situ hybridization with CHF1/Hey2 probe for left ventricle of E15.5 wild-type heart (n = 3). Arrows indicate epicardium. (b) The expression of CHF1/Hey2 RNA in left ventricle and primary isolated epicardial-derived cells (EPDCs) of CHF1/Hey2 knockout mice. Total RNA was obtained from E12.5 left ventricle of wild-type (n = 3) or CHF1/Hey2 knockout mice (n = 3), or EPDC of wild-type (n = 3) and CHF1/Hey2 knockout mice(n = 3) mice and was reverse-transcribed into cDNA and then subjected to real-time PCR using specific primers for CHF1/Hey2. The starting quantities were calculated and expressed as a ratio of each gene to that of Gapd. (c-f) Subepicardial space of left ventricle of wild-type mice (c, E11.5, n = 3; d, E13.5, n = 3) and CHF1/Hey2 knockout mice (e, E11.5, n = 3; f, E13.5, n = 3) were indistinguishable. In situ hybridization with WT1 probe for left ventricles of E12.5 wild-type (n = 3) (g) or CHF1/Hey2 knockout mice (n = 3) (h). Scale bars = 100 μm in a, c-f; 200 μm in g and h.
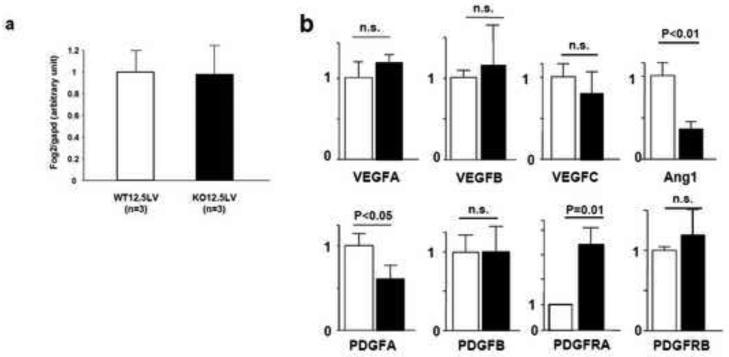
Coronary artery development is regulated by neighboring cells from the compact myocardium (Tevosian et al., 2000; Smart et al., 2007). Thymosin-β4, a G actin monomer binding protein, is secreted from the myocardium and stimulates EPDCs in a paracrine fashion to migrate to the myocardium. FOG-2 (Friend of GATA-2), a protein that physically associates with GATA-4, regulates the development of the coronary vessels. (Svensson et al., 2000; Tevosian et al., 2000). To determine the potential interactions between CHF1/Hey2 and Fog2 or thymosin β4, we examined expression levels in CHF1/Hey2 global knockout embryos. The expression level of Fog2 in left ventricles of E12.5 CHF1/Hey2 knockout mice was comparable to that of wild-type mice determined by qRT-PCR (Fig.8a). Also, E12.5 ventricles of Fog2 knockout mice showed no change of CHF1/Hey2 expression levels (Eric Svensson, personal communication). Unfortunately, the expression of thymosin β4 in E12.5 left ventricles was below the threshold of detection in our assays while the transcript was easily detected in the head region (data not shown).
Figure 8.
Identification of genes involved in vascular abnormalities of CHF1/Hey2 knockout mice. Total RNA obtained from E12.5 left ventricles of wild-type (n = 3) or CHF1/Hey2 knockout mice (n = 3) was reverse-transcribed into cDNA and then subjected to real-time PCR. The starting quantities were calculated and expressed as a ratio of each gene to that of Gapd. (a) Relative expression of Fog2 gene. (b) Relative expression of genes involved in VEGF, PDGF, Angiopoietin pathways. The values were calculated as relative expression levels. White bars indicate wild-type and black bars indicate knockout mice.
