Abstract
The FMN–heme intraprotein electron transfer (IET) kinetics in a human iNOS oxygenase/FMN (oxyFMN) construct co-expressed with NCaM, a calmodulin (CaM) construct that includes only its N-terminal globular domain, were determined by laser flash photolysis. The IET rate constant is significantly decreased by nearly 4-fold (compared to the iNOS oxyFMN co-expressed with full length CaM). This supports an important role of full length CaM in proper interdomain FMN/heme alignment in iNOS. The IET process was not observed with added excess EDTA, suggesting that Ca2+ depletion results in the FMN domain moving away from the heme domain. The results indicate that a Ca2+-dependent reorganization of the NCaM construct could cause a major modification of the NCaM/iNOS association resulting in a loss of IET.
Keywords: Heme–FMN electron transfer, Nitric oxide synthase, Intraprotein kinetics, Laser flash photolysis, Calmodulin
1. Introduction
Nitric oxide (NO) is a ubiquitous signaling molecule for vasodilation and neurotransmission at low concentrations and a defensive cytotoxin at higher concentrations [1,2]. NO's bioavailability is tightly regulated at the synthesis level by NOS. There are three mammalian NOS isoforms: endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS). Mammalian NOS is a homodimeric flavo-hemoprotein that catalyzes the oxidation of L-arginine (Arg) to NO and L-citrulline with NADPH and O2 as co-substrates [3]. Each subunit contains a C-terminal electron-supplying reductase domain with binding sites for NADPH (the electron source), FAD and FMN, and an N-terminal catalytic heme-containing oxygenase domain. The oxygenase and reductase domains are connected by a calmodulin (CaM)-binding region. The iNOS isoform binds CaM irreversibly while nNOS and eNOS bind CaM reversibly in response to intracellular Ca2+ concentration [3,4]. The major difference between eNOS/nNOS and iNOS is the presence of internal control elements [4], including an unique autoregulatory insert within the FMN domain of eNOS/nNOS [5] and the C-terminal tail [6].
The interdomain (intraprotein) electron transfer (IET) processes are key steps in NO synthesis by coupling reactions between the domains [3,4,7]. Uncoupled or partially coupled NOS results in synthesis of reactive oxygen species such as superoxide and peroxynitrite [8]. In particular, the CaM-controlled intersubunit FMN–heme IET is essential in coupling electron transfer in the reductase domain with NO synthesis in the heme domain by delivery of electrons required for O2 activation at the catalytic heme site [9]. It is generally accepted that CaM-binding has little or no effect on the thermodynamics of redox processes in NOS [10-13], indicating that the kinetic regulation of the IET processes within the enzyme by CaM binding is accomplished dynamically through controlling conformational changes required for effective IET. It is of current interest to study the CaM-modulated FMN–heme IET in the NOS isoforms [7,14,15].
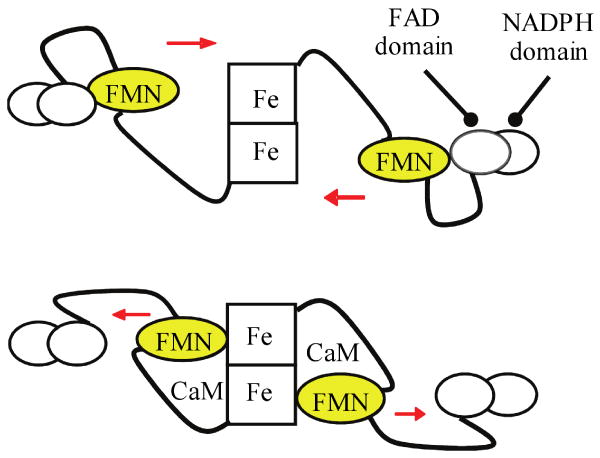
An “FMN-domain tethered shuttle” model (Fig. 1) was recently proposed [16,17], and supported by IET kinetic studies [17-26] and low temperature MCD data [27]. This model involves the swinging of the FMN domain from its original electron-accepting (input) state to a new electron-donating (output) state. This molecular rearrangement facilitates efficient IET between the FMN and the catalytic heme in the oxygenase domain. Calcium-dependent CaM binding to eNOS/nNOS unlocks the input state, thereby enabling the FMN domain to shuttle between the FAD and heme domains [17,20]. The structure of the functional output state has not yet been determined. To favor observation of the output state, bi-domain NOS oxyFMN constructs, in which only the oxygenase and FMN domains, along with the CaM binding region, are expressed, were recently designed and constructed [22,28,29]. Biochemical, kinetic and spectroscopic results have shown that these homologous dimeric oxyFMN constructs are validated models of the NOS output state for NO production [17,21,27,28].
