Abstract
CD4+ T cells of the Th17 subtype are over-represented in the aged immune system. Dendritic cells (DC) play a critical role in naive CD4+ T cell differentiation. However, expression of cytokines by aged DC that promote differentiation or survival of Th17 cells has not been extensively investigated. Using bone marrow-derived DC from C57BL/6 mice of different ages we compared cytokine production after DC activation by Toll-like receptor agonists for TLR4 and/or TLR7/8. DC-derived TNF-α and IL-12p70 production and expression of DC co-stimulatory molecules did not vary significantly by age indicating TLR expression, function and signal transduction were intact in aged DC. There were relatively minor age-related changes in TGF-β and IL-6 which promote Th17 differentiation, but IL-23, a Th17-suvival cytokine, increased more than 40-fold across the lifespan. DC-derived prostaglandin E2 (PGE2) also increased with age and the up-regulation of IL-23 expression by aged DC was blocked by indomethacin that prevents PGE2 production, and by antagonists of PGE2 receptors. Exogenous PGE2 added to DC cultures further enhanced IL-23 production from aged but not young DCs. These data indicate that age-related changes in DC PGE2 production are necessary, but not sufficient to induce DC IL-23 production. Such changes may play a role in the expansion of Th17 cells in the aged immune system.
Keywords: Aging, Dendritic cells, T helper cells, IL-23, PGE2, Th17
1. Introduction
Aging is associated with declining immune function, also known as immune senescence characterized by low level constitutive inflammation, poor vaccine responses, and multiple changes in T cell function. One major aspect of immune senescence is a change in CD4+ T helper (Th) subtypes; naïve Th cells typically differentiate into one of four Th subsets: Th1, Th2, Th17 or Treg cells (Bettelli et al., 2007; Bettelli et al., 2008; Dong, 2008; Gutcher and Becher, 2007). The Th17 subtype is of particular interest in aging research as recent data suggests this subtype is over-represented in aged mice (Huang et al., 2008) and humans (Van Duin et al., 2009), and Th17 cells are profoundly pro-inflammatory producing or inducing multiple pro-inflammatory cytokines, nitric oxide, metaloproteinases and chemokines, or stimulating the recruitment and activation of other immune cells (Kolls and Linden, 2004; Park et al., 2005; Shen et al., 2005; Ye et al., 2001b). Dendritic cells (DCs) play a pivotal role in directing Th differentiation by eliciting directing cytokines that promote or inhibit specific Th subtypes. Critical signals in mice for driving naïve Th cells toward Th17 differentiation include TGFβ and IL-6, but if TGFβ is present without IL-6 naïve Th cells tend to differentiate into Treg cells (Bettelli et al., 2007; Bettelli et al., 2008; Dong, 2008; Gutcher and Becher, 2007; Harrington et al., 2006; Mucida et al., 2007). DCs also produce IL-23, which does not directly differentiate naïve T cells into Th17 cells, but provides the essential stimulus required to establish and expand the Th17 subtype (Bettelli et al., 2007; Bettelli et al., 2008; Dong, 2008; Gutcher and Becher, 2007; Harrington et al., 2006; McGeachy and Cua, 2008; Mucida et al., 2007). Conditions favoring a one type of Th subset usually have an inhibitory effect on the other subsets (Dong, 2008; Schmidt-Weber et al., 2007).
The factors that lead to enhanced Th17 immune responses in the aged are unclear. Aged naïve T cells appear to be “pre-programmed” with a higher likelihood to differentiate into Th17 subtypes when compared to naïve T cells of young adults (Huang et al., 2008), however, there have been very limited studies of aged DCs that are likely to also play a role in this process. We recently described an age-related increase in production of the Th17 survival cytokine IL-23 by aged DCs stimulated with toll-like receptor (TLR) agonists. This change was accompanied by altered histone methylation at the IL-23 promoter site (El Mezayen et al., 2009). Other studies have reported conflicting results regarding cytokines produced by aged DCs that might promote or inhibit Th17 development. Using different TLR agonists, Tesar et al. reported similar levels of IL-6 by DCs isolated from young and old mice (Tesar et al., 2006). Grolleau-Julius et al. reported variable effects of age on IL-12p70, IL-10, and IL-6 production, using Gram-negative bacterial lipopolysaccharide (LPS), a TLR 4 agonist (Grolleau-Julius et al., 2006; Tesar et al., 2006).
Other factors influence Th17 differentiation. Prostaglandin E2 (PGE2) strongly promotes the differentiation of naïve T cells toward the Th17 phenotype (Steger et al., 1997), (Chizzolini et al., 2008; Steger et al., 1997), and advanced age is associated with increases in PGE2 by macrophages (Choudhry et al., 1999; Wu et al., 2004). PGE2 influences T cell function as a consequence of altered early signaling events in T cell activation and results in changes in the cytokine profile of T cells. PGE2 is known as an inflammatory mediator that suppresses Th1 differentiation by raising intracellular cyclic AMP (cAMP) concentration (Goodwin and Ceuppens, 1983; Hasler et al., 1983; Betz and Fox, 1991; Gold et al., 1994; Hilkens et al., 1995; Harris et al., 2002). A recent study reported that PGE2 targets four G protein-coupled receptors termed EP1, EP2, EP3, and EP4, and that the immunosuppressive effects of PGE2 in both T cells and DCs is mediated by EP2 and EP4 (Nataraj et al., 2001).
