Abstract
αA-crystallin is a lens chaperone that plays an essential role in the transparency and refractive properties of the lens. Mutations in αA-crystallin have been associated with the development of hereditary cataracts. The R49C mutation of αA-crystallin (αA-R49C) was identified in a four-generation Caucasian family with hereditary cataracts. The αA-R49C protein forms larger-than-normal oligomers in the lens and has decreased solubility. This aberrant αA-R49C oligomerization suggests that protein folding is altered. However, whether activation of the unfolded protein response (UPR) occurs during crystallin mutation-induced cataract formation and whether the UPR causes cell death under these conditions is unclear. We investigated UPR activation in an in vivo mouse model of αA-R49C using immunoblot analysis of lens extracts. We found that expression of the endoplasmic reticulum (ER) chaperone, BiP, was 5-fold higher in homozygous αA-R49C lenses than in wild type lenses. Analysis of proteins typically expressed during the UPR revealed that ATF-4 and CHOP levels were also higher in homozygous lenses than in wild type lenses, while the opposite was true of ATF-6 and XBP-1. Taken together, these findings show that mutation of αA-crystallin induces activation of the UPR during cataract formation. They also suggest that the UPR is an important mediator of cell death observed in homozygous αA-R49C lenses.
Keywords: Crystallin, cataract, mutation, lens, unfolded protein response
Introduction
The unfolded protein response (UPR) is a signaling pathway used by cells to deal with the production of misfolded proteins [1,2,3]. The UPR has been implicated in the pathogenesis of protein aggregation diseases such as neurodegenerative diseases (Alzheimer’s [4] and Parkinson’s [5]) and retinal degeneration [3]. The role of the UPR is also being investigated in other protein aggregation pathologies such as cataracts [6,7]. Cells respond to misfolded proteins by increasing ER chaperone expression, decreasing general transcription, and increasing ER-associated degradation [1]. An ER chaperone known as BiP is responsible for interacting with misfolded proteins. BiP is normally bound to IRE1, ATF-6, and PERK in the ER membrane; however, it dissociates from these molecules to aid in the folding of newly synthesized proteins and transport of these proteins from the ER. BiP dissociation allows for the activation of its binding partners and induction of the UPR [2]. Lens cells in diabetic cataracts [8] and from mice expressing collagen 4a mutant proteins [7] experience ER stress and subsequently activate the UPR.
αA-crystallin is a molecular chaperone that is highly expressed in the lens and prevents the non-specific aggregation and insolubilization of denaturing proteins [9,10]. αA-crystallin is responsible, along with other lens crystallins, for maintaining lens transparency throughout life [11]. Mutations in αA-crystallin lead to cell death [12]. In addition, constant production of misfolded proteins in the lens has been shown to lead to activation of the UPR and cataract onset [7]. Several mutations in αA-crystallin that lead to early onset cataract have been identified [12,13,14,15]. For example, the R49C mutation in αA-crystallin (αA-R49C) has been identified as the cause of hereditary cataract in a four-generation family [12]. To understand the mechanisms of cataract formation in an in vivo animal model, we have previously generated and characterized knock-in mice expressing αA-R49C [16,17,18,19]. We have found that αA-R49C has an increased oligomer size as well as decreased solubility [17]. Lenses expressing αA-R49C have been shown to develop cataracts in a gene dosage-dependent manner. Cataracts in the heterozygous mice become more severe with age probably because the amount of available wild type αA-crystallin is depleted by the unfolding of other proteins [19]. Cell death occurs in lenses homozygous for αA-R49C, and these lenses are smaller in size than wild type or heterozygous lenses. However, the mechanism underlying cell death in homozygous αA-R49C lenses is unknown. Here we investigated whether the expression of αA-R49C induces the UPR by comparing the expression of BiP and other UPR components between wild type and αA-R49C knock-in mouse lenses.
