Abstract
Symptoms of neuropathic spinal cord injury (SCI) pain include evoked cutaneous hypersensitivity and spontaneous pain, which can be present below the level of the injury. Adverse side-effects obtained with currently available analgesics complicate effective pain management in SCI patients. Voltage-gated Na+ channels expressed in primary afferent nociceptors have been identified to mediate persistent hyperexcitability in dorsal root ganglia (DRG) neurons, which in part underlies the symptoms of nerve injury-induced pain. Ambroxol has previously demonstrated antinociceptive effects in rat chronic pain models and has also shown to potently block Na+ channel current in DRG neurons. Ambroxol was tested in rats that underwent a mid-thoracic spinal cord compression injury. Injured rats demonstrated robust hind paw (below-level) heat and mechanical hypersensitivity. Orally administered ambroxol significantly attenuated below-level hypersensitivity at doses that did not affect performance on the rotarod test. Intrathecal injection of ambroxol did not ameliorate below-level hypersensitivity. The current data suggest that ambroxol could be effective for clinical neuropathic SCI pain. Furthermore, the data suggests that peripherally expressed Na+ channels could lend themselves as targets for the development of pharmacotherapies for SCI pain.
Keywords: allodynia, hyperalgesia, local anesthetic, mucolytic, off-label use, voltage-gated Na+ channel
1. Introduction
In patients with spinal cord injury (SCI), moderate to severe persistent pain is common and may worsen over time (Cruz-Almeida et al., 2005; Jensen et al., 2005; Siddall et al., 2003). Pain following SCI inhibits full participation in physical rehabilitation and greatly diminishes quality of life (Cruz-Almeida et al., 2005; Widerstrom-Noga et al., 2001). Although some pharmacotherapies provide pain relief to SCI patients, their regular usage is limited by both CNS and autonomic-mediated adverse side-effects (Turner et al., 2001; Warms et al., 2002). Thus, new analgesic drugs with the special needs of SCI patients in mind are needed.
Systemically administered voltage-gated Na+ channel blockers, such as the local anesthetic lidocaine, are effective in ameliorating central as well as peripheral neuropathic pain. However, debilitating side-effects such as dizziness, somnolence and motor dysfunction coincide with the efficacy in this class of drug (Attal et al., 2004; Finnerup et al., 2005). Sodium channel subtypes expressed predominantly in primary afferent nociceptive neurons, but not cardiovascular tissue or the CNS, are promising targets for analgesic drug development with reduced potential for target-mediated adverse side-effects (Cummins et al., 2007; Krafte and Bannon, 2008; McGowan et al., 2009). The efficacy of peripherally acting drugs in general has not been evaluated in neuropathic SCI pain.
Ambroxol (2-Amino-3,5-dibromo-N-(trans-4-hydroxycyclohexyl)benzylamine) has been marked in the EU and other countries (but not in the U.S.) since 1978 as a mucolytic drug (Weiser, 2008). Ambroxol clears mucus resulting from respiratory disease by increasing the clearance of mucus from the respiratory tract (Fig. 1). One way by which ambroxol does this is decreasing the viscosity of mucus by increasing the release of surfactant from alveoli (Malerba and Ragnoli, 2008). An early clinical observation in patients with “airway disorders” reported improvement in “painful complaint” with ambroxol applied via an inhaler to the oropharynx (Hoffmann, 1978). More recent double-blinded, placebo-controlled clinical trials in patients with acute pharyngitis confirmed pain relief with ambroxol treatment (de Mey et al., 2008; Fischer et al., 2002). Only recently, however, has a mechanism been proposed to explain the local anesthetic effect, which is unrelated to the mucolytic effect. Ambroxol has been shown to decrease the tetrodotoxin-resistant (TTX-R) Na+ channel current found in dorsal root ganglia (DRG) neurons and, furthermore, demonstrated antinociceptive effects in rat models of peripheral nerve injury, inflammation and in the formalin test (Gaida et al., 2005; Leffler et al., 2010; Weiser, 2006, 2008). In addition, since ambroxol does not cross the blood-brain barrier, the antinociceptive effect could be mainly due to blocking of Na+ channels expressed in primary afferent nociceptors.
Figure 1.
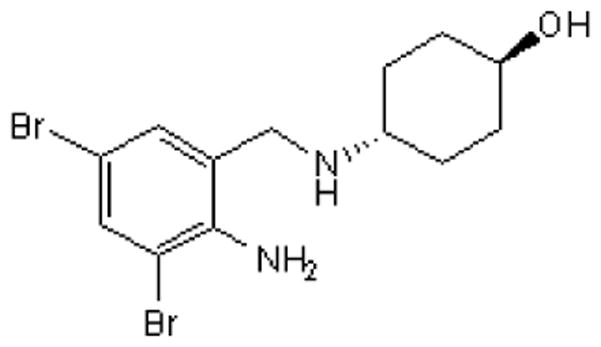
Chemical structure of ambroxol.
Some of the symptoms of chronic SCI pain resemble that of peripheral neuropathic pain, with the presence of spontaneous pain and hypersensitivity to cutaneous stimulation (Felix et al., 2007; Yezierski, 2000). Curiously, neuropathic symptoms may exist not only at-level but also below the level of SCI. A number of genetic and neurochemical changes in the spinal cord following SCI have been implicated in the initiation and maintenance of the chronic SCI pain state, resulting in increased spontaneous and evoked activity in dorsal horn neurons below the level of injury. Changes in glial function following SCI, observed several segments caudal to the lesion, have also been implicated in the maintenance of neuropathic SCI pain (Hains et al., 2002; Hains et al., 2003; Hulsebosch et al., 2008; Yezierski, 2000; Zhang et al., 1994). The development of treatments for neuropathic SCI pain has focused on these central changes.