In this study, we examined the effect of age on bone marrow-derived DC cytokine production, particularly those cytokines affecting Th17 differentiation. DCs from mice at four ages across the adult spectrum (2–18 months) were stimulated by TLR agonists, alone or in combination. Consistent with our previously reported study, DCs from mice 12 months or older secreted much greater amounts of IL-23 than did DCs from young mice after stimulation with TLR4 (LPS) or TLR7/8 agonist (R848) alone or in combination. Secretion of PGE2 by DCs was also increased with aging, and the age-related up-regulation of IL-23 was dependent upon PGE2. Young adult DCs were unaffected by the addition of exogenous PGE2 or inhibitors of PGE2 production/function. This suggests PGE2 is necessary, but not sufficient to induce the age-related changes in DC IL-23 production. Augmented production of both IL-23 and PGE2 by aged DCs may play a role in the shift toward a Th17 favorable milieu in the older adult immune system.
2. Materials and Methods
2.1. Animals
Male C57Bl/6 mice used in this study were catogerized as follows: young (2–3 months), early middle age (6 months), late middle age (12 months) and old (17–18 months). All mice were purchased from Harlan-Sprague-Dawley (Indianapolis, IN) or the National Institute of Aging and housed under specific pathogen-free conditions. All experiments were conducted according to the guidelines of Wake Forest University’s Animal Care and Use committee.
2.2. Cell culture
BMDCs were differentiated as described previously (Inaba et al., 1992; El Mezayen et al., 2009). Briefly, bone marrow progenitor cells were collected from murine femurs and tibias. After red blood cell lysis and filtration, cells were cultured for six days in RPMI 1640 medium (Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (Hyclone), 2mM glutamine, 2μM mercaptoethanol, 20 ng/mL GM-CSF (Invitrogen, Carlsbad, CA) and 0.8 μg/mL gentamicin. On days three and five, the medium was changed and supplemented with 20 ng/mL GM-CSF. PGE2 or Indomethacin (Sigma Aldrich, St. Louis, MO) at 10μM concentration were added to the DCs culture for 1 and 24 hours, respectively, prior to stimulation with TLR agonists. EP2 receptor antagonist AH 6809 or EP4 receptor antagonist AH 23848 ( Sigma Aldrich, St. Louis, MO) at 50 μM concentration were added to the cell culture for 1 hour before stimulation.
2.3. TLR stimulation
On day six, 2×106 cells/ml of differentiated cells (DCs) were stimulated with TLR agonists for 12 hours and the supernatants were collected for cytokine measurment. For mRNA analysis, cells were collected at 9 hours. The stimuli used were: 2.5 μg/mL of LTA, a TLR2 agonist (Sigma, St Louis, MO), 100 ng/mL of LPS (E. coli 0111:B4; Sigma, St. Louis, MO), a TLR4 agonist, , 3 μM of R848, a TLR7/8 agonist (3M, St. Paul, MN).
2.4. Costimulatory molecule expression
DCs were differentiated and stimulated as above. Cells were harvested, approximately 3×105 cells were suspended in BD Pharmingen stain buffer (BD Biosciences), stained for 30 minutes with PE conjugated antiCD80 or anti DCSIGN and FITC conjugated anti CD86 or anti CD40 antibodies from eBiosciences (San Diego, CA) and fixed in 200 μl of BD FACS Lysis Buffer (BD Biosciences). Samples were stored at 4 °C until data was collected on a BD FACS flow cytometer. Data were analyzed using FlowJo software.
2.5. Cytokine ELISA
All ELISA kits were purchased from eBioscience (San Diego, CA). PGE2 ELISA kit was purchased from R&D systems (Minneapolis, MN). The assays were performed according to manufacturer’s instructions.
2.6. Quantitative real-time PCR
Two micrograms of total RNA were reverse-transcribed to cDNA in a 25-μl reaction volume containing 0.2 μM dNTPs, 2.5 μM oligo (dT), 5 mM MgCl2, and 0.25 units/μl murine leukemia reverse transcriptase (Applied Biosystems, Foster City, CA). PCR was performed using the ABI prism 7000 Sequence Detection System (Applied Biosystems). The PCR reaction was carried out in a 25-μl volume containing 8 μl cDNA. Mouse IL-23, TGFβ, EP2 and EP4 pre-designed TaqMan primer/probe sets (Applied Biosystems) were used. Sample data were normalized to GAPDH mRNA level as an internal control. PCR conditions were: 1 cycle of 2 min at 50°C and 10 min at 95°C, followed by 40 cycles with 15s at 95°C and 1 min at 60°C.
2.7. Western blot analysis
Total protein extracts were prepared from DCs by incubation in lysis buffer (RIPA buffer, Pierce Biotechnology, Rockford, IL) and protease inhibitor cocktail. Supernatants were removed by centrifugation at 5,000 rpm for 10 min at 4°C. After incubation on ice for 15 min, lysates were centrifuged at 15,000 rpm for 15 min at 4°C. The supernatants were aliqoted and kept at −70°C, after measuring the total protein content. Equal amounts of proteins (20 μg) were resolved on SDS-PAGE and electroblotted onto PVDF membranes. The blots were blocked and probed overnight at 4°C with primary antobodies againts EP2 and EP4 (Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were washed and incubated with appropriate HRP-conjugated secondary antibodies. Proteins were detected using ECL reagent (Pierce Biotechnology, Rockford, IL).
2.8. Statistical analysis
Data were analyzed by SPSS software using ANOVA for multiple group comparisons, with least significant difference (LSD), and unpaired student t-test for comparison between individual groups. Data are expressed as mean ± SEM of three experiments. A p value of ± 0.05 was considered statistically significant.