Materials and Methods
Materials
ATF-6 antibody was purchased from ProSci (Poway, CA) (Cat 3683). Chemiluminescent reagent, anti-rabbit and anti-mouse horseradish peroxidase-conjugated secondary antibodies, and antibodies against activating transcription factor-4 (ATF-4)/CREB2 (sc-200), XBP-1 (sc-7160), and CEBP homologous protein (CHOP)/GADD153 (sc-575) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). BiP antibody (Cat 610978) was purchased from BD Biosciences (San Jose, CA). Protease inhibitor cocktail (P8340) was purchased from Sigma-Chemical Co. (St. Louis, MO). Pre-cast NuPAGE gels and SDS-PAGE reagents were obtained from Invitrogen (Carlsbad, CA).
Animals and tissue dissection
Wild type and αA-R49C knock-in mice were generated by our laboratory [19]. All animal protocols were in accordance with the Washington University institutional policy on the use of animals in research. Mice were used at 2 weeks to 4 months of age. Adult mice were euthanized by CO2 inhalation. Whole eyes were then removed, and whole lenses were dissected for immunoblot analysis, as described below. Two to ten lenses were analyzed per genotype at each age. The experiments were repeated twice.
SDS PAGE and immunoblotting
Whole lenses were homogenized on ice in 400 or 500 μl phosphate-buffered saline (PBS) containing protease inhibitors. Homogenates were centrifuged at 10,000 × g at 4°C, and the resulting supernatants were collected (water-soluble fraction). Pellets were resuspended in 125 μl of 8 M urea in PBS containing protease inhibitor, incubated at room temperature for 10 min, and centrifuged at 4°C. The supernatant was then collected (water-insoluble fraction). Protein concentrations were determined using the Bio-Rad Protein Assay. All samples were supplemented with sample buffer (4X) and reducing agent (10X) (Invitrogen). Soluble fraction samples were heated at 95°C for 5 min. Equal amounts of total protein (15 – 40 μg) were then separated on 10% or 4 – 12% pre-cast NuPAGE gels (Invitrogen). Soluble and insoluble fraction samples were analyzed on the same gel. For immunoblot analysis, proteins were transferred to a PVDF membrane and probed with antibodies specific for BiP, ATF-4, CHOP, ATF-6, and XBP-1. Protein bands were quantified by densitometry using the Alpha Innotech FluorChem SP Imager and Software (Cell Biosciences, Inc., Santa Clara, CA).
Immunofluorescence and microscopy
Immunohistochemistry was conducted on mid sagittal lens sections to analyze BiP distribution in the lens. Mouse eyes were fixed, embedded in paraffin, and cross-sectioned into 3-μm sections for examination of lens cells as described previously [20]. Briefly, tissue sections were successively labeled with anti-human BiP monoclonal antibody and Alexa568-conjugated goat anti-mouse IgG (Invitrogen). Nuclei were then counterstained with DRAQ5 (Invitrogen), and sections were visualized using a Zeiss 510 confocal microscope (Carl Zeiss, Jena, Germany). Sections were also labeled with hematoxylin and eosin, and visualized using an Olympus microscope equipped with a Spot camera (Spot Diagnostic Instruments Inc., Sterling Heights, MI.).
Results
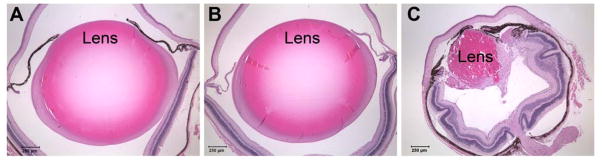
The effect of in vivo expression of the R49C mutant of αA-crystallin on lens histology is shown in Fig. 1. αA-R49C homozygous mutant lenses were smaller in size than the wild type or heterozygous mutant lenses. The heterozygous mutant lenses were protected against cell death [19]. We compared activation of the UPR in the lenses of αA-R49C mutants and age-matched, wild type controls by performing immunoblotting for BiP, CHOP, ATF-4, ATF-6, and XBP-1. Immunoblot analysis detected a BiP species with a molecular weight of 78 kDa, consistent with published reports [7,21]. Additional bands probably resulted from cross-reactivity with other proteins. BiP-specific bands were detected in the lenses of homozygous mutants and to a lesser extent, in wild type and heterozygous lenses (Fig. 2A). BiP was detected in the water-insoluble fractions, and in the homozygous mutant lenses, it was also detected in the water-soluble, cytoplasmic fraction. BiP levels were 5-fold higher in homozygous mutant lenses than in wild type lenses. Heterozygous αA-R49C mice had similar levels of BiP as wild type mice, indicating that the UPR is not activated in these lenses. Our previous work also showed that the αA-R49C heterozygous mutant mice also do not have small lenses and microphthalmia [16,18,19]. Thus, in heterozygous αA-R49C lenses, a single copy of wild type αA-crystallin, which is known to enhance cell survival [11,22,23], appears to ensure normal lens and eye growth, indicating that lens cells are able to cope with lower levels of αA-R49C.
