Abstract
Stress plays a role in many psychiatric disorders that are characterized by deficits in prepulse inhibition (PPI), a form of sensorimotor gating. Corticotropin-releasing factor (CRF) is one of the most important neurotransmitters involved in behavioral components of the stress response, and central infusion of CRF decreases PPI in rodents. We recently demonstrated that restraint stress decreases PPI and attenuates the increase in PPI caused by repeated testing. To broaden our investigation into how restraint affects PPI, we subjected Wistar-Kyoto (WKY) and Brown Norway (BN) rats to 10 consecutive days of 2-hour restraint, or to brief handling, prior to assessing PPI. We next examined the effects of 1 or 10 days of 2-hour restraint on plasma corticosterone levels in order to determine whether the endocrine response to stress parallels the behavioral effect of stress. Finally, we examined the effects of 1 or 10 days of 2-hour restraint on CRF and CRF receptor gene expression in the amygdala, hippocampus, frontal cortex, and hypothalamus in order to determine whether a temporal pattern of gene expression parallels the change in the behavioral response to stress. The major findings of the present study are that 1) restraint stress attenuates the increase in PPI caused by repeated testing in both WKY and BN rats, and BN rats are more sensitive to the effects of restraint on PPI than WKY rats, 2) restraint-induced increases in corticosterone levels mirror the effect of restraint on PPI in WKY rats but not in BN rats, 3) laterality effects on gene expression were observed for the amygdala, whereby restraint increases CRF gene expression in the left, but not right, amygdala, and 4) some restraint-induced changes in CRF and CRF receptor gene expression precede changes in PPI while other changes coincide with altered PPI in a rat strain- and brain region-dependent manner.
Keywords: Brown Norway, corticosterone, corticotropin-releasing factor, CRF receptor, mRNA, prepulse inhibition, restraint, Wistar-Kyoto
1. Introduction
The acoustic startle response (ASR) is a reflexive response to an unexpected and intense auditory stimulus and is characterized by contraction of facial and skeletal muscles (Koch, 1999). Despite the fact that the ASR is a reflex, it can be modulated. For example, startle magnitude is reduced if a non-startling acoustic stimulus is presented shortly prior to a startling stimulus (Hoffman and Ison, 1980; Hoffman and Searle, 1968). This phenomenon is referred to as prepulse inhibition (PPI) of the ASR. PPI is thought to result from the activation of brain mechanisms designed to briefly “protect” the information contained in the prepulse. When the startling stimulus is presented during this “protected” period, the startle response is reduced to allow for complete prepulse stimulus processing. Thus, PPI is a measure of sensorimotor gating (Braff and Geyer, 1990).
PPI deficits are observed in patients with a variety of psychiatric disorders, including schizophrenia (Braff et al., 1978; Braff et al., 1992), obsessive-compulsive disorder (Swerdlow et al., 1993), and post-traumatic stress disorder (Grillon et al., 1996). Many of these disorders are characterized by a reduced ability to suppress or “gate” intrusive sensory, motor, or cognitive information (Braff et al., 2001). Additionally, exposure to stressful events can trigger the onset or exacerbate symptoms of each of these disorders (Dinn et al., 1999; Keane et al., 2006; Walker and Diforio, 1997).
Corticotropin-releasing factor (CRF) is a 41-residue peptide that is synthesized in the paraventricular nucleus of the hypothalamus (PVN). During stress, CRF is released from the PVN and binds to CRF receptors located on the anterior pituitary to stimulate the release of adrenocorticotropic hormone (ACTH) (Vale et al., 1981), triggering an endocrine component of the stress response. Additionally, CRF is synthesized in extra-hypothalamic regions, including the cortex, hippocampus, central nucleus of the amygdala, and dorsal raphe nucleus (Swanson et al., 1983) and is released as a neurotransmitter/neuromodulator to mediate autonomic and behavioral aspects of the stress response (Bale and Vale, 2004; Gabr et al., 1994; Van Bockstaele et al., 1998). In the brain, CRF acts at two G-protein coupled receptors, CRF1 and CRF2 (Chang et al., 1993; Lovenberg et al., 1995), which are widely expressed in brain regions (Chalmers et al., 1995; Van Pett et al., 2000) known to modulate PPI, including the amygdala, hippocampus, and frontal cortex (Swerdlow et al., 2001). Central infusion of CRF causes specific behavioral and physiological effects that mimic those produced by stress, independently of endocrine effects (Czyrak et al., 2003; van den Buuse et al., 2004), including increased heart rate, increased locomotor activity in a familiar environment, and increased anxiety-like behaviors (Campbell et al., 2004; Dunn and Berridge, 1990; Jones et al., 1998; Spina et al., 2002).
We have shown that intracerebroventricular (ICV) infusion of CRF reduces PPI in both Wistar-Kyoto (WKY) and Brown Norway (BN) rats, which show different levels of baseline PPI (Conti et al., 2002; Palmer et al., 2000). Infusion of CRF reduces PPI in mice (Risbrough et al., 2004), and transgenic mice over-expressing CRF show reduced PPI compared to wild-type mice (Dirks et al., 2002). Interestingly, both typical and atypical antipsychotics reverse the effect of CRF on PPI (Conti et al., 2005) and improve PPI in CRF over-expressing mice (Dirks et al., 2003).
In a recently submitted manuscript (Sutherland and Conti, 2010), we have demonstrated that repeated restraint stress, a commonly used stressor in rodents (Glavin et al., 1994; Pare and Glavin, 1986), decreases PPI and attenuates the increase in PPI caused by repeated testing in BN rats. In that study, only BN rats were used and only five sessions of restraint stress (2 hour/session) were used. The first goal of this study was to broaden our investigation of the effect of restraint stress on PPI by increasing the number of days of restraint exposure to 10 and by examining an additional rat strain. Thus, we subjected both WKY and BN rats to 2-hour restraint stress, once a day for 10 consecutive days, or to brief handling (no restraint) prior to assessing PPI. Since WKY rats are less sensitive to the effect of ICV infusion of CRF on PPI than BN rats (Conti et al., 2002; Sutherland et al., 2008), we wanted to determine whether this strain was less sensitive to the effect of stress on PPI as well. The second goal of this study was to examine the effect of restraint stress on plasma corticosterone levels in order to determine whether the endocrine response to stress parallels the behavioral effect of stress on PPI or whether the two stress effects are dissociable. The final goal of this study was to determine the effects of restraint stress on CRF and CRF receptor mRNA levels in the left and right amygdala, hippocampus, frontal cortex, and hypothalamus in order to determine whether a temporal pattern of gene expression parallels the change in the behavioral response to stress. These brain regions were chosen because they play an important role in mediating the stress response (LeDoux, 2000; Roozendaal et al., 2009; Ulrich-Lai and Herman, 2009), synthesize CRF (Swanson et al., 1983; Vale et al., 1981), express CRF receptors (Chalmers et al., 1995; Van Pett et al., 2000), and, with the exception of the hypothalamus, modulate PPI (Swerdlow et al., 2001). We examined mRNA levels in the left and right amygdala separately to determine whether differences in gene expression existed between the two hemispheres since studies with rats suggest that laterality exists in the amygdala for processing of emotional memories (Baker and Kim, 2004; Coleman-Mesches and McGaugh, 1995a, b, c; Scicli et al., 2004).
2. Materials and methods
2.1 Animals
A total of 61 male WKY (Charles River, Raleigh, NC, USA) and 65 BN rats (Harlan Sprague-Dawley, Prattville, AL, USA) were 10 weeks old upon arrival and were maintained on a 12-hour light/dark cycle with food and water available ad libitum. Rats were group-housed for 2 weeks prior to restraint, and single-housed thereafter. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Experimental Design
In experiment 1, WKY and BN rats were restrained for 2 hours/day for 10 consecutive days by being placed into acrylic cylindrical restrainers designed for rodents weighing 250–500 grams (6.4 cm diameter, with adjustable length; Biomedical Research Instruments, Silver Springs, MD, USA). Control rats were handled briefly, but not restrained. Rats were tested for PPI and startle amplitude 30 minutes after restraint termination on days 1, 2, 3, 6, and 10. Grooming behavior was observed for 15 minutes, beginning immediately after restraint termination, as a secondary behavioral measure of stress (Spruijt et al., 1992). Rats were weighed on days 1 and 10 to assess stress-induced reduction in weight gain.
In experiment 2, the effect of restraint on plasma corticosterone levels was examined. Rats were exposed to either 1 or 10 days of 2-hour restraint, or to brief handling. Rats were decapitated and trunk blood was collected 30 minutes after termination of the last session of restraint.
In experiment 3, the effect of restraint on CRF and CRF receptor mRNA levels in the brain was examined. Rats were exposed to either 1 or 10 days of 2-hour restraint, or to brief handling. Thirty minutes after termination of the last session of restraint, rats were decapitated and brains were removed. The left and right amygdala, hippocampus, frontal cortex, and hypothalamus were dissected and frozen on dry ice. The hippocampus and hypothalamus were dissected from the brains of rats used for corticosterone assessment. The amygdala and frontal cortex were taken from the brains of a separate cohort of rats exposed to the same restraint paradigm. None of these rats underwent PPI testing. Tissue samples were stored at −80°C.