3. Results
3.1 DCs isolated from mice across the adult age spectrum maintain TLR responsiveness
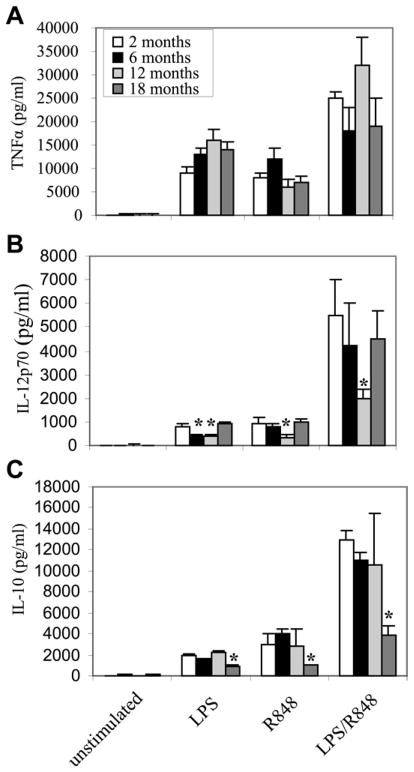
To test whether DCs isolated from all age groups retained sufficient TLR expression, signal transduction capacity and downstream events (up-regulation of well-established markers of DC maturation), expression of the inflammatory cytokine TNF-α (Figure 1A), surface expression of co-stimulatory molecules CD80 and CD86 (Figure 2), CD40 and DCSIGN (Table 1) after stimulation with the TLR4 agonist LPS and/or the TLR7/8 agonist R848. There were no statistically significant age-related changes in any of these parameters. Expression of the Th1 favorable cytokine IL-12 (Figure 1B) was decreased at 12 month compared to other age groups. In addition, the Th1-suppressive cytokine IL-10 declined (about 2 fold) from the youngest to the oldest age group (Figure 1C).
Figure 1. Age-related changes in DCs expression of TNF-α, IL-12 and IL-10.
Bone marrow progenitor cells were isolated from C57BL/6 mice (n = 4–5 mice), differentiated with GM-CSF for six days, and then stimulated with the indicated TLR agonist, individually or in combination. After 12 h, culture supernatants were collected, and cytokines levels were measured by ELISA. Samples were assayed in triplicate. Data represent the mean ± SEM from three experiments. *, statistically significant p≤0.05 (18.months vs. 2, 6, or 12 months).
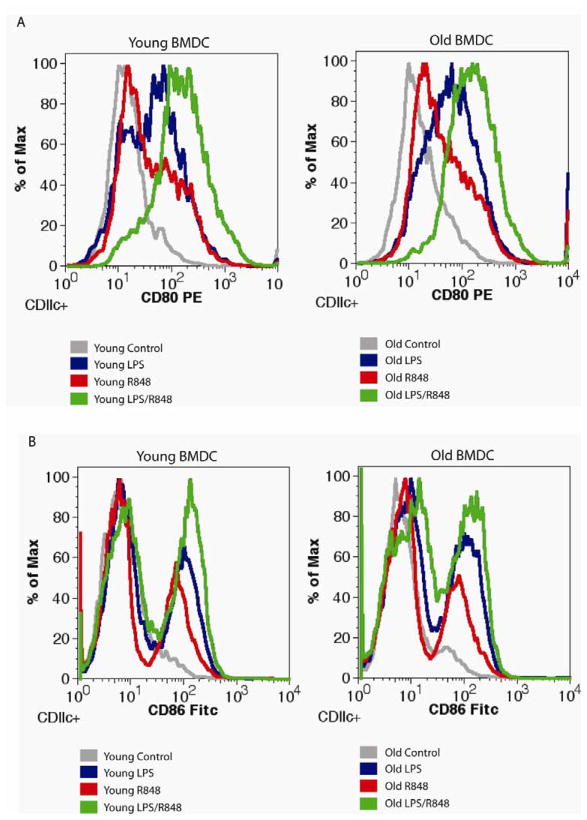
Figure 2. Expression of CD80 and CD86 on DCs from young and old mice after TLR stimulation.
Bone marrow progenitor cells from 2 and 18 months old C57BL/6 mice were differentiated with GM-CSF for six days, and then stimulated with the indicated TLR agonist, individually or in combination. After 24 hours (A) CD80 or (B) CD86 was measured by flow cytometry after labeling with fluorescent antibodies. These are representative experiments. (CD80 n= 3, CD86 n= 4).
Table 1.
Expression of CD40 and DCSIGN by BMDCs from young and old mice stimulated with TLR agonists.
| marker | CD40 | DCSIGN | ||
|---|---|---|---|---|
| group | young | old | young | old |
| LPS | 1.1±0.3 | 1.6±0.2* | 1.2±0.3 | 1.8±0.4* |
| R848 | 0.9±0.1 | 1.2±0.1* | 1.4±0.3 | 2.3±0.4* |
| LPS/R848 | 1.3±0.2 | 1.7±0.2* | 1.5±0.2* | 2.3±0.4* |
Bone marrow progenitor cells were isolated from C57BL/6 mice (n = 4 mice), differentiated with GM-CSF for six days then stimulated with agonists for TLR4 (LPS) and TLR7 (LPS/R848) alone or in combination for 24 hours. The expression of costimulatory molecules, CD40 and DCSIGN, was measured by flow cytometry with fluorescently labeled antibodies. The fold change over the control was calculated from the mean fluorescent intensity. (young n=4; old n=4). Data are presented as mean ± SEM of three experiments.
, statistically significant (p≤0.05; vs. control). None of the differences between young and old DCs were statistically significant.