Changes in peripheral nociceptor Na+ channel function and distribution have been reported in rat chronic pain models and in humans following nerve injury (Coward et al., 2000; Cummins et al., 2007; Hudmon et al., 2008; Yiangou et al., 2000). Compounds acting at peripheral sites may be clinically more favorable in terms of decreased side-effects (Krafte and Bannon, 2008). Changes in peripheral nerve function, many segments distal to a spinal lesion, have also been reported (Carlton et al., 2009; Lin et al., 2007). As with direct injury to the nerve, it is possible that gene and protein expression is also changed -- Na+ channel distribution within apparently undamaged peripheral nerves could also be altered following SCI.
The positive pre-clinical findings from various models of peripheral tissue injury-mediated pain suggests that ambroxol could be a clinically useful analgesic. Pharmacokinetic data in both humans and animals have been generated and ambroxol has an extensive clinical safety record (Table 1). However, since few drugs that are efficacious in these models are efficacious in a rat model of neuropathic SCI pain, it is not known if efficacy in peripheral tissue injury models will translate to efficacy in clinical neuropathic SCI pain (Hama and Sagen, 2007). Thus, the current study evaluated the efficacy of ambroxol in rats with neuropathic SCI pain.
Table 1.
Pharmacokinetic properties of orally administered ambroxol
| Rat | Human | |
|---|---|---|
| Peak plasma concentration (Cmax), μM | ||
| 300 mg/kg | 2.2 | -- |
| 1000 mg/kg | 7.2 | -- |
| 30 mg | -- | 0.2 |
| Time to peak Cmax (Tmax), h | 0.5 | 1.6 |
| Half-life (T1/2), h | n.a. | 9.7 |
| Mean residence time, h | 2 | 9.2 |
| Absolute bioavailability (F %) | n.a. | 79 |
| 50% lethal dose (LD50), mg/kg | 13,400 | -- |
Cmax was measured 90–130 min post-dosing Gaida et al. (2005). Pharmacological data compiled from Gaida et al., 2005; Kubo et al., 2004; Malerba and Ragnoli, 2008; Miyazaki et al., 2005.
n.a., not available
2. Material and methods
2.1 Animals
Male Sprague-Dawley rats (Harlan, IN) were used and had free access to food and water. Rats were housed in pairs, before and after surgery, and were kept in the vivarium on a 12 hr light:dark cycle. Rats were between 100–150 g upon arrival to the vivarium. The procedures were reviewed and approved by the University of Miami of Animal Care and Use Committee.
2.2 Spinal cord injury surgery
A previously described procedure utilizing a micro-vascular clip was used to induce a mid-thoracic SCI (Hama and Sagen, 2007). Rats were anesthetized with isoflurane in O2 and the back was shaved from the cervical to lumbar vertebral level. Rats were maintained on isoflurane in O2 and placed prone on the surgery table. Using aseptic technique, the paraspinal muscles were bluntly dissected to expose vertebrae T6-T7. Mid thoracic spinal segment, approximately T6-T7, was exposed via laminectomy. A micro-vascular clip (15–20 g; Harvard Apparatus, MA) was vertically applied over the exposed spinal segment and compressed the cord for 1 min. The clip was then removed and the muscles and skin closed. Rats were returned to their home cages to recover. No antibiotic or post-operative analgesic was administered to these rats. Rats with a SCI were tested beginning 4 wks following spinal surgery. At the end of the drug study rats were humanly euthanized with CO2 overdose.
2.21 Intrathecal catheters
Using aseptic technique, indwelling intrathecal (i.t.) catheters were implanted in 6 rats three weeks following SCI surgery (Yaksh and Rudy, 1976). Rats were anesthetized with isoflurane/O2 and the atlanto-occipital membrane was exposed. A catheter was introduced into the subarachnoid space and the internalized portion ended at the lumbar enlargement (ReCathCo, PA). Rats were tested one week after intrathecal surgery (i.e. four weeks after SCI surgery). The SCI rats had impaired hind limb function prior to i.t. surgery (see Results), but not complete paralysis. Thus, rats that displayed complete hind limb paralysis following i.t. surgery were excluded from the study. Rats were tested on two different days separated by at least 24 hrs. On day 1, rats were tested with heat and on day 2 rats were tested with von Frey filaments (see below).
At the end of behavioral testing, 5 μl of 1.5% (i.e. 75 μg) lidocaine HCl (in saline) was i.t. injected to confirm the placement of the internal end at the level of the lumbar enlargement. A bi-lateral flaccid paralysis of the hind limbs confirmed proper catheter placement.
2.3 Behavioral testing
Because of the need to acclimate rats to different chambers prior to testing, it was not possible to test each drug-treated rat with both mechanical and heat stimuli. Thus, separate groups of rats were assigned to a particular stimulus.
2.31 Mechanical hypersensitivity
Rats were placed in clear Plexiglas containers resting on an elevated wire mesh floor and allowed to acclimate to the chamber for at least 30 min. Eight specific calibrated von Frey filaments were used via the up-down method to determine the withdrawal threshold (grams) of the hind paws (Stoelting Co., IL). The filaments were applied to the plantar hind paw, bent slightly and held on the rat skin for six seconds (Chaplan et al., 1994). The 50% withdrawal threshold was calculated from the pattern of responses to the filaments (Chaplan et al., 1994). An upper limit of 15 g was used.
The amount of force needed to evoke a withdrawal was reported in “grams” in the current study, although force is usually reported in “newtons”. There is a lack of consistency in the use of units of force needed to evoke a response (withdrawal) across clinical and pre-clinical studies (Attal et al., 2004; Gaida et al., 2005; Jarvis et al., 2007; Wallace et al., 2000). In keeping with the original description of the method (Chaplan et al., 1994) as well as to facilitate comparison across the majority of pre-clinical pharmacological studies, the current study will report thresholds in grams.