2.3 Startle Chambers and PPI Testing
Startle amplitude and PPI were measured in two identical startle chambers (SR-LAB, San Diego Instruments, San Diego, CA, USA) consisting of a nonrestrictive Plexiglas cylinder (9 cm diameter, 18.5 cm length) mounted on a platform located inside a sound- and vibration-attenuating cabinet equipped with a 5-watt incandescent bulb and a fan for ventilation. A piezoelectric accelerometer, mounted under each cylinder, detected whole-body startle responses. From the onset of each startle stimulus, output signals from the accelerometer were recorded once/msec for 100 msec by the computer. Signals were rectified, digitized, and stored by the SR-LAB program. Startle response sensitivities were standardized across chambers using a standard calibration tube each day. White noise stimuli were delivered through a horn tweeter controlled by the SR-LAB program.
Following a 5-minute acclimation period, stimuli were delivered over a 70 dB white noise background. The first and last 6 trials of the session consisted of the startle stimulus alone (120 dB, 40 msec). Remaining trials occurred in a pseudorandom order and consisted of 12 startle alone trials (used to calculate % PPI and average startle amplitude), 12 prepulse + startle trials at each of 4 prepulse intensities (76, 82, 85, 88 dB), and 8 no stimulus trials. Prepulse stimuli (20 msec) preceded startle stimuli by 100 msec. The inter-trial interval averaged 20 seconds. Testing was performed between 10 a.m. and 4 p.m.
2.4 Corticosterone Radioimmunoassay (RIA)
Trunk blood was collected into heparinized tubes and was centrifuged at 3000 rpm for 10 minutes at 4°C and plasma was placed into chilled 1.5 ml microcentrifuge tubes. Corticosterone concentrations were assessed using a commercially available RIA kit (ImmuChem 07-120102, MP Biomedicals, Solon, OH, USA). The following modifications were made to the manufacturer’s instructions: 1) Reagents were used at half the recommended volumes, 2) 6 corticosterone calibrators were used at concentrations of 12.5, 25, 50, 100, 250, and 500 ng/ml; 3) Samples were processed in duplicate at 1:50 and 1:100. The precipitate was counted in the gamma counter for 2 minutes. All samples were assessed in one assay, and calculated using the weighted logit-log method (Davis et al., 1980).
2.5 Brain Dissection
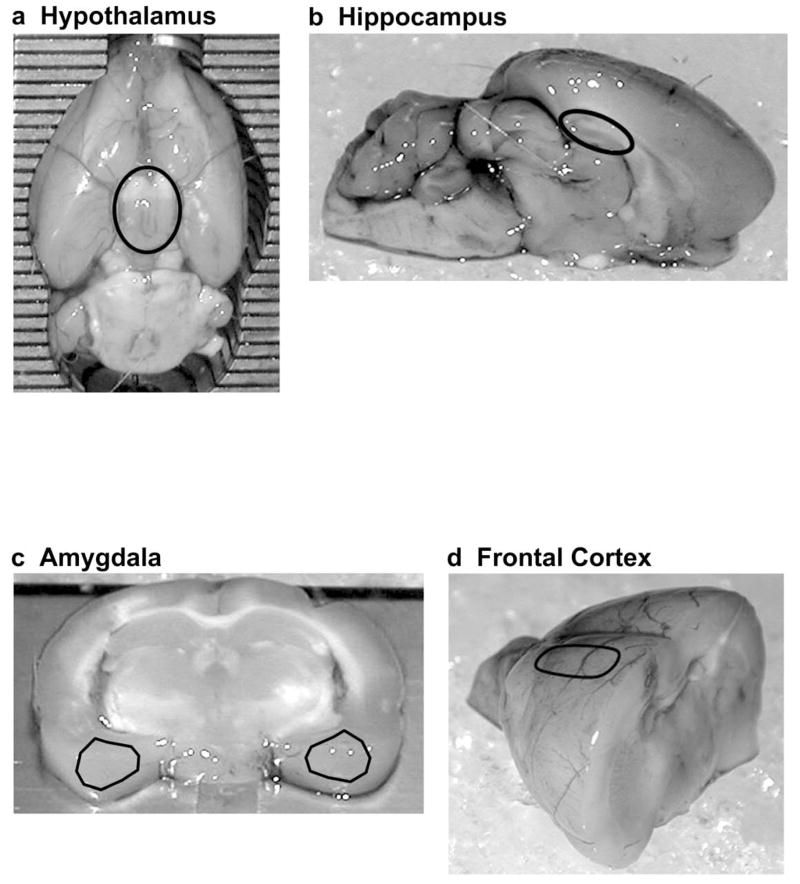
To dissect the hypothalamus, the brain was placed onto an ice-chilled petri dish, ventral side up. The entire hypothalamus, excluding optic nerves, was removed using forceps (Fig 1a). The brain was turned dorsal side up and hemisected. The hippocampus (approximately 2.0 to 5.0 mm posterior, 0 to 4.0 mm lateral, and 2.6 to 4.0 mm ventral to Bregma) was removed using forceps (Fig 1b) (Paxinos and Watson, 1986). To obtain the amygdala, the brain was placed ventral side up into an ice-chilled stainless steel rat brain slicer (1.0 mm coronal slices, Zivic Instruments, catalog #BSRS001.1, Pittsburgh, PA, USA). The first razor blade was placed approximately one-third of the way into the anterior edge of the hypothalamus and the second blade was placed 2.0 mm posterior to the first cut. Stereotaxic coordinates were approximately 2.0 to 4.0 mm posterior to Bregma. Using a surgical blade, the piriform cortex was removed from the amygdala and the entire left and right amygdala were dissected (approximately 3.0 to 5.4 mm lateral and 7.4 to 9.4 mm ventral to Bregma) (Fig 1c). The anterior portion of the remaining brain was hemisected and an approximately 1.0 mm thick slice of frontal cortex (approximately 3.0 mm anterior to 0.0 mm posterior and 1.0 to 4.0 mm lateral to Bregma) was removed from each hemisphere (Fig 1d). If necessary, white matter was scraped off of the ventral side of the cortical slice.
Fig 1.
Regions of the brain used for assessing mRNA levels. (a) The hypothalamus (black circle) was extracted from the ventral surface of the rat brain. (b) The hippocampi (black circle) were dissected from a mid-sagittal section of the rat brain. (c) 2.0 mm thick sections of amygdalae (black triangles) were dissected from a coronal section of the rat brain. (d) Frontal cortices (black oval) were dissected from an anterior portion of the rat brain. Dissection techniques and approximate stereotaxic coordinates for these brain regions are described in greater detail under “Brain Dissection” in the Methods.
2.6 Quantification of Brain CRF and CRF Receptor mRNA Levels
Total RNA was extracted from frozen tissue samples using Tri reagent (Sigma Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. Quantification of total RNA was performed using a BioMate 3 spectrophotometer (Thermo Scientific, Waltham, MA, USA).
cDNA synthesis was performed in 96-well plates using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions with the following modification: 5 μg of total RNA was used in a reaction volume of 80 μl. On each plate, one RNA sample was reacted without the addition of reverse transcriptase to control for DNA contamination. The Gene Amp PCR System 9700 thermal cycler (Applied Biosystems) was used at the following conditions: 1) 25°C for 10 minutes, 2) 37°C for 120 minutes, and 3) 85°C for 5 seconds.
Levels of mRNA were quantified using commercially available assays (Applied Biosystems; CRF mRNA: assay ID Rn01462137_m1; CRF1 receptor mRNA: assay ID Rn00578611_m1; CRF2 receptor mRNA: assay ID Rn00575617_ml). Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) was performed in triplicate using TaqMan Universal PCR Master Mix (Applied Biosystems) and a 7500 Real Time PCR System (Applied Biosystems). PCR was performed with the following cycling conditions: 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds and 60 °C for 60 seconds.
Endogenous control gene primers and probes were included in each assay well to provide for within-well normalization (CRF and CRF receptor mRNA assays incorporated FAM-labeled probes while control genes used HEX or Joe labels). Choice of endogenous reference gene was based on having a similar real-time PCR amplification Ct as the gene of interest, β-glucuronidase for CRF and β-2 microglobulin for CRF1 and CRF2 receptors. Primers and TaqMan fluorescent probes for endogenous control genes were designed with Primer Express 2.0 software (Applied Biosystems). Primers and probes for the β-glucuronidase TaqMan assay spanning exon 8–9 were Fw-TCTGAAACCTGCCGGATATTACTT, Rv-TGGTATTGCTCACAAAGGTCACA and HEX-AAAGCCCTGGACCCCACCCGTC-BHQ. For the β-2 microglobulin TaqMan assay spanning exons 2–4, primers and probes used were Fw-ACTCTGAAGGAGCCCAAAACC, Rv- GTCCAGATGATTCAGAGCTCCAT and JOE-ACCTGGGACCGAGACATGTAATCAAGCTC-BHQ.