3.2. Aging is associated with an increase in DC-derived Th17 favorable cytokines
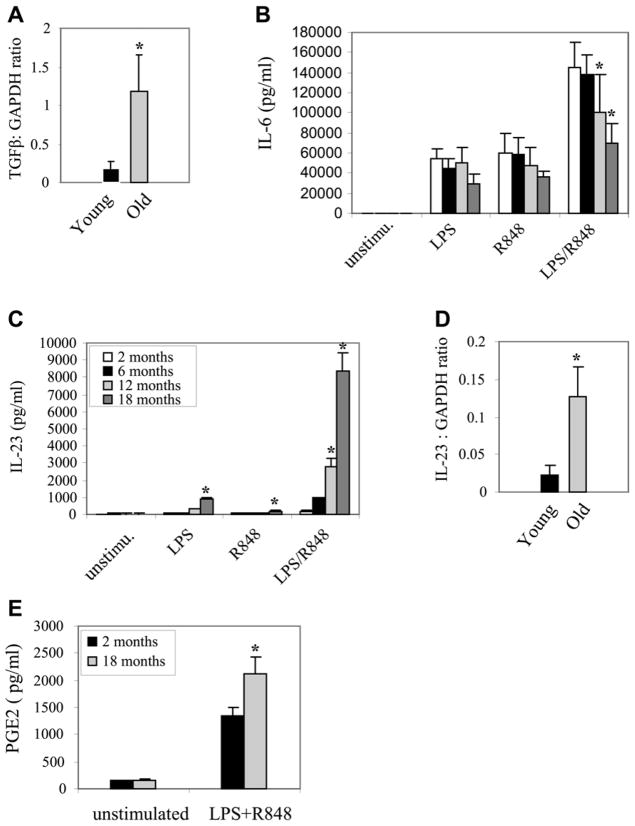
To assess the capacity of DCs to support the development of Th17 cells, we performed preliminary experiments using different TLR agonists to determine the most potent signal for inducing IL-23. We found that the TLR4 agonist, LPS and the TLR 7/8 agonist R848 were effective and that the combination of LPS + R848 was particularly potent (data not shown), with maximum IL-23 expression detected at 12 hours. Th17 differentiation in mice is supported by the simultaneous expression of TGFβ and IL-6, while the establishment and expansion of the Th17 subtype is dependent upon IL-23. Figure 3A shows that TGFβ expression, as measured by mRNA, is increased following TLR activation. (Note: protein was not examined as the serum in the media contains vast amounts of TGFβ limiting the ability to detect changes). IL-6 expression declines somewhat with age, but is maintained at ng/mL levels in all age groups (Figure 3B). The secretion of IL-23 progressively increased with advancing age from 2 months to 18 months of age (Figure 3C). This increase was most pronounced for the combination of LPS/R848 in which mean IL-23 production was more than 40-fold higher in 18 month-old mice (8245±918 pg/mL) than in 2 month-old mice (192±26 pg/mL; p<0.001).
Figure 3. Expression of Th17-favorable mediators (Compounds) IL-23, PGE2 and TGF-β is significantly increased in aged DCs upon TLR activation.
Bone marrow progenitor cells were isolated from C57BL/6 mice (n = 4–5 mice), differentiated with GM-CSF for six days, and then stimulated with the indicated TLR agonist, individually or in combination. After 9 h, total RNA was isolated and analyzed for gene expression by real-time PCR. Culture supernatants were collected after 12 hours, and cytokines levels were measured by ELISA. Samples were assayed in triplicate. Data represent the mean ± SEM from three experiments. *, statistically significant p≤0.05 (18 months vs. 2, 6, or 12 months).
To determine whether the increase in IL-23 production was due to changes in transcription or mRNA translation, we measured IL-23 mRNA levels 9 h after stimulation with LPS + R848. Since the significant increase in IL-23 production started at 12 months of age, real-time RT-PCR was performed on DCs isolated from 2 or 12 month-old mice. Twelve month-old mice showed about a 6-fold increase in IL-23 mRNA level compared with young mice (Figure 3D). These results suggested that the increase in IL-23 production in aged DCs was, at least in part, due to increases in gene transcription. In addition, we have reported that IL-23 mRNA was significantly increased after 3–6 hours of stimulation with LPS+R848 (El Mezayen et al., 2009).
Increased expression of PGE2 from macrophages and other APCs with age has been well documented, and PGE2 promotes the development of Th17 cells. We hypothesized that DC-derived PGE2 production may increase with age similar to IL-23. PGE2 in the culture media 12 hours after stimulation of DCs with LPS + R848 was significantly higher from DCs derived from 18 month-old mice vs. 2 month-old mice (2118 ± 242 vs. 1354 ± 149 pg/ml), respectively P = 0.04 (Figure 3E).
3.3. PGE2 modulates IL-23 production only from aged DCs
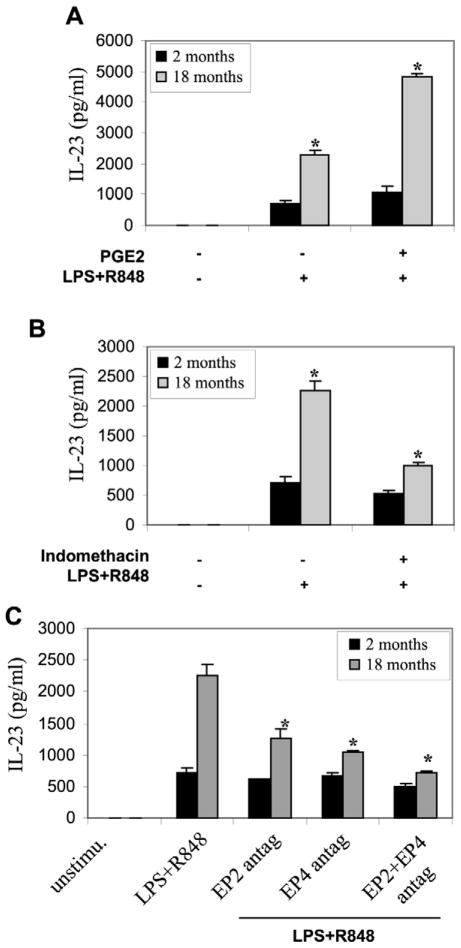
In prior experiments, maximum cytokine production occurred at 12 hours post-stimulation but the same pattern of age-related changes occurred at earlier time points, facilitating one-day time frame for the following experiments. Addition of PGE2 to the media 1 hour prior to and during stimulation of DCs significantly increased IL-23 production by aged DCs whereas IL-23 expression by young DCs was unaffected (Figure 4A). Further, addition of indomethacin to the culture for 24 h prior to stimulation significantly reduced the augmented production of IL-23 by aged DCs (Figure 4B), but again had little effect on young DC IL-23 production.