Fig. 1.
Tethered shuttle model. Top: input state; bottom: putative output state. During NOS catalysis, the FMN domain plays a central role by acting as both an electron acceptor (receiving electrons from FAD) and an electron donor (to the catalytic heme center), and is proposed to undergo large conformational movements and engage in two distinct interdomain interactions in the process. The output state is envisioned as an IET-competent complex between the heme and FMN-binding domains. CaM binding to eNOS/nNOS unlocks the input state, thereby enabling the FMN domain to shuttle between the FAD and heme domains. CaM is also required for proper alignment of the FMN and heme domains in iNOS, as indicated by this study.
Emerging evidence indicates that CaM is also important for proper alignment of the FMN and heme domains in iNOS [18,30]. It is challenging to study the CaM/iNOS interactions because CaM binds to iNOS very tightly, and the iNOS protein needs to be co-expressed with CaM due to its propensity to aggregate when residues of the highly hydrophobic CaM-binding domain are exposed to an aqueous environment [31]. The present study indicates that IET in a human iNOS oxyFMN protein co-expressed with NCaM (iNOS oxyFMN-NCaM) is Ca2+-sensitive, suggesting that Ca2+ depletion results in the FMN domain moving away from the heme domain. Note that NCaM includes only the N-terminal Ca2+-binding domains without the central linker region (CaM residues 1–75); circular dichroism spectroscopy showed that the truncated NCaM construct folds properly and is soluble in the absence of iNOS [32]. The IET kinetic results in this work support an important role of CaM in proper alignment of the FMN and heme domains in iNOS.
2. Materials and Methods
2.1. Expression and Purification of Human iNOS oxyFMN-NCaM
The human iNOS oxyFMN construct carries a deletion of the first 70 amino acids and an N-terminal polyhistidine tag; it consists of residues 71-723 of the human iNOS enzyme subcloned into pCWori+. The expression vector for iNOS oxyFMN was co-expressed with NCaM in E. coli BL21 (DE3) [13,26]. Purification of the iNOS oxyFMN-NCaM protein was performed as previously described [13]. The protein was characterized by UV-vis spectrum and gel electrophoresis (Figs. S1 and S2 in Supplementary Material).
2.2. Laser Flash Photolysis
CO photolysis experiments were performed at room temperature, and the sample was kept in ice between flashes to stabilize the protein, as previously described [17,20,21]; data from ∼ 20 laser flashes were averaged. Briefly, a solution containing 20 μM 5-deazariboflavin (dRF) and 5 mM fresh semicarbazide in pH 7.6 buffer (40 mM Bis-Tris propane, 400 mM NaCl, 2 mM L-Arg, 20 μM H4B, 1 mM Ca2+ and 10 % glycerol) was degassed in a laser photolysis cuvette by a mixture of Ar and CO (with a ratio of ∼ 3:1). Aliquots of concentrated iNOS oxyFMN protein (200 μM) were subsequently injected through a septum to achieve the desired protein concentration, and the solution was kept in ice and further purged by passing the Ar/CO mixture over the solution surface for 40 min to remove minor oxygen contamination before being subjected to illumination. The iNOS solution in cuvette was then illuminated for a certain period of time to obtain a partially reduced form of [Fe(II)–CO][FMNH͘], a process that can be followed spectrophotometrically by characteristic maxima of Fe(II)–CO and FMNH͘ at 446 nm and 580 nm, respectively. The partially reduced protein was subsequently flashed with 450 nm laser excitation to dissociate CO from Fe(II)–CO, and to generate a transient Fe(II) species that is able to transfer one electron to the FMNH͘ to produce FMNH2 and Fe(III). This latter IET process was followed spectrally by the time-resolved loss of absorbance of FMNH͘ at 580 nm.
3. Results and Discussion
3.1. The FMN–heme IET in iNOS oxyFMN construct co-expressed with NCaM
Upon CO photolysis, the absorption of the partially reduced iNOS oxyFMN-NCaM at 580 nm decays below the pre-flash baseline (due to the FMN–heme IET, eq 1, resulting in FMNH͘ depletion), with a rate constant of 93 ± 9 s-1 (Fig. 2a), followed by a slow recovery toward baseline (due to CO re-binding to Fe(II)) with a rate constant of 2.2 ± 0.5 s-1 (Fig. 2b). Note the spectral “transition” (i.e. a reversal in direction of absorption changes over time) in the 580 nm trace (Fig. 2b). Importantly, the rate constant of the rapid decay is independent of the signal amplitude, i.e. protein concentration (data not shown), indicating an intra-protein process.