Each qPCR assay plate included a standard curve dilution series of a cDNA pool derived from total RNA extracted from frontal cortices of naïve WKY and BN rats. Using this standard curve, Ct values were used to determine the equivalent quantity of frontal cortex cDNA pool for CRF or CRF receptor mRNA and for the paired endogenous control gene. Ratios of the relative quantity of target gene to endogenous control gene are shown in figures. A value of 1 indicates a gene expression level equivalent to the pooled WKY and BN frontal cortex reference sample. The level of CRF2 receptor expression was higher in amygdala and hypothalamus compared with frontal cortex.
2.7 Data and Statistical Analyses
Percent PPI was calculated for each rat at each prepulse intensity using the following equation: % PPI = 100 – (100 X [prepulse/startle]). Prepulse was the average startle amplitude on trials in which a prepulse stimulus preceded the startle stimulus. Startle was the average amplitude on trials in which the startle stimulus was presented alone, excluding the first and last 6 trials.
Since mRNA data are expressed as ratios, data were transformed using a logarithmic transformation (Log (X+1)) prior to statistical analyses (Steel and Torrie, 1980). Transformed data are shown in all mRNA figures. Data were analyzed using one-, two-, three-, or four-way analysis of variance (ANOVA), as discussed in detail in the Results section (SPSS 15.0 software, SPSS, Inc., Chicago, IL, USA). Tukey’s post-hoc tests were performed if significant main effects were found. Independent t-tests were used where appropriate. The alpha level was set at 0.05. Main effects of prepulse intensity, which occur because percent PPI increases with increasing prepulse intensity, are not reported since they are statistically significant in all analyses conducted. In experiment 3, rats exhibiting mRNA levels greater or less than two standard deviations from the mean were removed from analysis, resulting in no more than one rat removed per group.
3. Results
3.1 Experiment 1: Effect of 10 consecutive days of 2-hour restraint stress on PPI, startle amplitude, grooming behavior, and body weight
Initially, a four-way ANOVA was used to analyze PPI data from all 5 days of testing, with rat strain and restraint as between-subjects factors and day and prepulse intensity as within-subjects factors (Fig 2). A significant main effect of rat strain [F(1,57) = 34.404; p < 0.001] was revealed, with BN rats showing less PPI than WKY rats. Significant main effects of restraint [F(1,57) = 11.467; p < 0.001] and day [F(4,228) = 43.679; p < 0.001] on PPI were observed. There were significant interactions between day and rat strain [F(4,228) = 13.925; p < 0.001], prepulse intensity and rat strain [F(3,171) = 5.384; p = 0.001], prepulse intensity and restraint [F(3,171) = 5.329; p = 0.002], and among day, prepulse intensity, and rat strain [F(12,684) = 4.983; p < 0.001]. In order to understand these interactions, PPI data from each rat strain were examined separately using three-way ANOVAs.
Fig 2.

Effect of restraint stress on PPI in WKY and BN rats. Values shown are means ± SEMs. For all groups, n = 15–16. Rats were restrained for 2 hours, once a day for 10 consecutive days, or were handled briefly and returned to the home cage. PPI was assessed 30 minutes after restraint termination on days 1, 2, 3, 6, and 10. Prepulse stimulus intensities are 76, 82, 85, and 88 dB. (a) On day 1, restraint did not alter PPI in WKY or BN rats. (b) On day 2, restraint did not alter PPI in WKY or BN rats. (c) Exposure to 3 days of 2-hour restraint significantly attenuated the increase in PPI caused by repeated testing in BN rats (*p = 0.016). (d) Exposure to 6 days of 2-hour restraint also significantly attenuated the increase in PPI caused by repeated testing in BN rats (*p = 0.003). (e) Exposure to 10 days of 2-hour restraint significantly attenuated the increase in PPI caused by repeated testing in WKY (+p = 0.030) and BN rats (*p = 0.037).
In WKY rats, a three-way ANOVA revealed significant main effects of restraint [F(1,29) = 5.442; p = 0.027] and day on PPI [F(4,116) = 5.182; p = 0.001]. To determine on which days restraint reduced PPI, data from each day were examined separately using two-way ANOVAs. Restraint did not affect PPI on day 1 (Fig 2a), day 2 (Fig 2b), day 3 (Fig 2c), or day 6 (Fig 2d). However, restraint significantly attenuated the increase in PPI due to repeated testing in WKY rats on day 10 [F(1,29) = 5.191; p = 0.030] (Fig 2e).
In BN rats, a three-way ANOVA revealed significant main effects of restraint [F(1,28) = 6.037; p = 0.020] and day on PPI [F(4,112) = 53.610; p < 0.001]. PPI data from each day were examined separately using two-way ANOVAs. Restraint did not affect PPI on day 1 (Fig 2a) or day 2 (Fig 2b). However, restraint significantly attenuated the increase in PPI due to repeated testing in BN rats on day 3 [F(1,28) = 6.552; p = 0.016], day 6 [F(1,28) = 10.296; p = 0.003], and day 10 [F(1,28) = 4.800; p = 0.037] (Fig 2c–e, respectively). Significant prepulse intensity X restraint interactions on day 6 [F(3,84) = 4.663; p = 0.005] and day 10 [F(3,84) = 6.505; p = 0.001] indicated that the effect of restraint was greater at the 76, 82, and 85 dB prepulse intensities than at the 88 dB intensity.
Analysis of startle amplitude data (Fig 3) using a three-way ANOVA revealed a significant effect of strain [F(1,57) = 19.248; p < 0.001], with BN rats exhibiting a lower startle response than WKY rats. A significant effect of day [F(4,228) = 3.172; p = 0.015] and a significant day X rat strain interaction [F(4,228) = 9.425; p < 0.001] indicated that startle amplitude diminished as the days of testing progressed in BN rats only. Restraint stress did not alter startle amplitude in either rat strain on any day examined.
Fig 3.
Effect of restraint stress on baseline startle amplitude in WKY and BN rats. Values shown are means ± SEMs and were calculated from the startle stimulus alone trials that were used to calculate percent PPI. Startle amplitude was assessed 30 minutes after restraint termination on days 1, 2, 3, 6, and 10. BN rats exhibited a lower startle response (*p < 0.001 vs. WKY rats). Restraint did not alter startle amplitude in either strain, on any day examined.
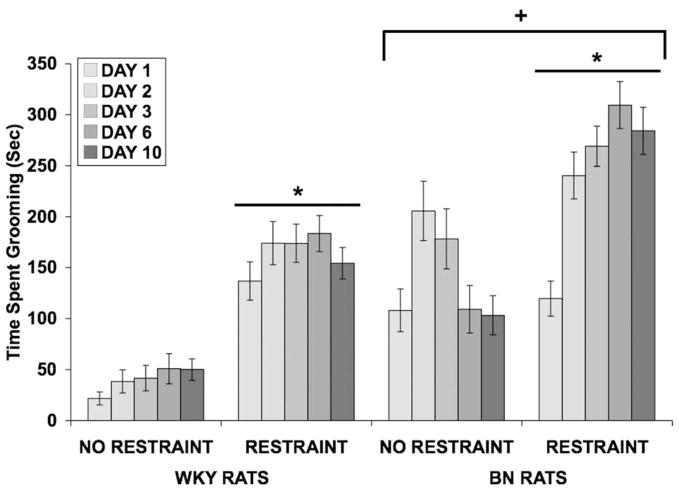
A three-way ANOVA revealed that the restrained groups spent more time grooming than the control groups [F(1,56) = 87.563; p < 0.001] (Fig 4). A significant effect of rat strain [F(1,56) = 55.025; p < 0.001] indicated that BN rats spent more time grooming than WKY rats. A significant effect of day [F(4,224) = 11.663; p < 0.001] and significant interactions between day and rat strain [F(4,224) = 3.752; p = 0.006], between day and restraint [F(4,224) = 5.860; p < 0.001], and among day, strain, and restraint [F(4,224) = 6.529; p < 0.001] revealed that restraint increased time spent grooming as the days progressed to a greater extent in WKY rats than in BN rats.
Fig 4.
Effect of restraint stress on time spent grooming (in seconds) in WKY and BN rats. Values shown are means ± SEMs. For all groups, n = 15–16. Grooming was assessed for 15 minutes, beginning immediately after restraint termination on days 1, 2, 3, 6, and 10. Restraint increased time spent grooming in both rat strains (*p < 0.001 vs. No Restraint). BN rats spent more time grooming overall (+p < 0.001 vs. WKY rats).
Analysis of weight gain over the 10 days of repeated restraint using a two-way ANOVA revealed a significant effect of strain [F(1,57) = 209.841; p < 0.001], with BN rats gaining less weight than WKY rats (data not shown). A significant effect of restraint [F(1,57) = 19.340; p < 0.001] revealed that rats exposed to 10 days of repeated restraint gained less weight than non-restrained controls, or lost weight in the case of BN rats.