Figure 4. PGE2 upregulates IL-23 production from aged DCs.
(A) PGE2 increases IL-23 production by aged DCs. Bone marrow progenitor cells were isolated from C57BL/6 mice (n = 5 mice), differentiated with GM-CSF for six days then cultured in media with or without 10 mM PGE2. After 1h, LPS+R848 was added for an additional 6 h. IL-23 levels in the culture supernatants were determined by ELISA. (B) Indomethacin decreases IL-23 production that is induced by LPS+R848 in aged DCs. DCs were isolated and grown with GMCSF for 6 days then treated with 10 μM Indomethacin, After 24 h, LPS+R848 was added for an additional 6 h. IL-23 levels in the culture supernatants were determined by ELISA. (C) EP2 and/or EP4 antagonist reduces IL-23 induction by LPS+R848. DCs were isolated and stimulated as above then treated with 50μM of EP2 antagonist (AH6809), EP4 antagonist (AH 23848), or EP2+EP4 antagonists. After 1 h, LPS+R848 was added for an additional 6 h, IL-23 levels in the culture supernatants were determined by ELISA. Samples were assayed in triplicate. Data represent the mean ± SEM from three experiments. *, statistically significant (p<0.05), 18 months vs. 2 months.
PGE2 exerts cellular changes through four subtypes of G protein-coupled receptors termed EP1, EP2, EP3, and EP4. EP2 and EP4 receptors are known to transduce PGE2 signal, and the immunosuppressive action of PGE2 on T cells has been linked to EP2 and EP4 receptors (Nataraj et al., 2001b; Sugimoto and Narumiya, 2007). Addition of the EP2 receptor antagonist (AH6809), EP4 receptor antagonist (AH23848), or EP2 plus EP4 antagonists in combination significantly reduced IL-23 production by aged DCs (Figure 4C), further demonstrating that the enhanced production of IL-23 by aged murine DCs is PGE2-dependent and that this effect is mediated through EP2 and EP4 receptors. In addition, we observed a modest decrease in IL-23 production in young DCs after the addition of EP2 plus EP4 antagonists, which was not statstically significant.
3.4. PGE2 receptors are equally expressed in young and aged DCs
To determine whether the increase in IL-23 expression in aged DCs was mediated by changes in PGE2 receptors, we compared EP2 and EP4 mRNA and protein levels in young and aged Dcs before and 6 hours after stimulation with LPS + R848. EP2 and EP4 mRNA was expressed in resting cells and was significantly increased after stimulation in both age groups (Figure 5A and B). However, we did not detect any significant differences in the levels of EP2 or EP4 mRNA levels between young and aged DCs after stimulation. In addition, we detected almost similar kinetic changes in the EP2 and EP4 protein levels (Figure 5C). These results suggest that the increase in IL-23 expression in aged DCs is likely driven by the increase in PGE2 secretion rather than changes in EP2 or EP4 expression.
Figure 5. Aging does not influence EP2 or EP4 expression in DCs.
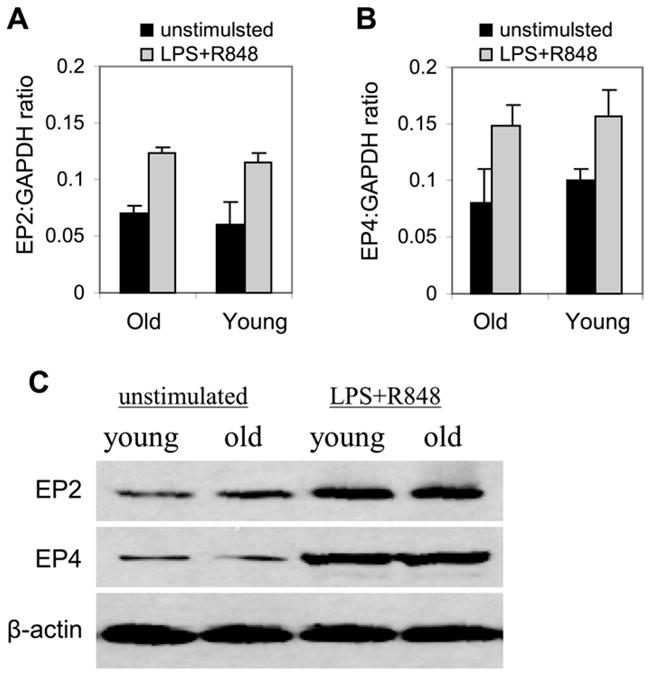
Bone marrow progenitor cells were isolated from C57BL/6 mice (n = 5 mice), differentiated with GM-CSF for six days were left unstimulated or stimulated with LPS+R848 for 6 hours. Total RNA was isolated and analyzed for EP2 and EP4 mRNA by real-time PCR and protein by western blotting. Data represent the mean ± SEM from three experiments. *, statistically significant (p≤0.05; 2 vs. 18 months).