Fig. 1.
Histology of wild type, αA-R49C heterozygous mutant, and αA-R49C homozygous mutant eye lenses. (A) Wild type lens, (B) αA-R49C heterozygous lens, and (C) αA-R49C homozygous lens. Note that the wild type and heterozygous mutant lenses were similar in size, but the αA-R49C homozygous lenses were smaller and had a highly disrupted morphology.
Fig. 2.
Immunoblot analysis of UPR-associated proteins in wild type, heterozygous αA-R49C (R49C Het), and homozygous αA-R49C (R49C Homo) lenses. (A) BiP expression. Note that BiP upregulation was detected in both the water-soluble and water-insoluble fractions of homozygous mutant lenses. The protein level was quantified by densitometry (see graph to right). (B) ATF-6 expression in the insoluble fraction. Loss of ATF-6 expression (50-kDa band) was accompanied by an increase in a 20-kDa species in homozygous αA-R49C lenses. (C) XBP-1 expression in the insoluble fraction. Note the loss of XBP-1 expression in αA-R49C homozygous mutant lenses. (D) CHOP expression in the soluble fraction. CHOP expression was 5-fold greater in homozygous αA-R49C lenses than in wild type lenses. (E) ATF-4 expression in the soluble fraction. Discrete bands with molecular weights between 55 kDa and 170 kDa were observed only in αA-R49C homozygous lenses. For each gel, equal amounts of wild type, αA-R49C heterozygous, and αA-R49C homozygous lens proteins were loaded in each lane. Error bars represent standard deviations from the mean of two to five experiments. Statistical analysis was performed by t-test. Asterisks indicate statistical significance with p ≤values 0.05.
To investigate the activation status of the UPR in mutant lenses, we analyzed ATF-6 expression in lenses. Immunoblot analysis revealed that ATF-6 was present as a 50-kDa species in wild type and heterozygous mutant lenses. However, this 50-kDa form of ATF-6 was absent in homozygous mutant lenses (Fig. 2B). The 50-kDa form of ATF-6 is considered to be the activated form of this transcription factor [1]. In homozygous lenses, ATF-6 was apparently present as a breakdown product with a molecular weight of approximately 20 kDa. To assess the activation status of IRE-1, we analyzed XBP-1 levels in wild type and mutant lenses. We detected an approximate 32-kDa band for XBP-1 in both wild type and heterozygous mutant lenses (Fig. 2C). However, XBP-1 levels in homozygous mutant lenses were about one-third of those in wild type or heterozygous lenses. Next, we investigated the activation of CHOP, a known pro-apoptotic molecule activated by the PERK branch of the UPR [24]. Immunoblot analysis of CHOP yielded bands with a molecular weight of approximately 22 kDa. These bands had 5-fold greater intensity in homozygous mutant lenses than in wild type or heterozygous mutant lenses (Fig. 2D). We also analyzed the expression of ATF-4, which was present as several discrete, high-molecular-weight species ranging from 55 – 170 kDa. All species were present at higher levels in homozygous αA-R49C lenses than in wild type lenses (Fig. 2E).