Fig. 2.
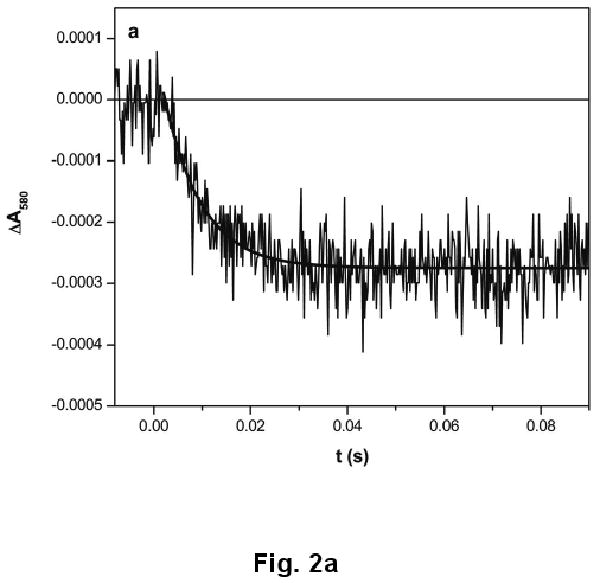
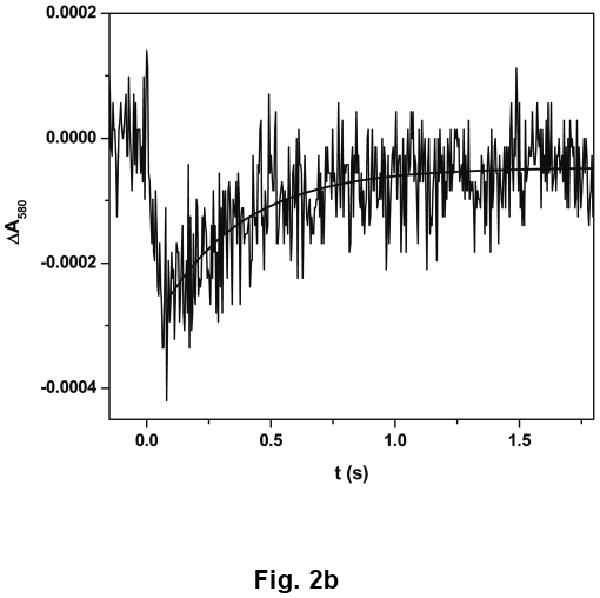
Transient trace at 580 nm at (a) 0 – 0.1 s and (b) 0 – 2 s obtained for the [Fe(II)–CO][FMNH͘] form of human iNOS oxyFMN-NCaM flashed by 450 nm laser excitation. The IET rate obtained from the decay (panel a) is independent of the signal amplitude (not shown), confirming an intramolecular process. The trace at a longer time scale (panel b) is due to CO-rebinding process. Anaerobic solutions contained 20 μM iNOS, ∼ 20 μM dRF and 5 mM fresh semicarbazide in pH 7.6 buffer (40 mM bis-Tris propane, 400 mM NaCl, 2 mM L-Arg, 1 mM Ca2+, and 10% glycerol). The sample was well degassed by Ar/CO (3:1) before illumination.
| eq 1 |
Note that the IET rate constant in the iNOS oxyFMN-NCaM construct is significantly decreased by approximately 4-fold (compared to human iNOS oxyFMN construct co-expressed with full length CaM [13]). This supports an important role of full length CaM in proper alignment of the FMN and heme domains in iNOS. Regarding the NOS regions involved in the eNOS/nNOS activation by CaM-binding, protein interaction with the canonical CaM-binding site alone is also insufficient to promote the NO production process [30,33].
That the deletion of the CaM C-terminal domains and central linker leads to a significant decrease in the FMN–heme IET rate constant is consistent with the diminished NO production when the iNOS enzyme was co-expressed with NCaM [26]. On the other hand, the amplitude of the IET kinetic traces for an autoregulatory (AR)-deletion mutant of nNOS was decreased two- to three-fold, whereas the AR deletion did not change the rate constant for the CaM-controlled IET. This indicates that the CaM/NOS interaction is a dominant factor in controlling the alignment of the FMN and heme domains, while the AR insert is involved in enhancing or stabilizing the productive docking.