4. Discussion
Previous studies have suggested that age has a relatively small effect on antigen presentation or co-stimulatory molecule expression by DCs (Grolleau-Julius et al., 2008; Grolleau-Julius et al., 2006; Steger et al., 1996; Steger et al., 1997; Tesar et al., 2006), but very limited data exist regarding specific Th-directing cytokine production by DCs in the aged immune system. Since DCs play a major role in T cell differentiation and survival and aging appears to be associated with increased numbers of Th17 cells, we examined the Th17-promoting cytokines produced by DCs across the adult age spectrum.
Data in figures 1 and 2 indicate the expression and function of TLR-receptors and subsequent signal transduction matures both young and old BMDCs. The combined TLR4 and TLR7/8 matures the greatest number of DCs and induces the greatest cytokine production. Our results showed an age-related change in the DCs cytokine profile with significant enhancement of the Th17-favoring cytokines IL-23, PGE2 and TGFβ. This shift was apparent by 12 months of age and maintained until at least 18 months of age. To ensure that the secretion changes were from DC, in preliminary studies, we purified BMDC cultures by magnetic bead separation (data not shown). These pure cultures of DC were found to have preserved or increased secretion of IL-23 after stimulation. It is therefore the DC that likely account for these age-associated IL-23 effects. Our culture conditions resulted in 85–95% pure DCs using magnetic bead-based cell separation techniques. Although we did not specifically purify DCs in these experiments, and the DC fraction accounted for essentially all the IL-23 expression (data not shown). However, PGE2 in the reported experiments could have derived from either the DCs or other cells present in the culture.
The most significant change was observed with IL-23 expression. The 40-fold increase in IL-23 across the adult age spectrum appeared to be due to changes in transcription as IL-23 mRNA levels increased at a time point prior to maximum secretion. While we found some variations in IL-12 and IL-10 cytokine production by DCs from different age groups, which showed significant decline at 12 and 18 months respectively. The changes in IL-6 expression were modest and did not show a specific age-related pattern.
Our results indicated that the increase in IL-23 expression in DCs was age dependent and not due to changes in physiological signaling by TLRs. In this regard, Tesar et al. (Tesar et al., 2006) found no change in secretion of TNF-α in aged DCs, suggesting all the necessary cellular machinery to sense TLR agonists and trigger signal transduction required for target gene expression were functional in older adults. Our studies extended these findings by including DCs from mice of intermediate ages and a combination of agonists. Interestingly, in Tesar’s study and our study, IL-6 was unchanged by age when TLR agonists were employed individually. However, our results indicated that the combination of TLR 4 and TLR 7/8 agonists may stretch the limits of signaling in older DCs as IL-6 production declined modestly in the oldest animals. Our findings also extend those of Grolleau-Julius et al. who showed no change in IL-12p70 secretion after DCs were stimulated with LPS under certain, but not all, conditions (Grolleau-Julius et al., 2008; Grolleau-Julius et al., 2006). We included stimulation with a TLR 7/8 agonist and a combination of TLR 4 and TLR 7/8 agonists. We also included middle aged mice, which was not investigated by Grolleau-Julius et al. (Grolleau-Julius et al., 2008; Grolleau-Julius et al., 2006). In addition, Grolleau-Julius et al. used DCs cultured with both GM-CSF plus IL-4, while we used GM-CSF only.
Th17 differentiation of naïve T cells is promoted by TGF-β in the presence of IL-6. While IL-6 declined somewhat with age in our studies, the effect of IL-6 in driving Th differentiation toward Th17 and away from Treg appears to be a threshold effect (i.e. presence or absence of IL-6), not a gradual increase across a range of concentrations (Bettelli et al., 2007; Bettelli et al., 2008; Dong, 2008; Gutcher and Becher, 2007; Harrington et al., 2006; McGeachy and Cua, 2008; Mucida et al., 2007; Schmidt-Weber et al., 2007). Thus, it is likely that both young and old DCs produce sufficient IL-6 to support Th17 differentiation. Further, T cell differentiation is influenced by the total cytokine milieu, not just those produced by DCs, and IL-6 is augmented with age in many other cell systems studied. PGE2 also supports Th17 differentiation (Chizzolini et al., 2008; Khayrullina et al., 2008) and was increased after stimulation of DCs isolated from older adult mice. Thus, our results suggest that aged DCs create a Th17 favorable milieu, particularly in the presence of combined TLR4 and TLR7/8 activation.
The most important finding of this study is that IL-23 production from DCs increases with age, and that this shift is very likely mediated by PGE2. Though IL-23 does not directly induce Th17 cell differentiation, it is essential for establishment and proliferation of the Th17 lineage (Bettelli et al., 2007; Bettelli et al., 2008; Dong, 2008; Gutcher and Becher, 2007; Harrington et al., 2006; McGeachy and Cua, 2008; Mucida et al., 2007; Schmidt-Weber et al., 2007). Previous studies reported that PGE2 has a differential effect on in vitro production of IL-23 and IL-12 by DCs, promoting IL-23 release and inhibiting IL-12p70 production (Sheibanie et al., 2004). In addition, recent studies reported that older macrophages produced higher levels of PGE2, and that inhibiting PGE2 production improved Th1 responses, suggesting that PGE2 significantly contributes to a Th17 favorable milieu (Beharka et al., 2002; Chizzolini et al., 2008; Han et al., 2000; Haynes, 2005; Khayrullina et al., 2008; Wu and Meydani, 2004). Our results clearly demonstrated that PGE2 significantly increased IL-23 production by aged DCs, and inhibiting PGE2 through two different methods reduced IL-23 expression to levels nearly equivalent to that of young DCs. In addition, our results indicated that aged DCs maintained the expression of PGE2 receptors EP2 and EP4. It appears, however, that all IL-23 expression is not totally dependent on PGE2, as IL-23 secretion was not abolished by these maneuvers, but reduced primarily in the aged DCs. This suggests only that portion of the IL-23 that is modified with age is modulated by PGE2.