The distribution of BiP in the mouse lens has not yet been reported. Therefore, we performed immunofluorescence labeling for BiP in wild type and homozygous mutant mouse lenses. In wild type lenses, BiP immunofluorescence was detected only in the lens epithelium and on the periphery of the fiber cell nuclei just before nuclear breakdown (Fig. 3A). On the other hand, in the homozygous mutant lenses, BiP was detected in the lens epithelium as well as lens fiber cells throughout the lens (Fig. 3B), including cells in the anterior epithelium (Fig. 3C) and the posterior lens fiber cells of the lens (Fig. 3D). These observations support the immunoblot data. They also clearly show that BiP expression is strongly upregulated and that the UPR is activated in homozygous mutant lenses.
Fig. 3.
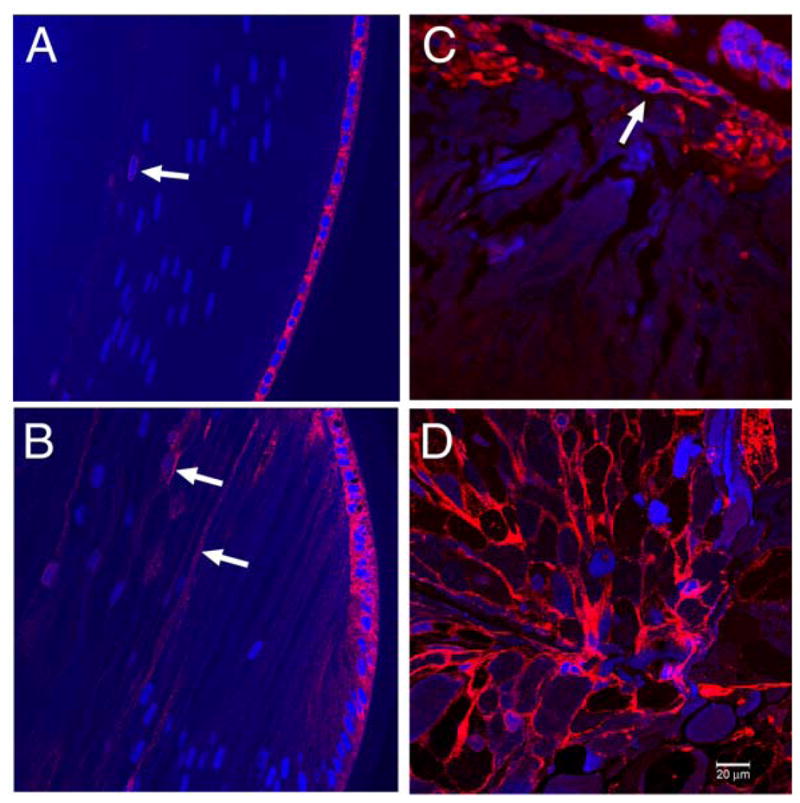
Immunofluorescence labeling for BiP in wild type and αA-R49C homozygous lenses. (A) Equatorial region of a wild type lens at 40× magnification. BiP labeling (red) was localized primarily to the region around the nuclei (stained with DRAQ5, blue) in lens epithelial cells. Some labeling was detected around the nuclei just before nuclear breakdown (arrow). (B–D) BiP labeling in the equatorial region (B), anterior region (C), and posterior region (D) of an αA-R49C homozygous lens. All images are 40× magnification. BiP was localized in lens epithelial and fiber cells (arrows). Bar = 20 μm for all images.
Discussion
Here we report that the UPR is activated in cataractous mouse lenses homozygous for αA-R49C, which is associated with hereditary cataract. We found that the PERK branch of the UPR was activated, whereas the IRE-1 and ATF-6 branches were downregulated. The PERK branch of the UPR is responsible for driving cells towards apoptosis under constitutive production of misfolded, aggregation-prone proteins. Our finding is particularly interesting in light of the reported increase in cell death observed in αA-R49C-expressing lens epithelial cells [12] and mouse lenses in vivo [19]. The IRE-1 branch of the UPR is known to elicit cytoprotective responses during ER stress [3]. Here, IRE-1 activity was downregulated in homozygous mutant lenses, as seen by lower XBP-1 levels. Initial activation of the UPR during cataractogenesis may be protective by virtue of its attempt to remove misfolded and aggregated proteins. However, prolonged ER stress can overwhelm the cellular protective mechanisms, ultimately triggering cell death [25].