2.32 Heat hypersensitivity
A commercially available apparatus was used to assess sensitivity of the plantar hind paws to a brief noxious heat stimulus (Hargreaves et al., 1988). A rat was placed in a Plexiglas enclosure that rested on an elevated glass floor. After an acclimating period of 30 min, an infrared emitter that was under the glass floor was positioned directly beneath the mid-plantar hind paw. A hind paw withdrawal stopped the infrared stimulus. The length of time between the onset of the stimulus and the paw withdrawal is the withdrawal latency (sec). The average of three trials per paw was used to report the final withdrawal latency. Testing of the same paw was separated by about 5 min. To prevent tissue damage, a cut-off of 20 sec. was used.
Following measurement of baseline withdrawal threshold or latency, rats were dosed p.o. with either drug or vehicle and tested 30, 60, 90, 120, 150 and 180 min post-dosing.
For rats with i.t. catheters, rats were tested 30, 60, 90, 120 min post-injection.
2.33 Rotarod test
The effect of ambroxol on coordinated motor function in uninjured rats was assessed with an accelerating rotarod apparatus (Columbus Instruments, OH; (Hama et al., 2003)). A group of uninjured rats were trained once a day for two days on the apparatus prior to testing. The rotarod accelerated 5–30 rpm over 60 sec. A baseline latency to fall (sec) was recorded prior to p.o. dosing with either 1000 mg/kg ambroxol or vehicle. Rats were tested 60 min following dosing.
2.34 The Basso, Beattie, Bresnahan (BBB) Locomotor Rating Scale
To assess possible acute effects of ambroxol on spontaneous hind limb function in SCI rats, two blinded observers scored SCI rats treated with either 1000 mg/kg ambroxol or vehicle 60 min post-dosing, using the Basso, Beattie, Bresnahan (BBB) Locomotor Rating Scale, a standardized rating system that has been developed to assess hind limb function following a SCI (Basso et al., 1995). Rats were placed in the center of a 1.2 m diameter arena, allowed to continuously locomote and were rated within a four minute test period. In this assessment, multiple factors were included, such as hind-paw/fore-paw coordination, toe clearance from the surface and paw position. These factors were considered when a assigning a BBB rating score.
The standardized scale ranges from 0–21. In normal, uninjured rats, the BBB score is 21, which is characterized by “consistent plantar surface paw stepping and coordinated gait” and “consistent toe clearance,” among other observations. By contrast, a score of 0 indicates complete absence of hind limb function. Increasing levels of hind limb function, such as (and not limited to) movement at the ankle, knee or hip joints, toe clearance and supporting of the weight with the hind limbs increases the BBB score.
2.4 Drugs
Ambroxol HCl (Sigma-Aldrich Co., MO) was dissolved in a vehicle of 10% Tween-80 in water. Ambroxol and its vehicle were administered p.o. in a volume of 5 ml/kg. Ambroxol up to a concentration of 60 mg/ml was a clear solution. However, over this concentration, ambroxol was administered as a suspension. Doses were based on a previous pharmacokinetic study, partially summarized in Table 1, and a previous behavioral study (Gaida et al., 2005).
In i.t. studies, the dose of ambroxol was 300 μg in a volume of 5 μl. Following injection of either ambroxol or vehicle, an additional 5 μl of vehicle was injected to flush the catheter.
2.5 Statistical analysis
Data are expressed as mean ± S.E.M. The effect of drug treatment over time was evaluated using a repeated measure two-way ANOVA (SigmaStat 3.1), followed by a Student-Newman-Keuls test for post-hoc comparisons. To compare the effect of ambroxol against vehicle administration in the rotarod test and BBB rating, the data were analyzed using a one-way ANOVA with Dunnett’s test for post-hoc comparison. Statistical significance was taken at P < 0.05.
At the time point at which maximal efficacy was observed (i.e. 60 min post-dosing for mechanical hypersensitivity and 90 min post-dosing for heat hypersensitivity), behavioral data were normalized in terms of baseline. Mechanical withdrawal thresholds were converted to a percent maximum possible effect (MPE):
Heat withdrawal latencies were converted to a percent reversal:
3. Results
3.1 Antinociceptive effect of ambroxol
The mechanical withdrawal threshold in uninjured rats was 15 g. After SCI, prior to p.o. dosing, the mean (± S.E.M.) hind paw withdrawal threshold was 2.0 ± 0.2 g, indicating significant below-level mechanical hypersensitivity. The highest dose of ambroxol, 1000 mg/kg, significantly reversed mechanical hypersensitivity beginning 30 min after dosing and persisted for at least 3 hrs post-dosing (P < 0.05 vs. vehicle; Fig 2A). Other doses of ambroxol did not significantly increase thresholds. By contrast, vehicle did not significantly alter withdrawal threshold at any time point. The maximum possible effect of ambroxol at 60 min post-dosing was 72% (Fig 2B). Rats were tested 24 hrs following dosing and withdrawal thresholds were as they were on the previous day prior to dosing.
Figure 2.