It is also important to note that NCaM (i.e. the N-terminal globular domain of CaM) still permits the IET to take place at a significant rate. This truncated CaM also supports NO production by holo-iNOS at about 70% of the normal rate, suggesting that NCaM contains important binding and activating elements for iNOS [31]. Taken together these results indicate that the N-terminal half of CaM is required for the IET and NO production in iNOS.
3.2. The FMN–heme IET in the iNOS oxyFMN-NCaM in the presence of EDTA
In order to ascertain the influence of Ca2+ on the IET process in the iNOS oxyFMN-NCaM construct, the effect of EDTA addition was determined. Fig. 3 shows the transient trace at 580 nm obtained by flashing the partially reduced iNOS oxyFMN-NCaM in the presence of 560 μM EDTA with 450 nm laser excitation. Note the absence of the rapid decay, indicating no observed FMN–heme IET. Upon adding excess calcium (final concentration = 1.2 mM), the IET phase was recovered (data not shown). The loss of NO production by holo-iNOS co-expressed with NCaM in the presence of excess EDTA has also been shown to be reversible, when assayed by the oxyhemoglobin capture assay [31]. These results suggest that Ca2+ depletion caused by the presence of EDTA results in the FMN domain moving away from the heme domain.
Fig. 3.
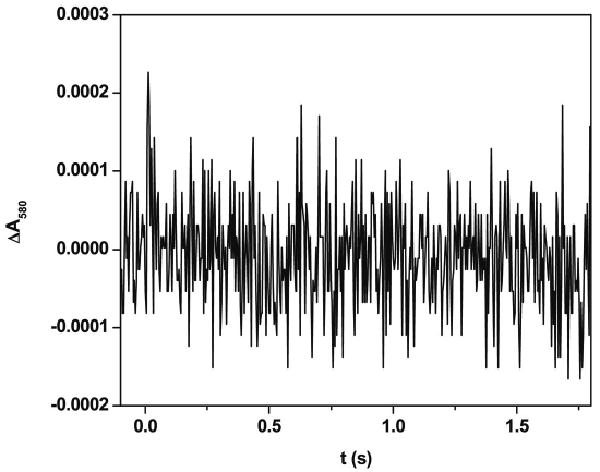
Transient trace at 580 nm at 0 – 2 s obtained for [Fe(II)–CO][FMNH͘] form of the iNOS oxyFMN-NCaM with added EDTA (560 μM) flashed by 450 nm laser excitation. Note that the trace does not have a marked transition phase, in contrast to the EDTA-free sample (Fig. 2b).
It was recently shown that the iNOS holoenzyme co-expressed with NCaM displayed no apparent activity in the presence of EDTA [31]. These results and the present IET results indicate that a Ca2+-dependent reorganization of the bound truncated NCaM construct could cause a major modification of the NCaM/iNOS association resulting in a loss of IET. It will be interesting to study the role of other CaM domains in the alignment of iNOS FMN and heme domains, a key component of NOS catalysis, by using iNOS proteins co-expressed with modified CaM constructs.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM081811 and HL091280 to C.F.), AHA Grant-in-Aid (09GRNT2220310 to C.F.), and NSERC (183521 to J.G.G.). The project described was also supported by Grant Number P20RR016480 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health.
Abbreviations
- NO
nitric oxide
- NOS
nitric oxide synthase
- iNOS
inducible NOS
- nNOS
neuronal NOS
- CaM
calmodulin
- NCaM
truncated N-terminal globular domain of calmodulin consisting of residues 1-75
- oxyFMN
bi-domain NOS construct in which only the heme-containing oxygenase and FMN domains along with the CaM binding region are present
- iNOS oxyFMN-NCaM
iNOS oxyFMN construct co-expressed with NCaM
- IET
intraprotein electron transfer
- dRF
5-deazariboflavin
- H4B
6R-5,6,7,8-ttrahydrobiopterin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Changjian Feng, Email: cfeng@salud.unm.edu.
Gordon Tollin, Email: gtollin@u.arizona.edu.