αA-crystallin is a survival-promoting protein that enhances cellular resistance to apoptosis [11,22]. Relevant to these findings, our current data demonstrate that no significant induction of the UPR occurs in wild type and αA-R49C heterozygous mutant lenses. This indicates that one copy of wild type αA-crystallin is sufficient to keep the UPR at basal levels in the heterozygous lenses, consistent with the normal size of these lenses [16,18,19].
The ER chaperone BiP serves as a sensor of alterations in ER homeostasis and plays a key role in ER stress signaling by binding to each of the transducers of ER stress (IRE1, ATF-6, and PERK). Because BiP is the master regulator of the UPR, relative levels of this protein can be used to assess UPR activity. UPR activation results in the transcriptional induction of genes encoding ER-localized stress response proteins, including BiP itself [26]. Our immunoblot data demonstrate that BiP protein levels are dramatically higher in αA-R49C homozygous mutant lenses than in wild type or heterozygous mutant lenses. BiP initially produces cytoprotective signals that result in reduced translation, enhanced ER folding capacity, and clearance of misfolded ER proteins. The acute UPR permits cells to adjust protein synthesis and chaperone levels to cope with the stress of misfolded proteins. If these steps fail to re-establish homeostasis, then IRE1 and ATF-6 signaling are successively attenuated, shifting the balance in favor of pro-apoptotic signals and inducing apoptosis [3]. A seminal study by Lin et al [3] showed that, under prolonged stress, the different branches of the UPR are activated in a time-dependent manner. That is, the IRE1 and ATF-6 branches of UPR are successively attenuated, while activation of the PERK signaling pathway is unyielding, eventually leading to apoptosis. We found that wild type and heterozygous mutant lenses had comparable levels of ATF-6, while ATF-6 was undetectable in αA-R49C homozygous mutant lenses. The 20-kDa species recognized by the ATF-6 antibody in homozygous lenses is presumably an ATF-6 breakdown product. Our data revealed that ATF-6 expression (50-kDa active forms) was reduced in homozygous αA-R49C lenses, which are exposed to persistent stress from the expression of αA-R49C protein. Moreover, reduced ATF-6 levels were accompanied by reduced expression of XBP-1. XBP-1 is activated by active ATF-6 and spliced by IRE-1 to its active form [27]. Thus, a decrease in XBP-1 necessarily indicates a decrease in IRE-1 activity in homozygous lenses. Attenuated IRE-1 signaling and ATF-6 signaling has been shown to create conditions favoring cell death [3]. This is consistent with the finding that cell death occurs in homozygous mutant lenses as well as with the smaller size of these lenses.
We found that the transcription factor ATF-4 (also known as CREB-2), a component of the PERK signaling pathway, was upregulated in αA-R49C homozygous mutant lenses. ATF-4 was not present at the expected size of 38 kDa. Instead, we found a significant increase in bands ranging from 55 – 170 kDa, suggesting that ATF-4 is in a complex with other proteins, as has been reported previously [28,29]. ATF-4 induces the expression of several genes involved in the UPR [3,30], including the pro-apoptotic transcription factor CHOP [24]. We found that CHOP was strongly upregulated in homozygous mutant cataractous lenses. CHOP influences the expression of genes favoring apoptosis in response to ER stress. Our data indicate that PERK signaling, as represented by induction of CHOP, is high. Thus, sustained expression of the αA-R49C mutant protein results not only in activation of ATF-4 signaling and induction of pro-apoptotic CHOP, but also in reduced ATF-6 signaling. These events would be expected to tilt the cell death/survival balance towards cell death, which has been reported to occur in αA-R49C lenses [19]. The idea that ER stress and UPR play a critical role in cell death in this cataract model is further supported by the finding that αA-R49C forms insoluble, high-molecular-weight protein aggregates in the lens in vivo [17]. The formation of high molecular weight protein aggregates has been linked to cell death in the pathology of diseases such as Parkinson’s and Alzheimer’s disease [31].