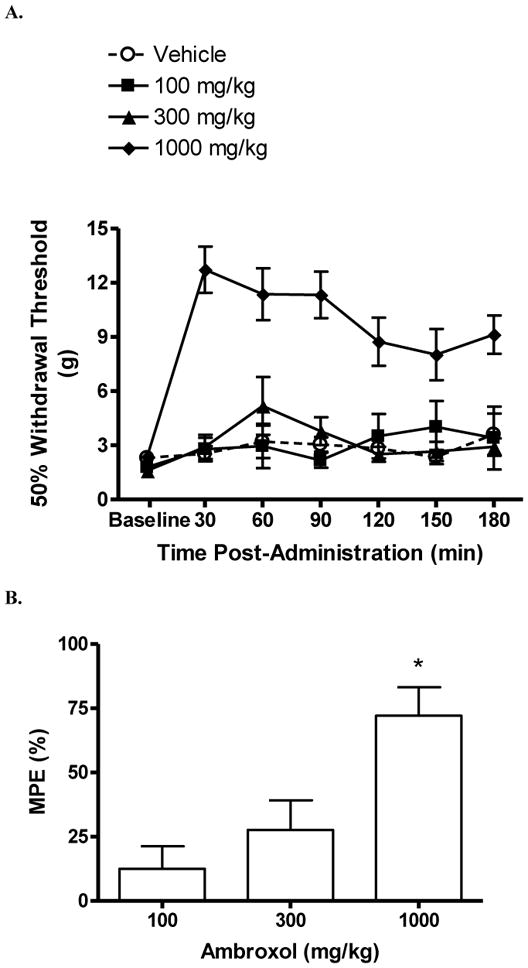
Effect of ambroxol on below-level mechanical hypersensitivity in rats with a spinal cord injury. Rats were tested prior to (“baseline”) and following p.o. dosing of either ambroxol or vehicle. (A) Effect over time of ambroxol on withdrawal threshold (g). The highest dose (1000 mg/kg) significantly increased withdrawal thresholds at all tested time points (*P < 0.05 vs. vehicle and baseline). (B) Percent maximum possible effect (MPE) of ambroxol 1 hr. post-dosing (*P < 0.05 vs. vehicle). Data are expressed as mean ± S.E.M. N = 7/group.
The heat withdrawal latency in uninjured rats was 11.9 ± 0.2 sec. Following SCI, but prior to dosing, the withdrawal latency was 7.8 ± 0.2 sec, indicating significant heat hypersensitivity (P < 0.05 vs. uninjured; Fig 3A). Beginning about 60 min post-dosing, increases in withdrawal latencies was observed in rats treated with 300 mg/kg ambroxol. A partial reversal of heat hypersensitivity was observed at 90 min with doses of 100 mg/kg (65%) and 300 mg/kg (61%) (P < 0.05 vs. vehicle; Fig 3B). The antinociceptive effect at these doses persisted up to 120 min post-dosing. By contrast, vehicle treatment did not significantly increase latencies at any time point. Rats were tested 24 hrs following dosing and withdrawal latencies were as they were on the previous day prior to dosing.
Figure 3.
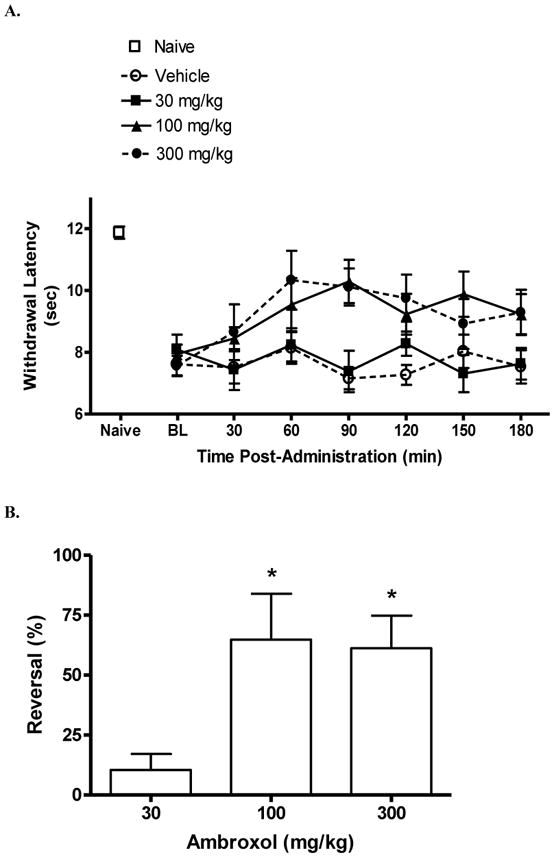
Effect of ambroxol on below-level heat hypersensitivity in rats with a spinal cord injury. Rats were tested prior to (“BL,” baseline) and following p.o. dosing of either ambroxol or vehicle. Note the decreased withdrawal latency of spinal cord-injured rats compared to “Naïve” rats. (A) Effect of ambroxol over time on withdrawal latency (sec). (B) Percent reversal of hypersensitivity of ambroxol at 90 min post-dosing (*P < 0.05 vs. vehicle). Data are expressed as mean ± S.E.M. N = 6/group.
There was no significant effect at any time point following i.t. injection of ambroxol or vehicle on either heat or mechanical hypersensitivity in SCI injured rats. Figure 4 shows the lack of effect of ambroxol and vehicle treatment at 30 min post-i.t. injection (P > 0.05 vs. vehicle).
Figure 4.
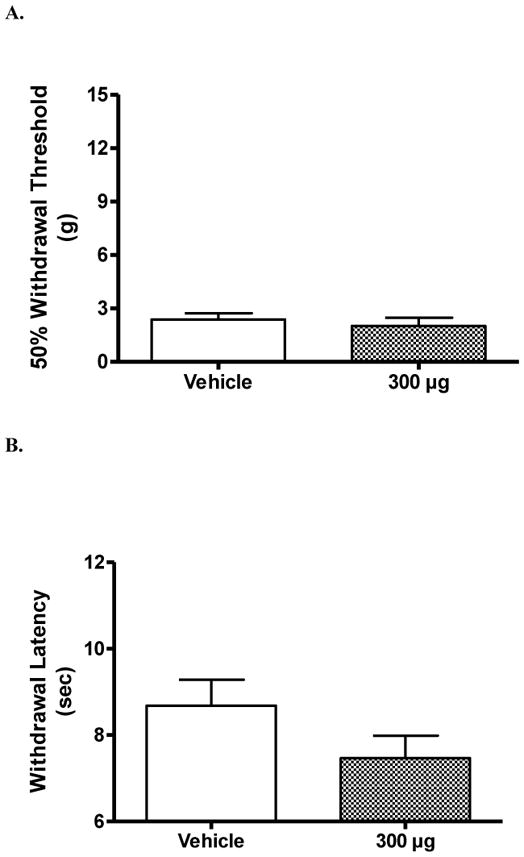
Effect of intrathecal ambroxol on below-level hypersensitivity in rats with a spinal cord injury. Lumbar intrathecal (i.t.) injection of 300 μg of ambroxol had no significant effect on either mechanical (A) or heat (B) hypersensitivity. There was no effect of i.t. injection of vehicle. Data are taken from rats 30 min following i.t. injection. There was a lack of effect at other time points as well. Data are expressed as mean ± S.E.M. N = 3/group.