We have previously made transgenic mice carrying an mlc2v-driven CHF1/Hey2 transgene that expresses diffusely in all 4 cardiac chambers, but not in epicardium. The transgene can rescue the myocardial phenotypes by suppressing ectopic atrial gene expression (Koibuchi and Chin, 2007), partially rescue the valvular and septation phenotypes in the knockout mice, and improve survival (Sakata et al., 2006). To assess whether this diffusely expressed transgene can restore the recruitment of vascular smooth muscle cells into coronary arteries or rescue vein identity in coronary veins, we performed immunohistochemistry on CHF1/Hey2 knockout embryos carrying this transgene at E18.5. Knockout embryos carrying the transgene showed rescue of the diminished αSMA signals in coronary arteries and restored expression of EphB4 in veins, but did not completely restore normal vessel patterning (Fig. 7). Since we have shown previously that small VSD and valve abnormalities still remained present in some knockout mice with the myocardial transgene (Sakata et al., 2006), it is not surprising that some aspects of the vascular phenotype might also be only partially rescued. Taken together with the finding that CHF1/Hey2 is not expressed in epicardium, the effect of CHF1/Hey2 on coronary vascular development is likely to be partially myocardially-dependent and non-cell autonomous but also may result from vascular cell-specific contributions.
Figure 7.
αSMA and EphB4 expression in coronary arteries of CHF1/Hey2 knockout mice rescued with a myocardial CHF1/Hey2 transgene. E18.5 left ventricles of either CHF1/Hey2 knockout mice (n = 4) (a, b), CHF1/Hey2 knockout with CHF1/Hey2 transgene mice (n = 4) (c, d) or wild-type without transgene mice (n=3)(e, f) were immunostained with anti-αSMA antibody (a, c, e) or anti-EphB4 antibody (b, d, f). Coronary arteries (a, c, e) and veins (b, d, f) were indicated by arrows. Scale bars = 200 μm.
Involvement of PDGF and angiopoietin-1/Tie2 signaling pathways in myocardial vascular phenotypes of CHF1/Hey2 knockout mice
To understand the molecular mechanisms by which CHF1/Hey2 regulates coronary vascular development, we next examined vascular endothelium growth factor (VEGF), platelet-derived growth factor (PDGF), and angiopoietin (Ang) signaling pathways that are known to be involved in vasculogenesis and angiogenesis. Our quantitative RT-PCR data indicated that the expression of Ang-1, PDGFA and PDGFRA were changed in E12.5 hearts of CHF1/Hey2 knockout mice, whereas the expression of all of VEGF genes, PDGFB, and PDGFRB remained relatively unchanged, (Fig. 8b), although Ang-2 was not detected. Of note, coronary artery ingrowth has not yet begun at E12.5, suggesting that these alterations are likely to be primary rather than secondary. Therefore, our data indicate potentially causal alterations in two important signaling pathways known to be involved in coronary maturation (Chen et al., 2008; Shindo et al., 2002), and provide a framework for establishing the CHF1/Hey2-mediated transcriptional network during coronary development.
Discussion
Impaired coronary maturation in CHF1/Hey2 knockout mice
Here we showed that CHF1/Hey2 knockout mice show vascular abnormalities in the developing myocardium characterized by impaired coronary artery development and altered microvascular and vein remodeling. Interestingly, knockout hearts demonstrated no alteration in the morphology of the epicardial and subepicardial layers or in epicardial marker expression at the stage in which EMT and subsequent EPDC migration initiate (Fig.6c-h). We have also found that CHF1/Hey2 is not expressed in the epicardium or in cultured EPDCs that eventually give rise to the coronary arteries (Fig.6a, b). In addition, we have confirmed that isolated EPDCs of CHF1/Hey2 knockout mice are able to differentiate into αSMA positive cells in similar fashion to those of wild-type mice (data not shown). These data suggest that a defect in early epicardial progenitor differentiation is unlikely.
In contrast, the left ventricles of E12.5 knockout demonstrated that the expression levels of pdgfa and Ang-1 were downregulated (Fig.8b). Angiopoietin-1/Tie-2 signaling is involved in myocardial vascular maturation (Chen et al., 2008). Pdgf-A is a downstream target of a zinc-finger transcription factor, KLF5, that regulates cardiovascular remodeling (Shindo et al., 2002). Since PDGF-A in its dimeric form, PDGF-AA, exclusively binds its receptor PDGFR-α (Andrae et al., 2008), upregulation of pdgfra (Fig.8b) might result from decreased PDGF-A by a negative feedback loop. Our data indicate that alterations in these pathways might explain alterations in myocardial vascular development resulting from CHF1/Hey2 loss of function. Although there is a possibility that the thinner myocardium of the mutant requires less perfusion and thus generates decreased signals for vessel growth and enlargement, our findings that both vascular smooth muscle and venous endothelial marker expression are impaired suggest a defect in endothelial cell maturation, smooth muscle recruitment and remodeling that is partially dependent on myocardium, as described below.