3.2 Experiment 2: Effect of 1 or 10 days of 2-hour restraint stress on plasma corticosterone levels
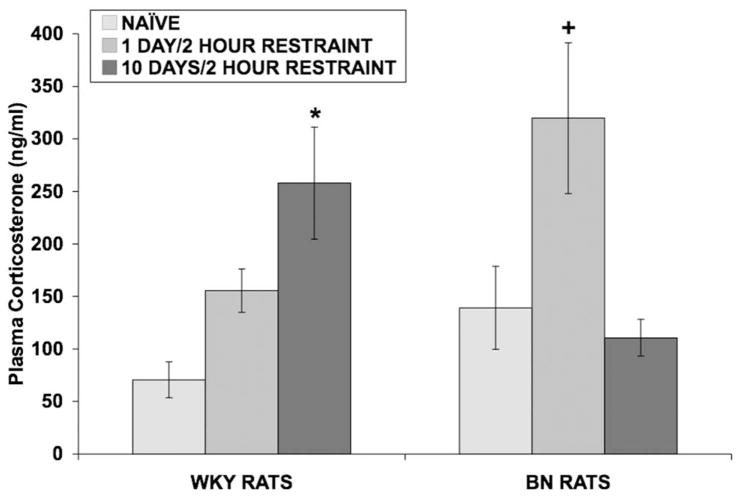
A two-way ANOVA, with rat strain and days of restraint (0, 1, or 10) as between-subjects factors, revealed that 2-hour restraint stress significantly increased plasma corticosterone levels [F(2,24) = 5.058; p = 0.015] (Fig 5). Since a significant rat strain X restraint interaction [F(2,24,) = 7.207; p = 0.004] was found, data from each rat strain were examined separately using one-way ANOVAs. In WKY rats, restraint increased corticosterone levels [F(2,12) = 7.397; p = 0.008], and this increase was only observed in the group that underwent 10 days of restraint (p = 0.006; Tukey’s test). In BN rats, restraint increased corticosterone levels [F(2,12) = 5.491; p = 0.020], and this increase was only observed after 1 day of 2-hour restraint (p = 0.053; Tukey’s test). Corticosterone levels on day 1 were also significantly different from day 10, indicating that levels returned to baseline after repeated restraint in BN rats (p = 0.025, Tukey’s test). To further characterize any stress-induced changes in corticosterone levels between the two rat strains, two separate independent t-tests were conducted on data from Day 1 and Day 10 of restraint. There was a trend for corticosterone levels to differ between WKY and BN rats on Day 1 (p = 0.059) while corticosterone levels were significantly different on Day 10 [t(8) = 2.623; p = 0.031], indicating the corticosterone levels returned to baseline by Day 10 in BN rats.
Fig 5.
Effect of restraint stress on plasma corticosterone levels in WKY and BN rats. Values shown are means ± SEMs. For all groups, n = 5. Rats were exposed to either: 1) brief handling (naïve controls), 2) one day of 2-hour restraint, or 3) ten consecutive days of 2-hour restraint. Rats were rapidly decapitated and trunk blood was collected 30 minutes after termination of the last session of restraint. In WKY rats, 10 days of 2-hour restraint increased corticosterone levels (*p = 0.006 vs. Naïve WKY rats, Tukey’s test). In BN rats, 1 day of 2-hour restraint increased corticosterone levels (+p 0.053 vs. Naïve BN rats, Tukey’s test).
3.3 Experiment 3: Effect of 1 or 10 days of 2-hour restraint stress on brain CRF and CRF receptor mRNA levels
Amygdala
In the left amygdala (Fig 6a), a two-way ANOVA, with rat strain and days of restraint (0, 1, or 10) as between-subjects factors, revealed a significant main effect of restraint on CRF mRNA levels [F(2,28) = 5.236; p = 0.012], with 1 day of restraint increasing CRF mRNA (p = 0.012; Tukey’s test). The Tukey’s test also revealed a significant difference in CRF mRNA levels between 1 and 10 days of restraint (p = 0.027), although it is evident that this difference occurred only in BN rats, as CRF mRNA levels in WKY rats were not different between the two restraint groups. In the right amygdala (Fig 6a), a two-way ANOVA indicated that there were no significant main effects of rat strain or restraint and no interaction between the two factors. However, a one-way ANOVA conducted only on the data from BN rats revealed a trend for 10 days of restraint to decrease CRF mRNA levels in the right amygdala (p = 0.091). Thus, restraint stress increased CRF gene expression in the left amygdala of both rat strains and had a tendency to decrease CRF gene expression in the right amygdala of BN rats.
Fig 6.
Effect of restraint stress on CRF and CRF receptor mRNA levels in the left and right amygdala of WKY and BN rats. Values are expressed as a ratio of CRF mRNA to β-glucuronidase mRNA and CRF1 (or CRF2) receptor mRNA to β-2 microglobulin mRNA in arbitrary units (means ± SEMs). For all groups, n = 5–7. Rats were exposed to either: 1) brief handling (naïve controls), 2) one day of 2-hour restraint, or 3) ten consecutive days of 2-hour restraint. Thirty minutes after termination of the last session of restraint, rats were rapidly decapitated and the amygdala was dissected. Note: the scale on the y-axis differs between CRF1 and CRF2 receptor mRNA graphs. (a) 1 day of 2-hour restraint increased CRF mRNA levels in the left amygdala of WKY and BN rats (*p = 0.012 vs. Naïve rats, Tukey’s test). In BN rats, CRF mRNA levels returned to baseline after 10 days of 2-hour restraint (+p = 0.027 vs. 1 Day/2 Hour Restraint, Tukey’s test). Restraint did not alter CRF mRNA levels in the right amygdala of either rat strain. (b) In the right hemisphere, BN rats had greater levels of CRF1 receptor mRNA (*p = 0.010 vs. WKY rats). (c) Restraint stress did not significantly alter CRF2 receptor mRNA levels in either the left or right amygdala of either rat strain.
Separate two-way ANOVAs were conducted on both CRF1 and CRF2 receptor mRNA data from the left and right amygdala. In the left hemisphere, there were no significant main effects of rat strain or restraint and no interaction between the two factors on CRF1 receptor mRNA levels (Fig 6b). In the right hemisphere, a main effect of rat strain [F(1,27) = 7.678; p = 0.010] revealed that BN rats have higher levels of CRF1 receptor mRNA in this region (Fig 6b). There were no significant main effects of rat strain or restraint and no strain X restraint interaction on CRF2 receptor mRNA levels in either the left or right amygdala (Fig 6c). Thus, neither 1 nor 10 days of restraint altered CRF1 or CRF2 receptor gene expression in the amygdala of either rat strain.
Hippocampus
In the hippocampus (Fig 7a), a two-way ANOVA, with rat strain and days of restraint (0, 1, or 10) as between-subjects factors, revealed significant main effects of strain (F(1,22) = 24.363; p < 0.001) and restraint [F(2,22) = 7.160; p = 0.004] on CRF mRNA levels. In WKY rats, a one-way ANOVA did not show any significant effect of restraint on CRF mRNA. In BN rats, a one-way ANOVA revealed a significant main effect of restraint [F(2,11) = 18.572; p < 0.001], with Tukey’s post hoc tests showing that both 1 day (p < 0.001) and 10 days (p = 0.008) of restraint increased CRF mRNA levels. The Tukey’s test also showed a trend for CRF mRNA levels to be lower after 10 days of restraint compared to 1 day of restraint (p = 0.080). Thus, both acute and repeated restraint increased CRF gene expression in BN rats, but not in WKY rats.
Fig 7.
Effect of restraint stress on CRF and CRF receptor mRNA levels in the hippocampus of WKY and BN rats. Values are expressed as a ratio of CRF mRNA to β-glucuronidase mRNA and CRF1 (or CRF2) receptor mRNA to β-2 microglobulin mRNA in arbitrary units (means ± SEMs). For all groups, n = 4–5. Rats were exposed to either: 1) brief handling (naïve controls), 2) one day of 2-hour restraint, or 3) ten consecutive days of 2-hour restraint. Thirty minutes after termination of the last session of restraint, rats were rapidly decapitated and the hippocampus was dissected. Note: the scale on the y-axis differs between CRF1 and CRF2 receptor mRNA graphs. (a) In BN rats, both 1 day (*p < 0.001 vs. Naïve BN rats, Tukey’s test) and 10 days (+p = 0.008 vs. Naïve BN rats, Tukey’s test) of restraint increased CRF mRNA levels. (b) In BN rats, 10 days of restraint increased CRF1 receptor mRNA levels (*p = 0.007 vs. Naïve BN rats, Tukey’s test). (c) Restraint did not affect CRF2 receptor mRNA levels in either rat strain.