As a general observation, i.t. injection of 75 μg lidocaine led to a rapid bi-lateral hind limb flaccid paralysis. However, i.t. injection of ambroxol did not.
3.2 Rotarod test
Prior to treatment, uninjured rats maintained positions on the rotarod for the entire 60 sec period. One hour after dosing, neither ambroxol nor vehicle significantly decreased time spent on the rotarod (P > 0.05; Table 2).
Table 2.
Effect of ambroxol on motor function in the rat
| Pre-dose | Post-dose | |
|---|---|---|
| Rotarod test (sec) | ||
| Vehicle | 60 (0.0) | 60 (0.0) |
| Ambroxol | 60 (0.0) | 60 (0.0) |
| BBB | ||
| Vehicle | 9.9 (0.7) | 9.4 (1.2) |
| Ambroxol | 7.5 (1.2) | 8.3 (0.7) |
Rats were tested 1 hr following oral dosing. The latency to fall (sec) in uninjured rats in the rotatod test was measured. BBB locomotor scores were measured on a scale of 0–21 in SCI rats. Neither vehicle nor ambroxol affected performance in either test. Data are mean (S.E.M.), N = 4/group.
3.3 BBB rating
The pre-SCI BBB rating of uninjured rats was 21. Four weeks after SCI and prior to dosing, the BBB rating of the SCI rats was 9 ± 1, indicating occasional weight-supported, dorsal hind paw stepping and weight supported only while stationary. One hour following dosing, neither ambroxol nor vehicle significantly altered BBB scores (P > 0.05; Table 2). Thus, an acute dose of ambroxol does not improve hind limb impairment due to SCI.
4. Discussion
The current study uncovered an antinociceptive effect of the mucolytic and local anesthetic drug ambroxol in a rat model of neuropathic SCI pain. Significant antinociception was obtained following oral dosing, reversing below-injury level tactile and noxious heat hypersensitivity. The highest tested dose of ambroxol did not lead to impairment of coordinated locomotion as assessed by the rotarod test. It has been previously reported that in vitro application of ambroxol potently blocked voltage-gated Na+ channel currents in DRG neurons, suggesting the antinociceptive effects in vivo is due to blocking of primary afferent nociceptor function. The lack of effect following i.t. injection of ambroxol excludes a direct lumbar spinal site of action in the systemic effect of ambroxol. The findings indicate that, not only could ambroxol be useful in treating neuropathic SCI pain, but also suggest that Na+ channels expressed on primary afferents could be useful drug targets for neuropathic SCI pain.
The mechanical and ischemic damages subsequent to SCI evoke changes in gene expression and neurochemistry in both neurons and glia, such as increased transcription factor expression, elevated concentrations of excitotoxic neurotransmitters, growth factors and cytokines (Hulsebosch et al., 2008). In addition to local changes in spinal dorsal horn, alterations in tissue homeostasis may spread distally from the epicenter to adjacent spinal cord segments and to brain somatosensory nuclei (Cummins et al., 2007; Yezierski, 2000; Zhou et al., 2002). Interestingly, nerve function outside the CNS may also be altered following an acute SCI, which could also underlie SCI-induced neuropathic pain (Carlton et al., 2009; Lin et al., 2007).
A possible point of mechanistic convergence of neuropathic pain due to injury of the peripheral or central nervous system could be the injury-induced abnormal and persistent function of dorsal horn wide-dynamic range (WDR) neurons, which respond to both noxious and innocuous stimuli (Carlton et al., 2009; Millan, 1999). Following tissue injury, these neurons display greatly exaggerated and long-lasting responses to noxious and innocuous cutaneous stimuli and increased spontaneous activity, which have been suggested to underlie hypersensitivity and spontaneous pain. Sodium channels expressed in peripheral nerves appear to be crucial in the maintenance of persistent injury-induced pain. Injection of a non-selective or a subtype selective Na+ channel blocker into lumbar DRG in rats with a peripheral injury reduced evoked WDR activity and cutaneous hypersensitivity (McGaraughty et al., 2008; Omana-Zapata et al., 1997; Sotgiu and Biella, 2000). These findings suggest that peripheral, pre-synaptic block of Na+ channels is sufficient in attenuating both abnormal WDR activity and nociception. It is possible that a similar treatment could ameliorate SCI-induced hypersensitivity.
Clinical use of non-selective Na+ channel blockers lidocaine and its analogue mexiletine appear to be effective on some neuropathic pain symptoms, the efficacy being non-dose dependent and the centrally-mediated side-effects are not well tolerated (Attal et al., 2004; Ferrante et al., 1996; Finnerup et al., 2005; Wallace et al., 2000). In a previous study, the highest tested dose of mexiletine (75 mg/kg) did not ameliorate either mechanical or heat hypersensitivity in SCI rats (Hama and Sagen, 2007). Furthermore, a slight increase in the dose of mexiletine (100 mg/kg) induced lethal convulsions in about 50% of the rats. Because of the central and peripheral adverse effects of non-selective Na+ channel block, systemically administered local anesthetic drugs may not be ideal for pain relief. Compared to currently available Na+ channel blockers, ambroxol is very well tolerated in humans and animals (Weiser, 2008). No effect was observed in the current study on coordinated locomotion following the highest tested dose of ambroxol, which was well below the LD50 (Table 1; Püschmann and Engelhorn, 1978).