Myocardial CHF1/Hey2 is indispensable for coronary maturation
In this study, transgenic expression of CHF1/Hey2 in myocardium partially rescued the vascular phenotypes in the knockout mice, as well as the previously reported thin walled myocardium (Sakata et al., 2006), which suggested that the effect on vascular development is at least partially myocardium-dependent. Recently, mice conditionally lacking CHF1/Hey2 only in the myocardium have been reported to develop an adult onset cardiomyopathy and show ectopic expression of atrial genes in the ventricular myocardium, while mice with endothelial specific deletion of CHF1/Hey2 have no apparent systemic vascular phenotype (Xin et al., 2007). Coronary vascular phenotypes were not assessed in these conditional knockout animals, however, and it remains unclear whether myocardial, endothelial or vascular smooth muscle expression of CHF1/Hey2 is essential for normal coronary vascular development.
CHF1/Hey2 is expressed in myocardium, endothelium and vascular smooth muscle, raising the possibility that the observed vascular defects can arise from defects in any of these tissues. Nkx2.5-driven cardiac muscle specific deletion of CHF1/Hey2 did not affect viability but resulted in later contractile dysfunction without VSDs, while SM22α-driven smooth and cardiac muscle specific deletion of CHF1/Hey2 also resulted in contractile dysfunction and VSDs, with subsequent perinatal death probably due to the presence of VSD (Xin et al., 2007). Assessment of coronary arteries was not done. Although we do not detect CHF1/Hey2 in the epicardium or cultured EPDCs, VEGFR2, Notch1, Jagged1, Dll4 are expressed in the mouse coronary artery (van den Akker et al., 2008) and potentially function upstream of CHF1/Hey2. Accordingly, we cannot exclude the possibility that CHF1/Hey2 becomes transcribed in the coronary vasculature differentiating from EPDCs and plays some role in coronary maturation in situ. Our data showing partial rescue with a myocardial transgene indicates that a significant effect is due to myocardial expression of CHF1/Hey2, although smooth muscle and endothelial effects cannot be ruled out.
Novel role of CHF1/Hey2 in coronary vascular maturation
To date there are no reports on whether CHF1/Hey2 regulates coronary vascular development, although CHF1/Hey2 is known to play a role in systemic vascular development. CHF1/Hey2 and CHF2/Hey1 double KO mice showed impaired arterial differentiation in the yolk sac and major arteries but normal venous differentiation (Fischer et al., 2004). These findings have suggested that CHF1/Hey genes promote systemic arterial differentiation, while venous differentiation is a default phenotype. Surprisingly, our study indicates that CHF1/Hey2 plays a role in coronary venous differentiation and remodeling, as CHF1/Hey2 knockout mice demonstrated less developed intramural coronary veins with downregulated EphB4 expression and hyperplastic major branches of the coronary veins running just below the epicardium. Although the vascular density did not seem to be altered in the myocardium of CHF1/Hey2 knockout mice, the patterning of the microvasculature was altered (Fig2, 3). These findings suggest increased immature and/or remodeled branching of the coronary veins in CHF1/Hey2 knockout mice. We have not been able to detect CHF1/Hey2 expression in coronary veins thus far, indicating that expression is below our limit of detection. The presence of venous defects in conjunction with absence of CHF1/Hey2 expression is consistent with our hypothesis that CHF1/Hey2 is involved in coronary maturation through unknown humoral factors at least partially dependent on myocardial expression of CHF1/Hey2.
Our findings reveal a novel role for CHF1/Hey2 in coronary vascular maturation during mouse development. This effect on coronary vascular development is partially dependent on myocardial expression of CHF1/Hey2, and likely involves as yet undetermined humoral factors. Further investigation to elucidate downstream pathways and tissue-specific requirements will allow delineation of a more precise role for CHF1/Hey2 in coronary vascular maturation.