A two-way ANOVA showed significant effects of rat strain [F(1,22) = 7.568; p = 0.012] and restraint [F(2,22) = 4.834; p = 0.018], and a trend towards a strain X restraint interaction (p = 0.068) on CRF1 receptor mRNA levels in the hippocampus (Fig 7b). In WKY rats, a one-way ANOVA did not show any significant effect of restraint on CRF1 receptor mRNA. In BN rats, a one-way ANOVA revealed a main effect of restraint [F(2,11) = 7.578; p = 0.009], with 10 days of restraint increasing CRF1 receptor mRNA levels (p = 0.007; Tukey’s test) (Fig 7b). When CRF2 receptor mRNA levels were examined, a two-way ANOVA did not reveal any significant main effects or an interaction (Fig 7c). Thus, repeated restraint increased gene expression of the CRF1, but not the CRF2, receptor, and this increase occurred only in BN rats.
Frontal Cortex
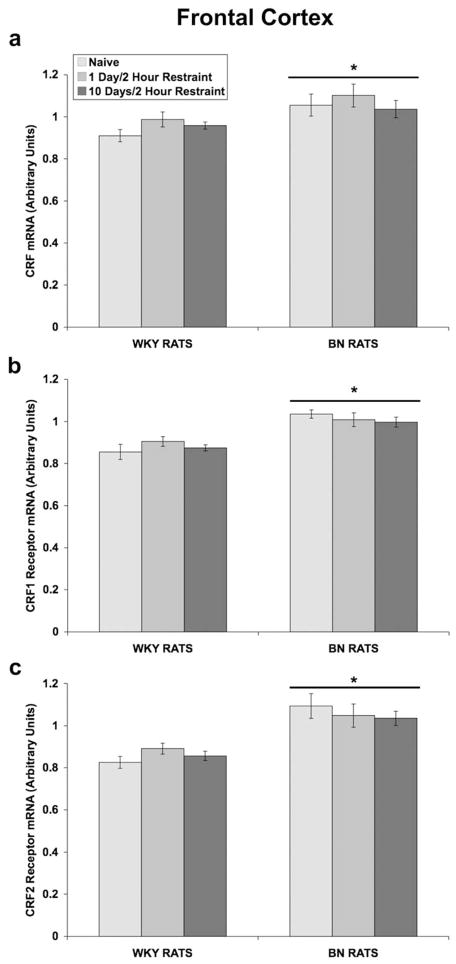
In the frontal cortex (Fig 8a), a two-way ANOVA, with rat strain and days of restraint (0, 1, or 10) as between-subjects factors, revealed a significant effect of rat strain on CRF mRNA levels [F(1,28) = 9.852; p = 0.004], with BN rats having higher levels in this region. There was no effect of restraint. A two-way ANOVA showed that BN rats have greater CRF1 receptor mRNA levels than WKY rats [F(1,21) = 30.123; p < 0.001] (Fig 8b). Similarly, BN rats had greater levels of CRF2 receptor mRNA in the frontal cortex, as indicated by a significant main effect of rat strain [F(1,27) = 35.868; p < 0.001] (Fig 8c). Although restraint had no effect on CRF or CRF receptor gene expression, BN rats had greater levels of CRF, CRF1, and CRF2 receptor mRNA than WKY rats.
Fig 8.
Effect of restraint stress on CRF and CRF receptor mRNA levels in the frontal cortex of WKY and BN rats. Values are expressed as a ratio of CRF mRNA to β-glucuronidase mRNA and CRF1 (or CRF2) receptor mRNA to β-2 microglobulin mRNA in arbitrary units (means ± SEMs). For all groups, n = 5–7. Rats were exposed to either: 1) brief handling (naïve controls), 2) one day of 2-hour restraint, or 3) ten consecutive days of 2-hour restraint. Thirty minutes after termination of the last session of restraint, rats were rapidly decapitated and the frontal cortex was dissected. (a) BN rats had greater levels of CRF mRNA in the frontal cortex (*p = 0.004 vs. WKY rats). (b) BN rats had greater levels of CRF1 receptor mRNA (*p < 0.001 vs. WKY rats). (c) BN rats had greater levels of CRF2 receptor mRNA (*p < 0.001 vs. WKY rats).
Hypothalamus
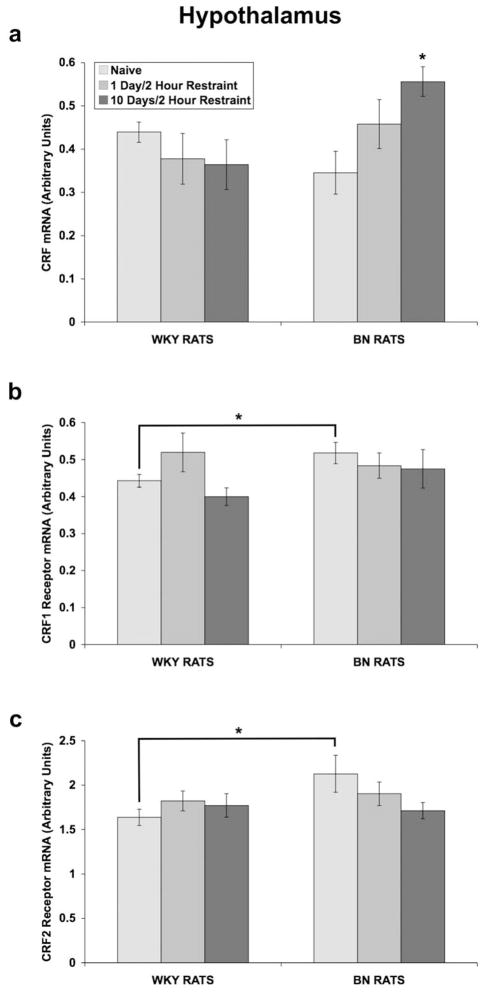
In the hypothalamus (Fig 9a), a two-way ANOVA, with rat strain and days of restraint (0, 1, or 10) as between-subjects factors, showed that there were no significant main effects of rat strain or restraint on CRF mRNA levels, but there was a significant strain X restraint interaction [F(2,24) = 4.401; p = 0.024]. Thus, one-way ANOVAs were conducted on data from each rat strain separately. In WKY rats, there was no significant effect of restraint. In BN rats, there was a significant main effect of restraint [F(2,12) = 4.871; p = 0.028], with 10 days of restraint increasing CRF mRNA levels (p = 0.022; Tukey’s test).
Fig 9.
Effect of restraint stress on CRF and CRF receptor mRNA levels in the hypothalamus of WKY and BN rats. Values are expressed as a ratio of CRF mRNA to β-glucuronidase mRNA and CRF1 (or CRF2) receptor mRNA to β-2 microglobulin mRNA in arbitrary units (means ± SEMs). For all groups, n = 5. Rats were exposed to either: 1) brief handling (naïve controls), 2) one day of 2-hour restraint, or 3) ten consecutive days of 2-hour restraint. Thirty minutes after termination of the last session of restraint, rats were rapidly decapitated and the hypothalamus was dissected. Note: the scale on the y-axis differs between CRF1 and CRF2 receptor mRNA graphs. (a) 10 days of 2-hour restraint increased CRF mRNA levels in BN rats (*p = 0.022 vs. Naïve BN rats, Tukey’s test). (b) Naïve BN rats had greater levels of CRF1 receptor mRNA (*p = 0.050 vs. Naïve WKY rats, independent t-test). (c) Naïve BN rats showed a trend towards having greater levels of CRF2 receptor mRNA (*p = 0.053 vs. Naïve WKY rats, independent t-test).
A two-way ANOVA showed that there was a trend towards a main effect of rat strain (p = 0.084) on CRF1 receptor mRNA levels (Fig 9b). An independent t-test comparing naïve WKY rats to naïve BN rats confirmed that BN rats have greater CRF1 receptor mRNA levels than WKY rats [t(7) = −2.363; p = 0.050] (Fig 9b). For CRF2 receptor mRNA levels, a two-way ANOVA showed that there was no significant main effect of rat strain or restraint and no interaction (Fig 9c). However, an independent t-test revealed a trend for naïve BN rats to have greater CRF2 receptor mRNA levels than naïve WKY rats (p = 0.053) (Fig 9c).
4. Discussion
The present study was designed to investigate whether there is a temporal parallel among the effects of restraint stress on PPI, corticosterone, and CRF and CRF receptor gene expression in two rat strains. In a recent study, we demonstrated that on days three and five of restraint stress, BN rats show less PPI than non-stressed controls. In the present study, we also examined the effect of restraint on PPI in WKY rats and expanded the number of stress sessions to ten because WKY rats are less sensitive to the effect of CRF on PPI than BN rats and might, thus, be less sensitive to the effect of stress. Since WKY rats show a prolonged endocrine response to stress (Rittenhouse et al., 2002), we also wanted to determine whether WKY rats would show a different hormonal response and/or a different pattern of gene expression following stress than BN rats. Plasma corticosterone levels and brain CRF and CRF receptor mRNA levels were assessed after rats were exposed to either 1 or 10 days of 2-hour restraint stress. The major findings of the present study are that 1) restraint stress attenuates the increase in PPI caused by repeated testing in both WKY and BN rats, and BN rats are more sensitive to the effects of restraint on PPI than WKY rats, 2) restraint-induced increases in corticosterone levels mirror the effect of restraint on PPI in WKY rats but not in BN rats, 3) laterality effects on CRF gene expression were observed for the amygdala, whereby restraint increases CRF gene expression in the left, but not right, amygdala, and 4) some restraint-induced changes in CRF and CRF receptor gene expression precede changes in behavior (PPI) while other changes in gene expression coincide with changes in behavior in a strain- and brain region-dependent manner.