Recently, we reported that the increase in IL-23 p19 expression in aged DCs was due to changes in the transcription associated with chromatin remodeling characterized by di- and trimethylation of histone H3K4 and an increase in c-Rel binding at the p19 promoter. In young DCs, the p19 subunit gene promoter was tri-methylated only at H3K4 and bound by both p65 and c-Rel (El Mezayen et al., 2009). The functional significance of this age-associated change in signal-activated gene expression is not clear. It is likely that IL-23 up-regulation and the subsequent effects on Th cell differentiation into Th17 favorable milieu is influenced by PGE2, and that the age-associated increase in PGE2 expression in BMDCs may signal epigentetic changes in IL-23 p19 promoter or co-activator/repressor activity. Cucinotta et al. reported that PGE2 stimulates the transcription of the IL-8 gene in human T cells by activating protein kinase C in a p38- and phosphatidylinositol 3-kinase (PI-3K)-dependent manner, resulting in changes in the activity of the transcription factor C/EBP homologous protein CHOP. More recently, the same group showed a PGE- dependent upregulation of the CHOP protein, which induced changes in transcription factors binding at the AP-1 binding site in the IL-8 promotor (Caristi et al., 2005; Cucinotta et al., 2008).
It has long been established that Th1 responses inhibit Th2 responses, and vice versa. There also appears to be a reciprocal relationship between Th1 and Th17 responses. Thus, another potential implication of our findings is that an age-related shift toward a Th17 milieu may contribute to the waning Th1 and/or Th2 responses noted with age, i.e. promoting immune senescence. To illustrate this concept, one can compare the ratio of Th1 mediating IL-12p70 vs. Th17-mediating IL-23 – the IL12:IL23 ratio declines by more than 30-fold from age 2 months to age 12 months and remains stable to at least 18 months of age. If a similar Th17-skew with aging occurs in vivo, the implications in older adults could be profound. Th17 immunity is essential for host defense against a variety of pathogens. Most well defined are models of K. pneumonia infection (Ye et al., 2001a) and the memory response of several vaccines (Higgins et al., 2006; Khader et al., 2007; Malley et al., 2006). Furthermore, we have reported increased host defense against Brucella abortus by old mice that was associated with shift toward a Th17 response (High et al., 2007). If Th17 immunity is maintained or favored in the aged and can be triggered with appropriate stimuli, it may be exploited to improve host defenses. This study indicates that a combination of TLR agonists, particularly TLR4 and TLR7/8, may be useful in triggering Th17 immunity in the aged. The aged respond poorly to current vaccine formulations (Haynes, 2005; High, 2004; Kovaiou et al., 2007) and the use of TLR agonists to improve vaccine responses for older adults has been proposed (Haynes, 2005;High, 2004;Kovaiou et al., 2007;van et al., 2006). This strategy would be supported by the intrinsic property of older naïve T cell predisposition toward Th17 differentiation (Huang et al., 2008). Studies of Agrawal et al. (Agrawal et al., 2007) in human monocyte-derived DCs from young and aged donors suggested a plausible mechanism for the changes in cytokine secretion we observed in our aged DCs. They found that the increases in cytokine secretion with age were associated with increased PTEN expression and that inhibition of PI-3K, the target of PTEN’s negative regulation, was able to convert young DCs to an aged phenotype. IL-23 was not measured in those studies, nor PTEN or PI3K in our study. This is an avenue of current investigation in our laboratory.