References
- 1.Schmidt H, Walter U. NO at work. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. British Journal of Pharmacology. 2006;147:S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: Structure, function and inhibition. Biochemical Journal. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roman LJ, Martasek P, Masters BSS. Intrinsic and extrinsic modulation of nitric oxide synthase activity. Chemical Reviews. 2002;102:1179–1189. doi: 10.1021/cr000661e. [DOI] [PubMed] [Google Scholar]
- 5.Salerno JC, et al. An autoinhibitory control element defines calcium-regulated isoforms of nitric oxide synthase. Journal of Biological Chemistry. 1997;272:29769–29777. doi: 10.1074/jbc.272.47.29769. [DOI] [PubMed] [Google Scholar]
- 6.Roman LJ, Martasek P, Miller RT, Harris DE, de la Garza MA, Shea TM, Kim JJP, Masters BSS. The C termini of constitutive nitric-oxide synthases control electron flow through the flavin and heme domains and affect modulation by calmodulin. Journal of Biological Chemistry. 2000;275:29225–29232. doi: 10.1074/jbc.M004766200. [DOI] [PubMed] [Google Scholar]
- 7.Feng CJ, Tollin G. Regulation of interdomain electron transfer in the NOS output state for NO production. Dalton Transactions. 2009:6692–6700. doi: 10.1039/b902884f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CA, Druhan LJ, Varadharaj S, Chen YR, Zweier JL. Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. Journal of Biological Chemistry. 2008;283:27038–27047. doi: 10.1074/jbc.M802269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panda K, Ghosh S, Stuehr DJ. Calmodulin activates intersubunit electron transfer in the neuronal nitric-oxide synthase dimer. Journal of Biological Chemistry. 2001;276:23349–23356. doi: 10.1074/jbc.M100687200. [DOI] [PubMed] [Google Scholar]
- 10.Dunford AJ, Rigby SEJ, Hay S, Munro AW, Scrutton NS. Conformational and thermodynamic control of electron transfer in neuronal nitric oxide synthase. Biochemistry. 2007;46:5018–5029. doi: 10.1021/bi7001339. [DOI] [PubMed] [Google Scholar]
- 11.Noble MA, et al. Potentiometric analysis of the flavin cofactors of neuronal nitric oxide synthase. Biochemistry. 1999;38:16413–16418. doi: 10.1021/bi992150w. [DOI] [PubMed] [Google Scholar]
- 12.Gao YT, et al. Thermodynamics of oxidation-reduction reactions in mammalian nitric-oxide synthase Isoforms. Journal of Biological Chemistry. 2004;279:18759–18766. doi: 10.1074/jbc.M308936200. [DOI] [PubMed] [Google Scholar]
- 13.Daff S, et al. Control of electron transfer in neuronal NO synthase. Biochemical Society Transactions. 2001;29:147–152. doi: 10.1042/0300-5127:0290147. [DOI] [PubMed] [Google Scholar]
- 14.Astashkin AV, Elmore BO, Fan W, Guillemette JG, Feng C. Pulsed EPR determination of the distance between heme iron and FMN centers in a human inducible nitric oxide synthase. Journal of the American Chemical Society. 2010;132:12059–12067. doi: 10.1021/ja104461p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuehr DJ, Tejero J, Haque MM. Structural and mechanistic aspects of flavoproteins: electron transfer through the nitric oxide synthase flavoprotein domain. FEBS Journal. 2009;276:3959–3974. doi: 10.1111/j.1742-4658.2009.07120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh DK, Salerno JC. Nitric oxide synthases: Domain structure and alignment in enzyme function and control. Frontiers in Bioscience. 2003;8:D193–D209. doi: 10.2741/959. [DOI] [PubMed] [Google Scholar]
- 17.Feng CJ, Tollin G, Holliday MA, Thomas C, Salerno JC, Enemark JH, Ghosh DK. Intraprotein electron transfer in a two-domain construct of neuronal nitric oxide synthase: The output state in nitric oxide formation. Biochemistry. 2006;45:6354–6362. doi: 10.1021/bi060223n. [DOI] [PubMed] [Google Scholar]