Experimental Procedures
Animals
The mice lacking CHF1/Hey2 and expressing CHF1/Hey2 under the control of mlc2v promoter on a C57BL/6 background were generated and bred as described previously. (Sakata et al., 2006)
Histology, Immunohistochemistry, and In Situ Hybridization
Preparation, fixation and staining of the tissue sections from embryos for histology and in situ hybridization were performed essentially as described (Sakata et al., 2002). For immunohistochemistry, the freshly dissected hearts were embedded in OCT compound and snap frozen in liquid nitrogen. The hearts were then sectioned serially in the transverse plane (from the base to the apex). The slides were fixed with cooled acetone for 2 min, and incubated in 0.3% hydrogen peroxide solution. After PBS wash, the antibodies were incubated with the tissues for overnight incubation; anti-mouse PECAM-1 (clone MEC13.3, BD Biosciences), anti-mouse α smooth muscle actin antibody (αSMA) (clone 1A4, SIGMA), anti-mouse Ephrin-B2 antibody (AF496, R&D), anti-mouse EphB4 antibody (AF446, R&D). For staining for PECAM-1 and αSMA, biotinylated second antibodies were used, followed by Vectastain ABC-peroxidase reagent and DAB visualization (Vector). The slides were then hydrated and mounted in toluene solution. Isolectin B4 labeling (Vector) was performed to detect endothelial cells of the myocardial vasculature. In situ hybridization was performed as described previously. (Koibuchi and Chin, 2007) The cDNAs used for generation of Digoxigenin-labeled mouse riboprobes were CHF1/Hey2, (Sakata et al., 2006), and WT1 (nucleotides 743 to 1793; GenBank no. NM_144783). At least 3 embryos were examined for each gene.
Whole-mount PECAM staining
After fixation with 4% PFA, tissues were dehydrated in a series of graded methanol baths. Then, the hearts were trimmed of other tissues, incubated in methanol/hydrogen peroxide, rehydrated, and blocked in PBSST (5% goat serum/PBS 0.1% Triton X-100). The primary antibody and biotinylated goat anti-rat IgG was used at 1:200 dilution in PBSST, followed by Vectastain ABC-peroxidase reagent and DAB visualization (Vector) as described in immunohistochemistry for sectioned tissues. Antibody and ABC reagent incubations were carried out overnight at 4°C. Following each overnight incubation, the hearts were washed five times (1 h each at 4°C) with PBSST. After PECAM staining, hearts were photographed and compared.
Culture of epicardial cells from embryonic hearts
Hearts from E12.5 embryos were dissected and ventricular chambers were placed epicardial side down on culture dishes coated with 1% gelatin. The hearts were cultured in DMEM containing 100 units/ml penicillin, 100 μg/ml streptomycin, 10% FBS, and 2 ng/ml basic fibroblast growth factor (B&D, Franklin Lakes, NJ) as described (Zamora et al., 2007). After 48 h, epicardial cell monolayers formed and the explanted hearts were removed. After 2-3 passages, cells were collected for RNA isolation.
RNA isolation and semi-quantitative PCR
RNA was isolated from E12.5 hearts or cultured EPDCs and then extracted with a commercially available kit (PicoPure RNA isolation, Arcturus) according to the manufacturer's instructions. RNA was reverse transcribed into cDNA using an iScript cDNA synthesis kit (Bio-Rad). Real-time PCR was carried out in an ABI7500 Fast Real-Time PCR System (Applied Biosystems) at 95°C for 5 min to activate Taq DNA polymerase, followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec using Power SYBR Green (Applied Biosystems). The expression level of each gene was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (Gapd). The sequences of the primers are shown as follows. Gapd_5′-CTTCCGTGTTCCTACCC-3′ (forward) and 5′-ACCTGGTCCTCAGTGTAGCC3′ (reverse); CHF1 5′-GAGTGCTTGACAGAAGTGGCTA-3′ (forward) and 5′-CACAGGTGCTGAGATGAGAGAC-3′ (reverse); Fog2 5′-ACCAGGAGAGCTAGAAGTGTTT-3′ (forward) and 5′-GGACCTGAGCCTTCGTCTT-3′ (reverse); Vegfa 5′-CTGCCGTCCGATTGAGACC-3′ (forward) and 5′-CCCCTCCTTGTACCACTGTC-3′ (reverse); Vegfb 5′-GCCAGACAGGGTTGCCATAC-3′ (forward) and 5′-GGAGTGGGATGGATGATGTCAG-3′ (reverse); Vegfc 5′-GAGGTCAAGGCTTTTGAAGGC-3′ (forward) and 5′-CTGTCCTGGTATTGAGGGTGG-3′ (reverse); Ang-1 5′-CACATAGGGTGCAGCAACCA-3′ (forward) and 5′-CGTCGTGTTCTGGAAGAATGA-3′ (reverse); Pdgf-a 5′-GAGGAGGAGACAGATGTGAGGT-3′ (forward) and 5′-GGCAATGAAGCACCATACATAG-3′ (reverse); Pdgf-b 5′-AAGTGTGAGACAATAGTGACCCC-3′ (forward) and 5′-CATGGGTGTGCTTAAACTTTCG-3′ (reverse); Pdgfra 5′-TCCATATTCGGTGGAGAAGATT-3′ (forward) and 5′-GCCAATGAACTCACACTAACCA-3′ (reverse); Pdgfrb 5′-CATATCATGAAGCCAGCAAGAG-3′ (forward) and 5′-CTGTCCTCACTGTCCATCATGT-3′ (reverse).