In the first experiment, stress-naïve rats exhibit an increase in PPI over the 5 testing sessions of the 10-day experiment. However, in repeatedly restrained rats, this increase in PPI is significantly blunted in both WKY and BN rats. Although we have previously shown that restraint stress attenuates the increase in PPI caused by repeated testing, in addition to decreasing PPI directly, in BN rats (Sutherland and Conti, 2010, submitted), this is the first time that the effect of restraint stress on PPI has been examined in WKY rats. Interestingly, the effect of restraint on PPI is first observed in BN rats on day 3 of restraint, while the effect is not observed in WKY rats until day 10 of restraint. It is intriguing that the sensitivity to the effects of restraint on PPI mirrors the sensitivity that the two rat strains exhibit to ICV infusion of CRF. A low dose of CRF (0.3 μg) decreases PPI in BN rats while WKY rats require a higher dose of CRF (3.0 μg) to achieve a reduction in PPI (Conti, 2005; Sutherland et al., 2008). The fact that BN rats have higher levels of CRF, CRF1 and CRF2 receptor mRNA in the frontal cortex, as observed in this study, and have higher levels of CRF protein and greater CRF receptor binding in the frontal cortex than WKY rats (Lahmame et al., 1997) may explain the sensitivity of the BN rats to CRF and possibly to stress.
Plasma corticosterone levels were assessed in order to determine whether the endocrine response to stress parallels the behavioral effect of stress on PPI or whether the two stress effects are dissociable. Restraint-induced increases in corticosterone levels mirror the effect of restraint on PPI in WKY rats, as neither was affected by 1 day of restraint while both were affected by 10 days of restraint. In BN rats, 1 day of restraint increases corticosterone but there is habituation of this response by day 10, even though PPI continues to be affected at this time. This suggests that the effect of restraint on PPI is not due to peripheral effects or to glucocorticoids in BN rats. Indeed, CRF administered peripherally does not decrease PPI (Conti, 2005) and glucocorticoids do not appear to mediate the decrease in PPI seen in CRF over-expressing mice (Groenink et al., 2008), suggesting that the effect of CRF on PPI is independent of its effects on HPA axis activity. Thus, restraint-induced increases in corticosterone levels mirror the effect of restraint on PPI in WKY rats but not in BN rats.
Studies with rats suggest that the right amygdala plays a more important role than the left for the processing of emotional memories (Baker and Kim, 2004; Coleman-Mesches and McGaugh, 1995y, b, c; Scicli et al., 2004). Thus, we thought it would be intriguing to examine whether such laterality exists with respect to stress-induced changes in CRF and CRF receptor gene expression. In the left amygdala, acute restraint increases CRF gene expression in both rat strains, with CRF mRNA levels returning to baseline in BN rats by day 10 while levels remain elevated in WKY rats by day 10. Thus, CRF mRNA levels in the left amygdala, corticosterone levels, and behavior (PPI) in WKY rats are each increasingly affected with increasing sessions of restraint. However, in BN rats, the behavioral effect remains in the face of habituated CRF mRNA and corticosterone levels. It is possible that CRF protein levels, which were not examined in this study, are still elevated on day 10 of restraint and that this is responsible for the decrease in PPI observed in BN rats on day 10. In the right amygdala, restraint did not significantly affect CRF mRNA levels in either rat strain. Thus, restraint increases CRF mRNA levels in the left amygdala only, revealing laterality effects on gene expression for the amygdala. It has been shown that the right amygdala is important for rapid stimulus assessment while the left amygdala is important for a slower, more sustained and detailed processing of emotional stimuli (Glascher and Adolphs, 2003; Wright et al., 2001). Thus, it is possible that increases in CRF gene expression in the right amygdala could have been detected if levels had been examined immediately after restraint termination or after fewer sessions of restraint.
Although we observed statistically significant restraint stress-induced increases in CRF gene expression in the left amygdala of both WKY and BN rats, it is possible that these increases would have been greater had we examined specific subregions of the amygdala, such as the central nucleus, instead of performing a dissection of the whole amygdala. Although the following studies did not examine hemispheric differences, it has been shown that acute and repeated restraint stress increases CRF mRNA levels (Hsu et al., 1998; Kalin et al., 1994), CRF-like immunoreactivity (Santibanez et al., 2006), and extracellular concentrations of CRF (Hand et al., 2002; Merali et al., 1998; Pich et al., 1995) in the central nucleus of the amygdala. It is also possible that in the present study, subtle variations in the dissection of the amygdala from the different brains led to subtle differences in each “amygdala” sample in which varying amounts of tissue from specific nuclei were included or excluded. The present study was designed to begin to characterize how restraint stress alters CRF and CRF receptor gene expression in a variety of brain regions that mediate the stress response, synthesize CRF, express CRF receptors, and modulate PPI and to determine whether these changes in gene expression parallel behavioral changes in response to stress. However, future studies will utilize immunohistochemical techniques to examine how restraint stress alters CRF and CRF receptor protein levels in brain sections, as this will allow us to examine specific nuclei within the amygdala and within other brain regions of interest.
In examining whether the temporal pattern of gene expression parallels the change in the behavioral response to stress, we saw the following patterns emerge (Table 1). In the first pattern, both stress-induced changes in gene expression and behavior are seen only following repeated stress. The second pattern shows that stress-induced increases in gene expression precede, and continue to be observed during, stress-induced behavioral changes. In the third pattern, stress-induced increases in gene expression precede stress-induced behavioral changes and these changes in gene expression return to basal levels at a time when stress-induced behavioral changes are observed.
Table 1.
Summary of temporal patterns observed in CRF and CRF receptor gene expression and behavior (PPI). In the first pattern, both stress-induced changes in gene expression and behavior are seen only following repeated stress. The second pattern shows that stress-induced increases in gene expression precede, and continue to be observed during, stress-induced behavioral changes. In the third pattern, stress-induced increases in gene expression precede stress-induced behavioral changes and these changes in gene expression return to basal levels at a time when stress-induced behavioral changes are observed. Abbreviations: HPC, hippocampus; Hypo, hypothalamus; LA, left amygdala.
| GENE EXPRESSION | PPI | |||
|---|---|---|---|---|
| Acute Restraint (1 Day) | Repeated Restraint (10 Days) | Acute Restraint (1 Day) | Repeated Restraint (10 Days) | |
| Pattern 1 | ||||
| Example 1: (Fig 7b) | No effect vs. controls | ↑ CRF1 receptor in HPC of BN rats | No effect vs. controls | ↓ PPI vs. controls |
| Example 2: (Fig 9a) | No effect vs. controls | ↑ CRF in Hypo of BN rats | ||
| Pattern 2 | ||||
| Example 1: (Fig 6a) | ↑ CRF in LA of WKY rats | ↑ CRF in LA of WKY rats | No effect vs. controls | ↓ PPI vs. controls |
| Example 2: (Fig 7a) | ↑ CRF in HPC of BN rats | ↑ CRF in HPC of BN rats | ||
| Pattern 3 | ||||
| Example 1: (Fig 6a) | ↑ CRF in LA of BN rats | No effect vs. controls | No effect vs. controls | ↓ PPI vs. controls |
In the present study, we observed increased CRF gene expression in response to acute and repeated restraint in the hippocampus. Since the hippocampus modulates PPI (Swerdlow et al., 2001), an increase in CRF release could result in increased activation of CRF receptors and decreased PPI. We also observed that acute restraint increases CRF gene expression in the amygdala, another PPI-modulating brain region (Swerdlow et al., 2001). Although PPI is not altered by acute restraint stress in BN rats, it is possible that an acute increase in CRF mRNA levels causes an increase in CRF protein levels and, presumably, increased CRF release at a time when restraint has been shown to alter PPI. For example, restraint alters PPI in BN rats as early as day 3. We do not know how restraint affects CRF gene expression on day 3, as this time-point was not assessed in this study. Thus, it may be that the acute increase in CRF gene expression observed in the amygdala of BN rats is important in initiating a cascade of events that results in restraint-induced changes in PPI. It seems unlikely that the repeated restraint-induced increase in CRF gene expression in the hypothalamus mediates the effect of restraint on PPI in BN rats, since this region does not modulate PPI (Swerdlow et al., 2001). It is more likely that hypothalamic CRF is mediating the endocrine effect of stress (Vale et al., 1981), which does not mirror the behavioral effect of stress in this study and suggests to us that the effect of restraint on PPI is not due to peripheral effects or to glucocorticoids in BN rats. Although repeated restraint also increases CRF1 receptor gene expression in the hippocampus, this most likely does not have an effect on PPI since CRF1 receptors do not appear to mediate the effect of restraint stress on PPI in BN rats (Sutherland and Conti, 2010, submitted). In fact, we have shown that CRF2 receptor blockade attenuates the effect of restraint on PPI in BN rats (Sutherland and Conti, 2010, submitted). Thus, increased CRF gene expression in the hippocampus and amygdala may affect PPI via CRF2 receptors.