- 18.Feng CJ, et al. Intraprotein electron transfer in inducible nitric oxide synthase holoenzyme. Journal of Biological Inorganic Chemistry. 2009;14:133–142. doi: 10.1007/s00775-008-0431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng CJ, Roman LJ, Hazzard JT, Ghosh DK, Tollin G, Masters BSS. Deletion of the autoregulatory insert modulates intraprotein electron transfer in rat neuronal nitric oxide synthase. FEBS Letters. 2008;582:2768–2772. doi: 10.1016/j.febslet.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng CJ, Tollin G, Hazzard JT, Nahm NJ, Guillemette JG, Salerno JC, Ghosh DK. Direct measurement by laser flash photolysis of intraprotein electron transfer in a rat neuronal nitric oxide synthase. Journal of the American Chemical Society. 2007;129:5621–5629. doi: 10.1021/ja068685b. [DOI] [PubMed] [Google Scholar]
- 21.Feng CJ, Thomas C, Holliday MA, Tollin G, Salerno JC, Ghosh DK, Enemark JH. Direct measurement by laser flash photolysis of intramolecular electron transfer in a two-domain construct of murine inducible nitric oxide synthase. Journal of the American Chemical Society. 2006;128:3808–3811. doi: 10.1021/ja0578606. [DOI] [PubMed] [Google Scholar]
- 22.Ilagan RP, et al. Regulation of FMN subdomain interactions and function in neuronal nitric oxide synthase. Biochemistry. 2009;48:3864–3876. doi: 10.1021/bi8021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Das A, Sibhatu H, Jamal J, Sligar SG, Poulos TL. Exploring the electron transfer properties of neuronal nitric oxide synthase by reversal of the FMN redox potential. Journal of Biological Chemistry. 2008;283:34762–34772. doi: 10.1074/jbc.M806949200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilagan RP, Tiso M, Konas DW, Hemann C, Durra D, Hille R, Stuehr DJ. Differences in a conformational equilibrium distinguish catalysis by the endothelial and neuronal nitric-oxide synthase flavoproteins. Journal of Biological Chemistry. 2008;283:19603–19615. doi: 10.1074/jbc.M802914200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welland A, Garnaud PE, Kitamura M, Miles CS, Daff S. Importance of the domain-domain interface to the catalytic action of the NO synthase reductase domain. Biochemistry. 2008;47:9771–9780. doi: 10.1021/bi800787m. [DOI] [PubMed] [Google Scholar]
- 26.Haque MM, Panda K, Tejero J, Aulak KS, Fadlalla MA, Mustovich AT, Stuehr DJ. A connecting hinge represses the activity of endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9254–9259. doi: 10.1073/pnas.0700332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sempombe J, Elmore BO, Sun X, Dupont A, Ghosh DK, Guillemette JG, Kirk ML, Feng C. Mutations in the FMN domain modulate MCD spectra of the heme site in the oxygenase domain of inducible nitric oxide synthase. Journal of the American Chemical Society. 2009;131:6940–6941. doi: 10.1021/ja902141v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh DK, Holliday MA, Thomas C, Weinberg JB, Smith SME, Salerno JC. Nitric-oxide synthase output state - Design and properties of nitric-oxide synthase oxygenase/FMN domain constructs. Journal of Biological Chemistry. 2006;281:14173–14183. doi: 10.1074/jbc.M509937200. [DOI] [PubMed] [Google Scholar]
- 29.Li HY, Igarashi J, Jamal J, Yang WP, Poulos TL. Structural studies of constitutive nitric oxide synthases with diatomic ligands bound. Journal of Biological Inorganic Chemistry. 2006;11:753–768. doi: 10.1007/s00775-006-0123-8. [DOI] [PubMed] [Google Scholar]
- 30.Newman E, et al. Differential activation of nitric-oxide synthase isozymes by calmodulin-troponin C chimeras. Journal of Biological Chemistry. 2004;279:33547–33557. doi: 10.1074/jbc.M403892200. [DOI] [PubMed] [Google Scholar]
- 31.Spratt DE, Newman E, Mosher J, Ghosh DK, Salerno JC, Guillemette JG. Binding and activation of nitric oxide synthase isozymes by calmodulin EF hand pairs. FEBS Journal. 2006;273:1759–1771. doi: 10.1111/j.1742-4658.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- 32.Spratt DE, Taiakina V, Palmer M, Guillemette JG. Differential binding of calmodulin domains to constitutive and inducible nitric oxide synthase enzymes. Biochemistry. 2007;46:8288–8300. doi: 10.1021/bi062130b. [DOI] [PubMed] [Google Scholar]
- 33.Su ZZ, Blazing MA, Fan D, George SE. The calmodulin-nitric oxide synthase interaction - Critical role of the calmodulin latch domain in enzyme activation. Journal of Biological Chemistry. 1995;270:29117–29122. doi: 10.1074/jbc.270.49.29117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.