Acknowledgements
This work was supported by a grant from Mitsubishi Pharma Research Foundation (to T.W.), a John L. Locke Foundation grant (to M.T.C.) and NIH grants HL081088 and HL076232 to M.T.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JX, Stinnett A. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arterioscler Thromb Vasc Biol. 2008;28(9):1606–13. doi: 10.1161/ATVBAHA.108.169235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MT, Maemura K, Fukumoto S, Jain MK, Layne MD, Watanabe M, Hsieh CM, Lee ME. Cardiovascular basic helix loop helix factor 1, a novel transcriptional repressor expressed preferentially in the developing and adult cardiovascular system. Role of platelet-derived growth factors in physiology and medicine. J Biol Chem. 2000;275:6381–6387. doi: 10.1074/jbc.275.9.6381. [DOI] [PubMed] [Google Scholar]
- Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM, Yancopoulos GD. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230(2):151–60. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- Huang X, Gao X, Diaz-Trelles R, Ruiz-Lozano P, Wang Z. Coronary development is regulated by ATP-dependent SWI/SNF chromatin remodeling component BAF180. Dev Biol. 2008;319:258–266. doi: 10.1016/j.ydbio.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Iso T, Sartorelli V, Chung G, Shichinohe T, Kedes L, Hamamori Y. HERP, a new primary target of Notch regulated by ligand binding. Mol Cell Biol. 2001;21:6071–6079. doi: 10.1128/MCB.21.17.6071-6079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SJ, Hutson DR, Kubalak SW. Analysis of the proepicardium-epicardium transition during the malformation of the RXRalpha−/− epicardium. Dev Dyn. 2005;233:1091–1101. doi: 10.1002/dvdy.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koibuchi N, Chin MT. CHF1/Hey2 plays a pivotal role in left ventricular maturation through suppression of ectopic atrial gene expression. Circ Res. 2007;100:850–855. doi: 10.1161/01.RES.0000261693.13269.bf. [DOI] [PubMed] [Google Scholar]
- Kokubo H, Lun Y, Johnson RL. Identification and expression of a novel family of bHLH cDNAs related to Drosophila hairy and enhancer of split. Biochem Biophys Res Commun. 1999;260:459–465. doi: 10.1006/bbrc.1999.0880. [DOI] [PubMed] [Google Scholar]
- Leimeister C, Externbrink A, Klamt B, Gessler M. Hey genes: a novel subfamily of hairy- and Enhancer of split related genes specifically expressed during mouse embryogenesis. Mech Dev. 1999;85:173–177. doi: 10.1016/s0925-4773(99)00080-5. [DOI] [PubMed] [Google Scholar]
- Mahtab EA, Wijffels MC, Van Den Akker NM, Hahurij ND, Lie-Venema H, Wisse LJ, Deruiter MC, Uhrin P, Zaujec J, Binder BR, Schalij MJ, Poelmann RE, Gittenberger-De Groot AC. Cardiac malformations and myocardial abnormalities in podoplanin knockout mouse embryos: Correlation with abnormal epicardial development. Dev Dyn. 2008;237:847–857. doi: 10.1002/dvdy.21463. [DOI] [PubMed] [Google Scholar]
- Manner J. Extracardiac tissues and the epigenetic control of myocardial development in vertebrate embryos. Ann Anat. 2006;188:199–212. doi: 10.1016/j.aanat.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Manner J, Schlueter J, Brand T. Experimental analyses of the function of the proepicardium using a new microsurgical procedure to induce loss-of-proepicardial-function in chick embryos. Dev Dyn. 2005;233:1454–1463. doi: 10.1002/dvdy.20487. [DOI] [PubMed] [Google Scholar]