Interestingly, initial pre-clinical characterization of ambroxol did not uncover a robust antinociceptive effect (Püschmann and Engelhorn, 1978). Ambroxol was tested in mice using the tail-pinch and acetic acid-induced writhing assays. The dose range tested was 50—200 mg/kg (p.o.). It is possible that the mice were under-dosed. A higher dose was later used (1000 mg/kg) with no significant antinociceptive effects in the tailflick and hot plate assays (Gaida et al., 2005). This same dose, however, demonstrated efficacy in tissue-injury models of pain. Thus, dosing was not likely an issue.
The lack of a positive antinociceptive effect in uninjured rats suggests that these assays (e.g. tailflick, hot plate) are weakly able to detect antinociceptive effects of systemic local anesthetics (Wiesenfeld-Hallin and Lindblom, 1985). Other local anesthetic drugs have demonstrated variable effects in suppressing the electrically induced mono-synaptic (c-fiber mediated) or poly-synaptic (myelinated afferents mediated) reflexes in vivo (Woolf and Wiesenfeld-Hallin, 1985). Ambroxol did not alter either of these. The only in vivo assay that indicated that ambroxol had local anesthetic activity was in the suppression of the rabbit corneal reflex, which is c-fiber mediated (Püschmann and Engelhorn, 1978). Clinical studies confirmed a robust anesthetic effect based on the suppression of painful cough, also c-fiber mediated, an unexpected finding since ambroxol was developed and tested for mucolysis, a non-neurological indication (de Mey et al., 2008; Fischer et al., 2002; Hoffmann, 1978). The experience with ambroxol, a mucolytic and local anesthetic drug, demonstrates the need for careful selection of in vivo assays for Na+ channel blockers in particular and analgesic drugs in general for discovery. The experience also suggests the need to be prepared for the serendipitous (“chance favors the prepared mind”).
It was previously shown that ambroxol potently blocks the TTX-R current in vitro, which are mediated by Nav1.8 and Nav1.9, typically found in primary afferent nociceptors (Weiser, 2006). A more recent study confirmed ambroxol preferentially blocking Nav1.8 channels compared to heterologously expressed neuronal channel subtypes Nav1.3 and Nav1.7 (Leffler et al., 2010). Unlike local anesthetic drugs, however, ambroxol does not cross the blood-brain barrier. To ascertain a possible spinal site of action, ambroxol was injected into the lumbar intrathecal space – there was no effect on below-level hypersensitivity. Thus, one possible mechanism of ambroxol’s effect in the current and previous in vivo studies is through block of peripherally expressed Na+ channels, particularly those channels expressed following injury.
In the current study, ambroxol ameliorated below-level mechanical and noxious heat hypersensitivity in SCI rats. Ambroxol’s effect on mechanical hypersensitivity was restricted to the highest tested dose, similar to that observed in peripheral nerve-injured rats (Gaida et al., 2005). The all-or-none effect with Na+ channel blocking drugs has also been clinically observed (Ferrante et al., 1996). The high dose-only effect could be related to the limited distribution of Nav1.8 in large-diameter mechano-sensitive primary afferents in contrast to the abundant expression of Nav1.8 in small-diameter nociceptors. Antinociceptive effects are obs1erved with Na+ channel subtype selective compounds, but not ambroxol, in uninjured rats with tests of acute nociception (Jarvis et al., 2007; Weiser, 2008). An increase in Nav1.8 in peripheral nociceptor axons following hind paw inflammation appears to underlie the increased potency of the Nav1.8 selective compound, compared to that of uninflamed rats (Coggeshall et al., 2004). An increased expression Nav1.8 and other Na+ channels in SCI rats could explain the differential doses needed and efficacy between noxious heat and tactile hypersensitivity.
The current in vivo data demonstrated a long lasting antinociception following p.o. dosing. Maximal plasma concentration of ambroxol is rapidly attained following oral dosing and the mean residence time is 2 hrs (Table 1). However, efficacy was observed for at least 2–3 hrs. A similar prolonged analgesia has been reported following lidocaine administration (Chaplan et al., 1995). Furthermore, the plasma concentration of lidocaine (2.6 μM) needed for a prolonged analgesia was much lower than that needed to block DRG neuron Na+ channels in vitro, usually in the mM range, and much lower than that needed to block nerve conduction (Weiser, 2006). The mechanism explaining the prolonged analgesia of local anesthetics has yet to be fully elaborated. Perhaps the effect in the current study was due to a novel blocking effect on Na+ channels or an active metabolite. Ambroxol has been shown to block other ion channels at high concentrations and has anti-inflammatory properties, which could also underlie the antinociceptive effect in the current study. However, the importance of these mechanisms in the antinociceptive of ambroxol has yet to be established (Malerba and Ragnoli, 2008).
Because of the added health burdens due to SCI, a peripherally acting analgesic would be ideal in these patients (Turner et al., 2001; Warms et al., 2002). A number of subtype selective compounds that block primary afferent-expressed Na+ channels have been developed with the goal of pain relief without the liability obtained with non-selective, brain penetrant Na+ channel blockers (Krafte and Bannon, 2008; McGowan et al., 2009). As a part of the drug discovery process, the safety of these novel chemical entities will need extensive and time-consuming evaluation. In the mean time, drugs that are already marketed, such as ambroxol, could be considered for clinical use (Chong and Sullivan, 2007).