Several studies have reported activation of the UPR in mutant lenses and cultured lens cells, including the induction of the UPR by the G98R mutant of αA-crystallin [6,7,8,32]. Our data support these findings and further show that specific branches of the UPR are activated or suppressed in response to the expression of the αA-R49C mutant protein. The human cataract caused by the R49C mutation is due to one dominant allele of mutant αA-crystallin, and patients heterozygous for the αA-R49C mutation develop cataracts. In this study, activation of the UPR observed in homozygous mouse lenses may still be relevant to human disease because the human protein might be more susceptible to disulfide bonding-induced protein crosslinking and aggregation due to the presence of two cysteine residues in human versus one cysteine in the mouse αA-crystyallin.
In conclusion, we have demonstrated that, in homozygous αA-R49C lenses, the UPR pathways are selectively induced, as seen by a dramatic upregulation of BiP and an upregulation of PERK pathway components [3,30]. We have also shown that the XBP-1 and ATF-6 levels are reduced, pointing to an attenuation of IRE-1 and ATF-6 signaling in αA-R49C homozygous lenses. Activation of the UPR in αA-R49C lenses is most probably initiated by the accumulation of unfolded polypeptides in the ER [33]. Although the initial activation of the UPR may be cytoprotective, persistence of ER stress probably shifts the final outcome towards cell death [12,19], small lenses [16,19], and severe cataracts [16,18,19].
Research Highlights.
αA-crystallin is highly expressed in eye lenses. The mutation arginine 49 to cysteine in αA-crystallin causes hereditary cataracts.
We investigated whether the unfolded protein response (UPR) was activated in αA-R49C mutant mouse lenses.
We found that ER chaperone, BiP, was 5-fold higher in homozygous αA-R49C lenses than in wild type lenses.
ATF-4 and CHOP levels were higher in homozygous lenses, but levels of ATF-6 and XBP-1 were reduced.
These findings suggest that the UPR is an important mediator of cell death observed in homozygous αA-R49C lenses.
Acknowledgments
This work was supported by NIH grant R01EY05681 to UPA, NIH Core grant EY-02687, and a Research to Prevent Blindness grant to the Department of Ophthalmology and Visual Sciences at Washington University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 2.Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 3.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 5.Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell. 2001;105:891–902. doi: 10.1016/s0092-8674(01)00407-x. [DOI] [PubMed] [Google Scholar]
- 6.Mulhern ML, Madson CJ, Danford A, Ikesugi K, Kador PF, Shinohara T. The unfolded protein response in lens epithelial cells from galactosemic rat lenses. Invest Ophthalmol Vis Sci. 2006;47:3951–3959. doi: 10.1167/iovs.06-0193. [DOI] [PubMed] [Google Scholar]
- 7.Firtina Z, Danysh BP, Bai X, Gould DB, Kobayashi T, Duncan MK. Abnormal expression of collagen IV in lens activates unfolded protein response resulting in cataract. J Biol Chem. 2009;284:35872–35884. doi: 10.1074/jbc.M109.060384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikesugi K, Yamamoto R, Mulhern ML, Shinohara T. Role of the unfolded protein response (UPR) in cataract formation. Exp Eye Res. 2006;83:508–516. doi: 10.1016/j.exer.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 9.Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Piatigorsky J. Multifunctional lens crystallins and corneal enzymes. More than meets the eye. Ann N Y Acad Sci. 1998;842:7–15. doi: 10.1111/j.1749-6632.1998.tb09626.x. [DOI] [PubMed] [Google Scholar]
- 11.Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the alphaA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–793. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- 13.Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, Weleber RG. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum Mol Genet. 1998;7:471–474. doi: 10.1093/hmg/7.3.471. [DOI] [PubMed] [Google Scholar]