- Nakagawa O, Nakagawa M, Richardson JA, Olson EN, Srivastava D. HRT1, HRT2, and HRT3: a new subclass of bHLH transcription factors marking specific cardiac, somitic, and pharyngeal arch segments. Dev Biol. 1999;216:72–84. doi: 10.1006/dbio.1999.9454. [DOI] [PubMed] [Google Scholar]
- Ratajska A, Czarnowska E, Ciszek B. Embryonic development of the proepicardium and coronary vessels. Int J Dev Biol. 2008;52:229–236. doi: 10.1387/ijdb.072340ar. [DOI] [PubMed] [Google Scholar]
- Sakata Y, Kamei CN, Nakagami H, Bronson R, Liao JK, Chin MT. Ventricular septal defect and cardiomyopathy in mice lacking the transcription factor CHF1/Hey2. Proc Natl Acad Sci U S A. 2002;99:16197–16202. doi: 10.1073/pnas.252648999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata Y, Koibuchi N, Xiang F, Youngblood JM, Kamei CN, Chin MT. The spectrum of cardiovascular anomalies in CHF1/Hey2 deficient mice reveals roles in endocardial cushion, myocardial and vascular maturation. J Mol Cell Cardiol. 2006;40:267–273. doi: 10.1016/j.yjmcc.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Shindo T, Manabe I, Fukushima Y, Tobe K, Aizawa K, Miyamoto S, Kawai-Kowase K, Moriyama N, Imai Y, Kawakami H, Nishimatsu H, Ishikawa T, Suzuki T, Morita H, Maemura K, Sata M, Hirata Y, Komukai M, Kagechika H, Kadowaki T, Kurabayashi M, Nagai R. Krüppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat Med. 2002;8(8):856–63. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- Svensson EC, Huggins GS, Lin H, Clendenin C, Jiang F, Tufts R, Dardik FB, Leiden JM. A syndrome of tricuspid atresia in mice with a targeted mutation of the gene encoding Fog-2. Nat Genet. 2000;25:353–356. doi: 10.1038/77146. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, Fujiwara Y, Orkin SH. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101:729–739. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- van den Akker NM, Caolo V, Wisse LJ, Peters PP, Poelmann RE, Carmeliet P, Molin DG, Gittenberger-de Groot AC. Developmental coronary maturation is disturbed by aberrant cardiac vascular endothelial growth factor expression and Notch signaling. Cardiovasc Res. 2008;78:366–375. doi: 10.1093/cvr/cvm108. [DOI] [PubMed] [Google Scholar]
- Wu H, Lee SH, Gao J, Liu X, Iruela-Arispe ML. Inactivation of erythropoietin leads to defects in cardiac morphogenesis. Development. 1999;126:3597–3605. doi: 10.1242/dev.126.16.3597. [DOI] [PubMed] [Google Scholar]
- Xin M, Small EM, van Rooij E, Qi X, Richardson JA, Srivastava D, Nakagawa O, Olson EN. Essential roles of the bHLH transcription factor Hrt2 in repression of atrial gene expression and maintenance of postnatal cardiac function. Proc Natl Acad Sci U S A. 2007;104:7975–7980. doi: 10.1073/pnas.0702447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora M, Manner J, Ruiz-Lozano P. Epicardium-derived progenitor cells require beta-catenin for coronary artery formation. Proc Natl Acad Sci U S A. 2007;104:18109–18114. doi: 10.1073/pnas.0702415104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414:216–220. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Rosenberg M, Mohideen MA, Weinstein B, Fishman MC. gridlock, an HLH gene required for assembly of the aorta in zebrafish. Science. 2000;287:1820–1824. doi: 10.1126/science.287.5459.1820. [DOI] [PubMed] [Google Scholar]