Acknowledgments
We are grateful to Dr. Maria Collado and Ms. Lyudmila Rusakova for technical assistance. Supported by NIH grant 61172 and by the Craig H. Nielsen Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attal N, Rouaud J, Brasseur L, Chauvin M, Bouhassira D. Systemic lidocaine in pain due to peripheral nerve injury and predictors of response. Neurology. 2004;62:218–25. doi: 10.1212/01.wnl.0000103237.62009.77. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Du J, Tan HY, Nesic O, Hargett GL, Bopp AC, et al. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain. 2009;147:265–76. doi: 10.1016/j.pain.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Shafer SL, Yaksh TL. Prolonged alleviation of tactile allodynia by intravenous lidocaine in neuropathic rats. Anesthesiology. 1995;83:775–85. doi: 10.1097/00000542-199510000-00017. [DOI] [PubMed] [Google Scholar]
- Chong CR, Sullivan DJ., Jr New uses for old drugs. Nature. 2007;448:645–6. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Tate S, Carlton SM. Differential expression of tetrodotoxin-resistant sodium channels Nav1.8 and Nav1.9 in normal and inflamed rats. Neurosci Lett. 2004;355:45–8. doi: 10.1016/j.neulet.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Coward K, Plumpton C, Facer P, Birch R, Carlstedt T, Tate S, et al. Immunolocalization of SNS/PN3 and NaN/SNS2 sodium channels in human pain states. Pain. 2000;85:41–50. doi: 10.1016/s0304-3959(99)00251-1. [DOI] [PubMed] [Google Scholar]
- Cruz-Almeida Y, Martinez-Arizala A, Widerstrom-Noga EG. Chronicity of pain associated with spinal cord injury: A longitudinal analysis. J Rehabil Res Dev. 2005;42:585–94. doi: 10.1682/jrrd.2005.02.0045. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: Implications for mechanisms of pain. Pain. 2007;131:243–57. doi: 10.1016/j.pain.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mey C, Peil H, Kolsch S, Bubeck J, Vix JM. Efficacy and safety of ambroxol lozenges in the treatment of acute uncomplicated sore throat. EBM-based clinical documentation. Arzneimittelforschung. 2008;58:557–68. doi: 10.1055/s-0031-1296557. [DOI] [PubMed] [Google Scholar]
- Felix ER, Cruz-Almeida Y, Widerstrom-Noga EG. Chronic pain after spinal cord injury: What characteristics make some pains more disturbing than others? J Rehabil Res Dev. 2007;44:703–16. doi: 10.1682/jrrd.2006.12.0162. [DOI] [PubMed] [Google Scholar]
- Ferrante FM, Paggioli J, Cherukuri S, Arthur GR. The analgesic response to intravenous lidocaine in the treatment of neuropathic pain. Anesth Analg. 1996;82:91–7. doi: 10.1097/00000539-199601000-00016. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Biering-Sorensen F, Johannesen IL, Terkelsen AJ, Juhl GI, Kristensen AD, et al. Intravenous lidocaine relieves spinal cord injury pain: a randomized controlled trial. Anesthesiology. 2005;102:1023–30. doi: 10.1097/00000542-200505000-00023. [DOI] [PubMed] [Google Scholar]
- Fischer J, Pschorn U, Vix JM, Peil H, Aicher B, Muller A, et al. Efficacy and tolerability of ambroxol hydrochloride lozenges in sore throat. Randomised, double-blind, placebo-controlled trials regarding the local anaesthetic properties. Arzneimittelforschung. 2002;52:256–63. doi: 10.1055/s-0031-1299889. [DOI] [PubMed] [Google Scholar]
- Gaida W, Klinder K, Arndt K, Weiser T. Ambroxol, a Nav1.8-preferring Na(+) channel blocker, effectively suppresses pain symptoms in animal models of chronic, neuropathic and inflammatory pain. Neuropharmacology. 2005;49:1220–7. doi: 10.1016/j.neuropharm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Hains BC, Everhart AW, Fullwood SD, Hulsebosch CE. Changes in serotonin, serotonin transporter expression and serotonin denervation supersensitivity: involvement in chronic central pain after spinal hemisection in the rat. Exp Neurol. 2002;175:347–62. doi: 10.1006/exnr.2002.7892. [DOI] [PubMed] [Google Scholar]
- Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J Neurosci. 2003;23:8881–92. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama A, Sagen J. Behavioral characterization and effect of clinical drugs in a rat model of pain following spinal cord compression. Brain Res. 2007;1185:117–28. doi: 10.1016/j.brainres.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Hama A, Woon Lee J, Sagen J. Differential efficacy of intrathecal NMDA receptor antagonists on inflammatory mechanical and thermal hyperalgesia in rats. Eur J Pharmacol. 2003;459:49–58. doi: 10.1016/s0014-2999(02)02828-5. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hoffmann H. Inhalation therapy with the new antisecretory drug ambroxol (metabolite VIII of bromhexine) in otorhinolaryngology. Arzneimittelforschung. 1978;28:931–3. [PubMed] [Google Scholar]
- Hudmon A, Choi JS, Tyrrell L, Black JA, Rush AM, Waxman SG, et al. Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci. 2008;28:3190–201. doi: 10.1523/JNEUROSCI.4403-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2008 doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Honore P, Shieh CC, Chapman M, Joshi S, Zhang XF, et al. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci U S A. 2007;104:8520–5. doi: 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Hoffman AJ, Cardenas DD. Chronic pain in individuals with spinal cord injury: a survey and longitudinal study. Spinal Cord. 2005;43:704–12. doi: 10.1038/sj.sc.3101777. [DOI] [PubMed] [Google Scholar]