- 14.Richter L, Flodman P, Barria von-Bischhoffshausen F, Burch D, Brown S, Nguyen L, Turner J, Spence MA, Bateman JB. Clinical variability of autosomal dominant cataract, microcornea and corneal opacity and novel mutation in the alpha A crystallin gene (CRYAA) Am J Med Genet A. 2008;146:833–842. doi: 10.1002/ajmg.a.32236. [DOI] [PubMed] [Google Scholar]
- 15.Andley UP. Crystallins and hereditary cataracts: molecular mechanisms and potential for therapy. Expert Rev Mol Med. 2006;8:1–19. doi: 10.1017/S1462399406000111. [DOI] [PubMed] [Google Scholar]
- 16.Andley UP, Reilly MA. In vivo lens deficiency of the R49C alphaA-crystallin mutant. Exp Eye Res. 2010;90:699–702. doi: 10.1016/j.exer.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andley UP, Hamilton PD, Ravi N. Mechanism of insolubilization by a single-point mutation in alphaA-crystallin linked with hereditary human cataracts. Biochemistry. 2008;47:9697–9706. doi: 10.1021/bi800594t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reilly MA, Andley UP. Quantitative biometric phenotype analysis in mouse lenses. Mol Vis. 2010;16:1041–1046. [PMC free article] [PubMed] [Google Scholar]
- 19.Xi JH, Bai F, Gross J, Townsend RR, Menko AS, Andley UP. Mechanism of small heat shock protein function in vivo: a knock-in mouse model demonstrates that the R49C mutation in alpha A-crystallin enhances protein insolubility and cell death. J Biol Chem. 2008;283:5801–5814. doi: 10.1074/jbc.M708704200. [DOI] [PubMed] [Google Scholar]
- 20.Andley UP. AlphaA-crystallin R49Cneo mutation influences the architecture of lens fiber cell membranes and causes posterior and nuclear cataracts in mice. BMC Ophthalmol. 2009;9:4. doi: 10.1186/1471-2415-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vernia S, Rubio T, Heredia M, Rodriguez de Cordoba S, Sanz P. Increased endoplasmic reticulum stress and decreased proteasomal function in lafora disease models lacking the phosphatase laforin. PLoS ONE. 2009;4:e5907. doi: 10.1371/journal.pone.0005907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andley UP, Song Z, Wawrousek EF, Bassnett S. The molecular chaperone alphaA-crystallin enhances lens epithelial cell growth and resistance to UVA stress. J Biol Chem. 1998;273:31252–31261. doi: 10.1074/jbc.273.47.31252. [DOI] [PubMed] [Google Scholar]
- 23.Andley UP, Song Z, Wawrousek EF, Fleming TP, Bassnett S. Differential protective activity of alpha A- and alphaB-crystallin in lens epithelial cells. J Biol Chem. 2000;275:36823–36831. doi: 10.1074/jbc.M004233200. [DOI] [PubMed] [Google Scholar]
- 24.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 25.Hoozemans JJ, van Haastert ES, Eikelenboom P, de Vos RA, Rozemuller JM, Scheper W. Activation of the unfolded protein response in Parkinson's disease. Biochem Biophys Res Commun. 2007;354:707–711. doi: 10.1016/j.bbrc.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 26.Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 28.He CH, Gong P, Hu B, Stewart D, Choi ME, Choi AM, Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J Biol Chem. 2001;276:20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- 29.Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 31.Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 32.Gong B, Zhang LY, Pang CP, Lam DS, Yam GH. Trimethylamine N-oxide alleviates the severe aggregation and ER stress caused by G98R alphaA-crystallin. Mol Vis. 2009;15:2829–2840. [PMC free article] [PubMed] [Google Scholar]
- 33.Sargsyan E, Baryshev M, Mkrtchian S. The physiological unfolded protein response in the thyroid epithelial cells. Biochem Biophys Res Commun. 2004;322:570–576. doi: 10.1016/j.bbrc.2004.07.155. [DOI] [PubMed] [Google Scholar]