We and others have shown that ICV CRF does not decrease PPI via its effects on serotonin (5-HT) (Sutherland et al., 2008) or dopamine (DA) acting at D1 or D2 receptors (Vinkers et al., 2007). It remains possible that stress alters PPI via effects on neurotransmitters other than CRF that are known to be released during stress (De Souza and Van Loon, 1986; Glavin et al., 1983; Mo et al., 2008; Shimizu et al., 1992) and to decrease PPI, including 5-HT, DA, and/or norepinephrine (Alsene et al., 2006; Carasso et al., 1998; Johansson et al., 1995; Mansbach et al., 1988; Martinez and Geyer, 1997; Sipes and Geyer, 1994).
Despite the wide array of strain differences on behavior, corticosterone levels, and gene expression, it should be noted that WKY rats were purchased from Charles River while BN rats were purchased from Harlan-Sprague Dawley in this study. Although it is possible that the observed strain differences may reflect differences in the rearing and/or shipping environments between the two vendors, we believe that the genetic influence is stronger than the potential influence of vendor. In the present study, and in previous studies from our laboratory (Conti, 2005; Sutherland et al., 2008), BN rats purchased from Harlan show less PPI than WKY rats purchased from Charles River. BN rats purchased from Harlan also consistently show less PPI than WKY rats bred in a La Jolla colony at the University of California at San Diego (Conti et al., 2002; Palmer et al., 2000). Additionally, we have purchased BN rats from Charles River and examined the effects of ICV infusion of CRF on PPI. When these data (unpublished observations) and data from two separate experiments in which BN rats were purchased from Harlan (Sutherland et al., 2008) are compared, the average percent PPI in saline-treated rats in the three experiments is 20–23%. When PPI is assessed 30 minutes after infusion of 0.3 μg CRF, the average percent PPI in all three experiments is reduced to 8–9%. Not only are the basal levels of PPI very similar between BN rats purchased from two different vendors, but also their behavioral response (PPI) to the same dose of CRF infusion is also extremely similar. Thus, it appears that the genetic influence is important in mediating behavior while the effect of vendor is negligible.
It should also be noted that during the 2-hour restraint period, rats did not have access to food. However, food was not withheld from control rats during the restraint interval. Although it is possible that food deprivation may have had some effects on PPI, corticosterone levels, and/or gene expression, this seems unlikely given that all experimental procedures were performed during the light cycle, when little food consumption occurs. In rats, up to 80% of caloric intake occurs at night, during the dark cycle (Balagura et al., 1975; Teitelbaum and Campbell, 1958).
In this study, behavior (PPI) and corticosterone/gene expression were examined in separate cohorts of animals in order to avoid any potential effects of PPI testing itself on the other measures. It would be interesting to examine the effects that the PPI testing procedure itself has on the endocrine stress response and gene expression in WKY and BN rats in future studies as this may allow us to collect data on behavior, stress hormones, and gene expression in the same animals so that correlational analyses could be conducted. Additionally, it would be extremely interesting to examine whether anxiolytics, antipsychotics, or CRF receptor antagonists block the effects of restraint stress on CRF gene expression. We have previously shown that antipsychotics block the CRF-induced decrease in PPI (Conti et al., 2005) and that a selective CRF2 receptor antagonist blocks the restraint stress-induced decrease in PPI (Sutherland and Conti, 2010, submitted) but we have yet to examine how these treatments may affect gene expression.
In conclusion, our results demonstrate that restraint stress attenuates the increase in PPI caused by repeated testing in both WKY and BN rats, and that BN rats are more sensitive to the effects of restraint on PPI than WKY rats. Restraint-induced increases in corticosterone levels mirror the effect of restraint on PPI in WKY rats but not in BN rats. Laterality effects on CRF gene expression were observed for the amygdala, whereby restraint increases CRF gene expression in the left, but not right, amygdala. Some restraint-induced changes in CRF and CRF receptor gene expression precede changes in behavior (PPI) while other changes in gene expression coincide with changes in behavior in a rat strain- and brain region-dependent manner. Since PPI can be assessed in both humans and rodents under nearly identical parameters, it is a useful tool for investigating human information processing deficits using animal models. This and future studies aimed at uncovering how restraint stress alters sensorimotor gating at the behavioral and genetic levels may have important clinical applications and bring us closer to understanding stress-sensitive psychiatric disorders in which information processing deficits are a hallmark.
Restraint stress decreases prepulse inhibition in two inbred rat strains.
Restraint differentially affects corticosterone levels in the two inbred rat strains.
Restraint increases CRF gene expression in the left, but not right, amygdala.
Restraint alters CRF gene expression in a strain- and brain region-dependent manner.
Acknowledgments
We thank Dr. Richard Mains and Dr. Danielle Moore for their help assessing plasma corticosterone levels using RIA. We thank Mr. Andrew Breglio for assessing CRF1 and CRF2 receptor mRNA levels. We also thank Ms. Verica Milivojevic for taking photographs of the rat brain. These studies were supported by MH065467, AA017367, and 5T32NS041224-08. The authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsene KM, Carasso BS, Connors EE, Bakshi VP. Disruption of prepulse inhibition after stimulation of central but not peripheral alpha-1 adrenergic receptors. Neuropsychopharmacology. 2006;31:2150–61. doi: 10.1038/sj.npp.1300989. [DOI] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Amygdalar lateralization in fear conditioning: evidence for greater involvement of the right amygdala. Behav Neurosci. 2004;118:15–23. doi: 10.1037/0735-7044.118.1.15. [DOI] [PubMed] [Google Scholar]
- Balagura S, Harrell LE, Roy E. Effect of the light-dark cycle on neuroendocrine and behavioral responses to scheduled feeding. Physiol Behav. 1975;15:245–7. doi: 10.1016/0031-9384(75)90243-7. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–43. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–8. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–15. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Morrison JL, Walker EL, Merchant KM. Differential regulation of behavioral, genomic, and neuroendocrine responses by CRF infusions in rats. Pharmacol Biochem Behav. 2004;77:447–55. doi: 10.1016/j.pbb.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Carasso BS, Bakshi VP, Geyer MA. Disruption in prepulse inhibition after alpha-1 adrenoceptor stimulation in rats. Neuropharmacology. 1998;37:401–4. doi: 10.1016/s0028-3908(98)00051-3. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–50. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CP, Pearse RV, 2nd, O’Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–95. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- Coleman-Mesches K, McGaugh JL. Differential effects of pretraining inactivation of the right or left amygdala on retention of inhibitory avoidance training. Behav Neurosci. 1995a;109:642–7. doi: 10.1037//0735-7044.109.4.642. [DOI] [PubMed] [Google Scholar]
- Coleman-Mesches K, McGaugh JL. Differential involvement of the right and left amygdalae in expression of memory for aversively motivated training. Brain Res. 1995b;670:75–81. doi: 10.1016/0006-8993(94)01272-j. [DOI] [PubMed] [Google Scholar]
- Coleman-Mesches K, McGaugh JL. Muscimol injected into the right or left amygdaloid complex differentially affects retention performance following aversively motivated training. Brain Res. 1995c;676:183–8. doi: 10.1016/0006-8993(95)00108-3. [DOI] [PubMed] [Google Scholar]
- Conti LH. Characterization of the effects of corticotropin-releasing factor on prepulse inhibition of the acoustic startle response in Brown Norway and Wistar-Kyoto rats. Eur J Pharmacol. 2005;507:125–34. doi: 10.1016/j.ejphar.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Conti LH, Costill JE, Flynn S, Tayler JE. Effects of a typical and an atypical antipsychotic on the disruption of prepulse inhibition caused by corticotropin-releasing factor and by rat strain. Behav Neurosci. 2005;119:1052–60. doi: 10.1037/0735-7044.119.4.1052. [DOI] [PubMed] [Google Scholar]
- Conti LH, Murry JD, Ruiz MA, Printz MP. Effects of corticotropin-releasing factor on prepulse inhibition of the acoustic startle response in two rat strains. Psychopharmacology. 2002;161:296–303. doi: 10.1007/s00213-002-1025-2. [DOI] [PubMed] [Google Scholar]
- Czyrak A, Mackowiak M, Chocyk A, Fijal K, Gadek-Michalska A, Wedzony K. 8-OHDPAT-induced disruption of prepulse inhibition in rats is attenuated by prolonged corticosterone treatment. Neuropsychopharmacology. 2003;28:1300–10. doi: 10.1038/sj.npp.1300165. [DOI] [PubMed] [Google Scholar]