- Krafte DS, Bannon AW. Sodium channels and nociception: recent concepts and therapeutic opportunities. Curr Opin Pharmacol. 2008;8:50–6. doi: 10.1016/j.coph.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Leffler A, Reckzeh J, Nau C. Block of sensory neuronal Na+ channels by the secreolytic ambroxol is associated with an interaction with local anesthetic binding sites. Eur J Pharmacol. 2010;630:19–28. doi: 10.1016/j.ejphar.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Lin CS, Macefield VG, Elam M, Wallin BG, Engel S, Kiernan MC. Axonal changes in spinal cord injured patients distal to the site of injury. Brain. 2007;130:985–94. doi: 10.1093/brain/awl339. [DOI] [PubMed] [Google Scholar]
- Malerba M, Ragnoli B. Ambroxol in the 21st century: pharmacological and clinical update. Expert Opin Drug Metab Toxicol. 2008;4:1119–29. doi: 10.1517/17425255.4.8.1119. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Scanio MJ, Kort ME, Faltynek CR, Jarvis MF. A selective Nav1.8 sodium channel blocker, A-803467 [5-(4-chlorophenyl-N-(3,5-dimethoxyphenyl)furan-2-carboxamide], attenuates spinal neuronal activity in neuropathic rats. J Pharmacol Exp Ther. 2008;324:1204–11. doi: 10.1124/jpet.107.134148. [DOI] [PubMed] [Google Scholar]
- McGowan E, Hoyt SB, Li X, Lyons KA, Abbadie C. A peripherally acting Na(v)1.7 sodium channel blocker reverses hyperalgesia and allodynia on rat models of inflammatory and neuropathic pain. Anesth Analg. 2009;109:951–8. doi: 10.1213/ane.0b013e3181b01b02. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Omana-Zapata I, Khabbaz MA, Hunter JC, Bley KR. QX-314 inhibits ectopic nerve activity associated with neuropathic pain. Brain Res. 1997;771:228–37. doi: 10.1016/s0006-8993(97)00770-1. [DOI] [PubMed] [Google Scholar]
- Püschmann S, Engelhorn R. Pharmacological study on the bromhexine metabolite ambroxol (author’s transl) Arzneimittelforschung. 1978;28:889–98. [PubMed] [Google Scholar]
- Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–57. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Sotgiu ML, Biella G. Contribution of central sensitization to the pain-related abnormal activity in neuropathic rats. Somatosens Mot Res. 2000;17:32–8. doi: 10.1080/08990220070274. [DOI] [PubMed] [Google Scholar]
- Turner JA, Cardenas DD, Warms CA, McClellan CB. Chronic pain associated with spinal cord injuries: a community survey. Arch Phys Med Rehabil. 2001;82:501–9. doi: 10.1053/apmr.2001.21855. [DOI] [PubMed] [Google Scholar]
- Wallace MS, Magnuson S, Ridgeway B. Efficacy of oral mexiletine for neuropathic pain with allodynia: a double-blind, placebo-controlled, crossover study. Reg Anesth Pain Med. 2000;25:459–67. doi: 10.1053/rapm.2000.8583. [DOI] [PubMed] [Google Scholar]
- Warms CA, Turner JA, Marshall HM, Cardenas DD. Treatments for chronic pain associated with spinal cord injuries: many are tried, few are helpful. Clin J Pain. 2002;18:154–63. doi: 10.1097/00002508-200205000-00004. [DOI] [PubMed] [Google Scholar]
- Weiser T. Comparison of the effects of four Na+ channel analgesics on TTX-resistant Na+ currents in rat sensory neurons and recombinant Nav1.2 channels. Neurosci Lett. 2006;395:179–84. doi: 10.1016/j.neulet.2005.10.058. [DOI] [PubMed] [Google Scholar]
- Weiser T. Ambroxol: a CNS drug? CNS Neurosci Ther. 2008;14:17–24. doi: 10.1111/j.1527-3458.2007.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widerstrom-Noga EG, Felipe-Cuervo E, Yezierski RP. Chronic pain after spinal injury: interference with sleep and daily activities. Arch Phys Med Rehabil. 2001;82:1571–7. doi: 10.1053/apmr.2001.26068. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Lindblom U. The effect of systemic tocainide, lidocaine and bupivacaine on nociception in the rat. Pain. 1985;23:357–60. doi: 10.1016/0304-3959(85)90005-3. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Wiesenfeld-Hallin Z. The systemic administration of local anaesthetics produces a selective depression of C-afferent fibre evoked activity in the spinal cord. Pain. 1985;23:361–74. doi: 10.1016/0304-3959(85)90006-5. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–6. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yezierski RP. Pain following spinal cord injury: pathophysiology and central mechanisms. In: Sandkühler J, Bromm B, Gebhart GF, editors. Progress in Brain Research. New York: Elsevier; 2000. pp. 429–49. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Birch R, Sangameswaran L, Eglen R, Anand P. SNS/PN3 and SNS2/NaN sodium channel-like immunoreactivity in human adult and neonate injured sensory nerves. FEBS Lett. 2000;467:249–52. doi: 10.1016/s0014-5793(00)01166-2. [DOI] [PubMed] [Google Scholar]
- Zhang AL, Hao JX, Seiger A, Xu XJ, Wiesenfeld-Hallin Z, Grant G, et al. Decreased GABA immunoreactivity in spinal cord dorsal horn neurons after transient spinal cord ischemia in the rat. Brain Res. 1994;656:187–90. doi: 10.1016/0006-8993(94)91383-8. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Abdelhady M, Mourad MS, Hassouna MM. Change of vanilloid receptor 1 following neuromodulation in rats with spinal cord injury. J Surg Res. 2002;107:140–4. doi: 10.1006/jsre.2002.6481. [DOI] [PubMed] [Google Scholar]