Acknowledgments
This work was supported by National Institutes of Health (NIAID) Grant AI057952 and National Institutes on Aging (NIA)R21/AG 003382501 (to KPH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- Beharka AA, Wu D, Serafini M, Meydani SN. Mechanism of vitamin E inhibition of cyclooxygenase activity in macrophages from old mice: role of peroxynitrite. Free Radic Biol Med. 2002;32:503–511. doi: 10.1016/s0891-5849(01)00817-6. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146:108–113. [PubMed] [Google Scholar]
- Caristi S, Piraino G, Cucinotta M, Valenti A, Loddo S, Teti D. Prostaglandin E2 induces interleukin-8 gene transcription by activating C/EBP homologous protein in human T lymphocytes. J Biol Chem. 2005;280:14433–14442. doi: 10.1074/jbc.M410725200. [DOI] [PubMed] [Google Scholar]
- Chizzolini C, Chicheportiche R, Alvarez M, de RC, Roux-Lombard P, Ferrari-Lacraz S, Dayer JM. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry MA, Ahmed Z, Sayeed MM. PGE(2)-mediated inhibition of T cell p59(fyn) is independent of cAMP. Am J Physiol. 1999;277:C302–C309. doi: 10.1152/ajpcell.1999.277.2.C302. [DOI] [PubMed] [Google Scholar]
- Cucinotta M, Visalli M, Aguennouz M, Valenti A, Loddo S, Altucci L, Teti D. Regulation of interleukin-8 gene at a distinct site of its promoter by CCAAT enhancer-binding protein homologous protein in prostaglandin E2-treated human T cells. J Biol Chem. 2008;283:29760–29769. doi: 10.1074/jbc.M803145200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- El Mezayen R, El Gazzar M, Myer R, High KP. Aging-dependent upregulation of IL-23p19 gene expression in dendritic cells is associated with differential transcription factor binding and histone modifications. Aging Cell. 2009;8:553–565. doi: 10.1111/j.1474-9726.2009.00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold KN, Weyand CM, Goronzy JJ. Modulation of helper T cell function by prostaglandins. Arthritis Rheum. 1994;37:925–933. doi: 10.1002/art.1780370623. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983;3:295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Grolleau-Julius A, Garg MR, Mo R, Stoolman LL, Yung RL. Effect of aging on bone marrow-derived murine CD11c+CD4−CD8alpha- dendritic cell function. J Gerontol A Biol Sci Med Sci. 2006;61:1039–1047. doi: 10.1093/gerona/61.10.1039. [DOI] [PubMed] [Google Scholar]
- Grolleau-Julius A, Harning EK, Abernathy LM, Yung RL. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. 2008;68:6341–6349. doi: 10.1158/0008-5472.CAN-07-5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutcher I, Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest. 2007;117:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SN, Wu D, Ha WK, Beharka A, Smith DE, Bender BS, Meydani SN. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology. 2000;100:487–493. doi: 10.1046/j.1365-2567.2000.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MC, Liao JJ, Bonasera S, Longo DL, Geotzl EJ. Nuclear factor -kappa B-dependent reversal of aging-induced alteration in T cell cytokines. FaSEB J. 2008;22:2142–2150. doi: 10.1096/fj.07-103721. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- Hasler F, Bluestein HG, Zvaifler NJ, Epstein LB. Analysis of the defects responsible for the impaired regulation of EBV-induced B cell proliferation by rheumatoid arthritis lymphocytes. II. Role of monocytes and the increased sensitivity of rheumatoid arthritis lymphocytes to prostaglandin E. J Immunol. 1983;131:768–772. [PubMed] [Google Scholar]
- Haynes L. The effect of aging on cognate function and development of immune memory. Curr Opin Immunol. 2005;17:476–479. doi: 10.1016/j.coi.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- High KP. Infection as a cause of age-related morbidity and mortality. Ageing Res Rev. 2004;3:1–14. doi: 10.1016/j.arr.2003.08.001. [DOI] [PubMed] [Google Scholar]
- High KP, Prasad R, Marion CR, Schurig GG, Boyle SM, Sriranganathan N. Outcome and immune responses after Brucella abortus infection in young adult and aged mice. Biogerontology. 2007;8:583–593. doi: 10.1007/s10522-007-9106-6. [DOI] [PubMed] [Google Scholar]
- Hilkens CM, Vermeulen H, van Neerven RJ, Snijdewint FG, Wierenga EA, Kapsenberg ML. Differential modulation of T helper type 1 (Th1) and T helper type 2 (Th2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. Eur J Immunol. 1995;25:59–63. doi: 10.1002/eji.1830250112. [DOI] [PubMed] [Google Scholar]
- Huang MC, Liao JJ, Bonasera S, Longo DL, Goetzl EJ. Nuclear factor-kappaB-dependent reversal of aging-induced alterations in T cell cytokines. FASEB J. 2008;22:2142–2150. doi: 10.1096/fj.07-103721. [DOI] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181:721–735. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Kovaiou RD, Herndler-Brandstetter D, Grubeck-Loebenstein B. Age-related changes in immunity: implications for vaccination in the elderly. Expert Rev Mol Med. 2007;9:1–17. doi: 10.1017/S1462399407000221. [DOI] [PubMed] [Google Scholar]
- Malley R, Srivastava A, Lipsitch M, Thompson CM, Watkins C, Tzianabos A, Anderson PW. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006;74:2187–2195. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Nataraj C, Thomas DW, Tilley SL, Nguyen MT, Mannon R, Koller BH, Coffman TM. Receptors for prostaglandin E(2) that regulate cellular immune responses in the mouse. J Clin Invest. 2001;108:1229–1235. doi: 10.1172/JCI13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;120:247–254. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Sheibanie AF, Tadmori I, Jing H, Vassiliou E, Ganea D. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J. 2004;18:1318–1320. doi: 10.1096/fj.03-1367fje. [DOI] [PubMed] [Google Scholar]
- Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- Steger MM, Maczek C, Grubeck-Loebenstein B. Morphologically and functionally intact dendritic cells can be derived from the peripheral blood of aged individuals. Clin Exp Immunol. 1996;105:544–550. doi: 10.1046/j.1365-2249.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger MM, Maczek C, Grubeck-Loebenstein B. Peripheral blood dendritic cells reinduce proliferation in in vitro aged T cell populations. Mech Ageing Dev. 1997;93:125–130. doi: 10.1016/s0047-6374(96)01835-0. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S. Prostaglandin E receptors 1. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- Tesar BM, Walker WE, Unternaehrer J, Joshi NS, Chandele A, Haynes L, Kaech S, Goldstein DR. Murine [corrected] myeloid dendritic cell-dependent toll-like receptor immunity is preserved with aging. Aging Cell. 2006;5:473–486. doi: 10.1111/j.1474-9726.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- Van Duin D, Siegel HK, Kramer KL, Fairchild RL. Th17 cells in human aging. Infectious Diseases Society of America; Philadelphia: 2009. p. 1067. [Google Scholar]
- Wu D, Meydani SN. Mechanism of age-associated up-regulation in macrophage PGE2 synthesis. Brain Behav Immun. 2004;18:487–494. doi: 10.1016/j.bbi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001a;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001b;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]