- Davis SE, Munson PJ, Jaffe ML, Rodbard D. Radioimmunoassay data processing with a small programmable calculator. J Immunoassay. 1980;1:15–25. doi: 10.1080/01971528008055773. [DOI] [PubMed] [Google Scholar]
- De Souza EB, Van Loon GR. Brain serotonin and catecholamine responses to repeated stress in rats. Brain Res. 1986;367:77–86. doi: 10.1016/0006-8993(86)91581-7. [DOI] [PubMed] [Google Scholar]
- Dinn WM, Harris CL, Raynard RC. Posttraumatic obsessive-compulsive disorder: a three-factor model. Psychiatry. 1999;62:313–24. doi: 10.1080/00332747.1999.11024877. [DOI] [PubMed] [Google Scholar]
- Dirks A, Groenink L, Schipholt MI, van der Gugten J, Hijzen TH, Geyer MA, et al. Reduced startle reactivity and plasticity in transgenic mice overexpressing corticotropin-releasing hormone. Biol Psychiatry. 2002;51:583–90. doi: 10.1016/s0006-3223(01)01323-3. [DOI] [PubMed] [Google Scholar]
- Dirks A, Groenink L, Westphal KG, Olivier JD, Verdouw PM, van der Gugten J, et al. Reversal of startle gating deficits in transgenic mice overexpressing corticotropin-releasing factor by antipsychotic drugs. Neuropsychopharmacology. 2003;28:1790–8. doi: 10.1038/sj.npp.1300256. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Gabr RW, Gladfelter WE, Birkle DL, Azzaro AJ. In vivo microdialysis of corticotropin releasing factor (CRF): calcium dependence of depolarization-induced neurosecretion of CRF. Neurosci Lett. 1994;169:63–7. doi: 10.1016/0304-3940(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Glascher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci. 2003;23:10274–82. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavin GB, Pare WP, Sandbak T, Bakke HK, Murison R. Restraint stress in biomedical research: an update. Neurosci Biobehav Rev. 1994;18:223–49. doi: 10.1016/0149-7634(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Glavin GB, Tanaka M, Tsuda A, Kohno Y, Hoaki Y, Nagasaki N. Regional rat brain noradrenaline turnover in response to restraint stress. Pharmacol Biochem Behav. 1983;19:287–90. doi: 10.1016/0091-3057(83)90054-0. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Southwick SM, Davis M, Charney DS. Baseline startle amplitude and prepulse inhibition in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1996;64:169–78. doi: 10.1016/s0165-1781(96)02942-3. [DOI] [PubMed] [Google Scholar]
- Groenink L, Dirks A, Verdouw PM, de Graaff M, Peeters BW, Millan MJ, et al. CRF1 not glucocorticoid receptors mediate prepulse inhibition deficits in mice overexpressing CRF. Biol Psychiatry. 2008;63:360–8. doi: 10.1016/j.biopsych.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Hand GA, Hewitt CB, Fulk LJ, Stock HS, Carson JA, Davis JM, et al. Differential release of corticotropin-releasing hormone (CRH) in the amygdala during different types of stressors. Brain Research. 2002;949:122–30. doi: 10.1016/s0006-8993(02)02972-4. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–89. [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic and temporal factors in the evocation of startle. J Acoust Soc Am. 1968;43:269–82. doi: 10.1121/1.1910776. [DOI] [PubMed] [Google Scholar]
- Hsu D, Chen F, Takahashi L, Kalin N. Rapid stress-induced elevations in corticotropin-releasing hormone mRNA in rat central amygdala nucleus and hypothalamic paraventricular nucleus: An in situ hybridization analysis. Brain Research. 1998;788:305–10. doi: 10.1016/s0006-8993(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Johansson C, Jackson DM, Zhang J, Svensson L. Prepulse inhibition of acoustic startle, a measure of sensorimotor gating: effects of antipsychotics and other agents in rats. Pharmacol Biochem Behav. 1995;52:649–54. doi: 10.1016/0091-3057(95)00160-x. [DOI] [PubMed] [Google Scholar]
- Jones DN, Kortekaas R, Slade PD, Middlemiss DN, Hagan JJ. The behavioural effects of corticotropin-releasing factor-related peptides in rats. Psychopharmacology. 1998;138:124–32. doi: 10.1007/s002130050654. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Takahashi LK, Chen FL. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Research. 1994;656:182–6. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- Keane TM, Marshall AD, Taft CT. Posttraumatic stress disorder: etiology, epidemiology, and treatment outcome. Annu Rev Clin Psychol. 2006;2:161–97. doi: 10.1146/annurev.clinpsy.2.022305.095305. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–28. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Lahmame A, Grigoriadis DE, De Souza EB, Armario A. Brain corticotropin-releasing factor immunoreactivity and receptors in five inbred rat strains: relationship to forced swimming behaviour. Brain Res. 1997;750:285–92. doi: 10.1016/s0006-8993(96)01368-6. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995;92:836–40. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology. 1988;94:507–14. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- Martinez DL, Geyer MA. Characterization of the disruptions of prepulse inhibition and habituation of startle induced by alpha-ethyltryptamine. Neuropsychopharmacology. 1997;16:246–55. doi: 10.1016/S0893-133X(96)00240-0. [DOI] [PubMed] [Google Scholar]
- Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. The Journal of Neuroscience. 1998;18:4758–66. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo B, Feng N, Renner K, Forster G. Restraint stress increases serotonin release in the central nucleus of the amygdala via activation of corticotropin-releasing factor receptors. Brain Res Bull. 2008;76:493–8. doi: 10.1016/j.brainresbull.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AA, Dulawa SC, Mottiwala AA, Conti LH, Geyer MA, Printz MP. Prepulse startle deficit in the Brown Norway rat: a potential genetic model. Behav Neurosci. 2000;114:374–88. doi: 10.1037//0735-7044.114.2.374. [DOI] [PubMed] [Google Scholar]
- Pare WP, Glavin GB. Restraint stress in biomedical research: a review. Neurosci Biobehav Rev. 1986;10:339–70. doi: 10.1016/0149-7634(86)90017-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- Pich EM, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. The Journal of Neuroscience. 1995;15:5439–47. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004;24:6545–52. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse PA, Lopez-Rubalcava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology. 2002;27:303–18. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–33. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Santibanez M, Gysling K, Forray M. Desipramine prevents the sustained increase in corticotropin-releasing hormone-like immunoreactivity induced by repeated immobilization stress in the rat central extended amygdala. Journal of Neuroscience Research. 2006;84:1270–81. doi: 10.1002/jnr.21023. [DOI] [PubMed] [Google Scholar]
- Scicli AP, Petrovich GD, Swanson LW, Thompson RF. Contextual fear conditioning is associated with lateralized expression of the immediate early gene c-fos in the central and basolateral amygdalar nuclei. Behav Neurosci. 2004;118:5–14. doi: 10.1037/0735-7044.118.1.5. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Take S, Hori T, Oomura Y. In vivo measurement of hypothalamic serotonin release by intracerebral microdialysis: significant enhancement by immobilization stress in rats. Brain Res Bull. 1992;28:727–34. doi: 10.1016/0361-9230(92)90252-s. [DOI] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacology. 1994;33:441–8. doi: 10.1016/0028-3908(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Spina MG, Merlo-Pich E, Akwa Y, Balducci C, Basso AM, Zorrilla EP, et al. Time-dependent induction of anxiogenic-like effects after central infusion of urocortin or corticotropin-releasing factor in the rat. Psychopharmacology (Berl) 2002;160:113–21. doi: 10.1007/s00213-001-0940-y. [DOI] [PubMed] [Google Scholar]
- Spruijt BM, van Hooff JA, Gispen WH. Ethology and neurobiology of grooming behavior. Physiol Rev. 1992;72:825–52. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Steel R, Torrie J. Principles and procedures of statistics: a biometrical approach. New York: McGraw-Hill; 1980. [Google Scholar]
- Sutherland JE, Page ME, Conti LH. The effect of corticotropin-releasing factor on prepulse inhibition is independent of serotonin in Brown Norway and Wistar-Kyoto rats. Pharmacol Biochem Behav. 2008;89:324–37. doi: 10.1016/j.pbb.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–86. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biol Psychiatry. 1993;33:298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Campbell BA. Ingestion patterns in hyperphagic and normal rats. J Comp Physiol Psychol. 1958;51:135–41. doi: 10.1037/h0042719. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10:743–57. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Morris M, Chavez C, Martin S, Wang J. Effect of adrenalectomy and corticosterone replacement on prepulse inhibition and locomotor activity in mice. Br J Pharmacol. 2004;142:543–50. doi: 10.1038/sj.bjp.0705511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Risbrough VB, Geyer MA, Caldwell S, Low MJ, Hauger RL. Role of dopamine D1 and D2 receptors in CRF-induced disruption of sensorimotor gating. Pharmacol Biochem Behav. 2007;86:550–8. doi: 10.1016/j.pbb.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104:667–85. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–83. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]