Abstract
A major emphasis of our studies has been on developing a better understanding of how and why the skin serves as a target for immune reactions as well as how the skin evades becoming a target for destruction. For these studies we developed transgenic mice that express a membrane-tethered form of a model self-antigen, chicken ovalbumin (mOVA), under the control of a keratin 14 (K14) promoter. K14-mOVA transgenic mice that express OVA mRNA and protein in the epithelia have been assessed for their immune responsiveness to OVA and are being used as targets for T cells obtained from OT-1 transgenic mice whose CD8+ T cells carry a Va2/Vb5-transgenic T cell receptor with specificity for the OVA257-264-peptides (OVAp) in association with class I MHC antigens. Some of the K14-mOVA transgenic mice develop a graft-vs-host-like disease (gvhd) when the OT-1 cells are injected while others appear to be tolerant to the OT-1 cells. We found that γc cytokines, especially IL-15, determine whether autoimmunity or tolerance ensues in K14-mOVATg mice. We also developed transgenic mice that express soluble OVA under the control of a K14 promoter (K14-sOVA) that die within 5–8 days after adoptive transfer of OT-1 cells and identified these mice as a model for more acute gvhd-like reactions. Spontaneous autoimmunity occurs when these sOVA-mice are crossed with the OT-I mice. In contrast, we found that preventive or therapeutic OVAp injections induced a dose-dependent increase in survival. In this review the characterization of 5 strains of K14-OVATg mice and underlying mechanisms involved in autoimmune reactions in these Tg mice are discussed. We also describe a strategy to break tolerance and describe how the autoimmunity can be obviated using OVAp. Finally, a historical overview of using transgenic mice to assess the mechanisms of tolerance is also provided.
1. Introduction
Deletion of self-reactive T cells through negative selection in the thymus (central tolerance) is a major mechanism that contributes to the prevention of autoimmunity. However, this mechanism is not complete and some self-reactive T cells escape central tolerance and exist in the periphery. In the periphery, these cells undergo surveillance by peripheral tolerance mechanisms including ignorance, anergy, and suppression by regulatory T cells [1]. As a consequence of the activation of these self-reactive T cells destruction of single or multiple target tissues ensues in what we call autoimmune disease.
Elucidating the mechanisms involved in autoimmunity and tolerance has been a great challenge. It is not only difficult to identify the target antigens recognized by T cells in most human autoimmune diseases, it has also been difficult to study specific lymphocyte responses to these autoantigens. Also, there are few lymphocytes specific for any antigen (Ag) (self or foreign) in a normal immune system and this makes it difficult to define biochemical alterations in antigen-specific cells exposed to the relevant cognate antigen [2–4].
Thus, transgenic (Tg) mouse models have become valuable tools because they provide both defined, tissue-specific cognate Ags and well-characterized T cells with a T-cell receptor (TCR) that recognizes these Ags. Utilizing TCR Tg T cells can overcome the challenge of using low frequency peptide-specific T cells in normal animals and enables the isolation and quantitation of self-reactive T cells as well as the assessment of their responses to Ag in vitro and in vivo [2,4]. In addition, use of Tg mouse models, in which the autoantigen is known, facilitates the direct assessment of Ag-specific therapeutic interventions (see sections 5 and 7 in this review). Furthermore, the role of various genes can be determined by specifically deleting them from the T cells or selected cells from the Ag-expressing mice by either crossing them with appropriate knockout strains (see section 5) or through the use of oligonucleotide-specific therapeutics. Thus, both genetic or cellular manipulations can be used to disrupt or induce immunity or tolerance, and analyze the self-Ag-specific T cells to identify the specific mechanisms involved [2,3]. One big caveat that must be kept in mind when using Tg mouse models is that, despite their huge contribution to understanding basic mechanisms in immune responses, the lymphocyte repertoire of Ag-receptor Tg mice is not normal, and therefore results with such models must be interpreted with caution.
Two major approaches have utilized Tg mouse models to understand the mechanisms involved in the pathogenesis of autoimmune disease. One is adoptive transfer of self-reactive T cells into Tg mice which express self-Ag (see sections 3 and 5). The other is the generation of double Tg (DTg) mice by crossing the Tg mice that express self-Ag with those expressing the TCR that recognizes the self-Ag (see section 6). In the adoptive transfer model, the T cells encounter the antigen for the first time. Thus, the kinetics of responses of specific T cells to the self-antigen can be studied from the earliest encounter [2]. As well, central tolerance mechanisms can be avoided with this model. In contrast, in DTg models, lymphocytes are exposed to the self-antigen in utero and throughout life, mimicking what normally occurs and this facilitates the study of established tolerance [2]. In other words, in this model one cannot study the initial encounter of T cells with the self-Ag. Therefore, both of these experimental models provide complementary approaches.
During the past 8 years the major emphasis of our laboratory has been on developing a better understanding of how and why the skin serves as a target for immune reactions as well as how the skin evades becoming a target for destruction. In addition, we have been focusing on how we can modulate the effector functions of CD8+ T cells so as to obviate tissue destruction. For these studies we developed transgenic mice that express a model self-antigen, chicken ovalbumin (OVA) in either membrane-tethered forms (mOVA) or soluble forms (sOVA), under the control of a keratin 14 (K14) promoter. K14-mOVA transgenic mice that express OVA mRNA and protein in the epithelia have been assessed for their immune responsiveness to OVA and are being used as targets for T cells obtained from OT-1 transgenic mice whose CD8+ T cells carry a Vα2/Vβ5-transgenic T cell receptor with specificity for the OVA257–264-peptides (OVAp) in association with class I MHC antigens [5]. Although Tg mouse models are used for the analysis of mechanisms of both central and peripheral tolerance, this review will concentrate on peripheral tolerance mechanisms. In addition, we are going to focus on CD8 T cell-mediated disease models and not CD4 T cell-mediated models to study peripheral tolerance and will describe our models as well as others reported over the past years.
2. Historical overview of analyses of mechanisms of central and peripheral tolerance using transgenic mice
Considerable progress was made in elucidating mechanisms of central tolerance in the late 1980s. This was first shown using monoclonal antibodies against certain Vβ genes that are expressed with unusually high frequency on T cells specific for certain class II MHC-associated alloantigens. Using these antibodies, Kappler et al. and MacDonald et al. demonstrated that, in mice expressing the relevant class II MHC-associated antigens, cells expressing the particular Vβ gene products were deleted in the thymus [6–8]. Development of transgenic mice offered another means to analyse the mechanisms of central tolerance. One of the earliest Tg experiments used TCR Tg mice in which most T cells express a receptor specific for the male (H–Y) antigen in the context of MHC class I [9]. These studies demonstrated that only H–Y-specific T cells were deleted at the CD4+CD8+ double positive stage in the thymus of male mice though not in female mice. However, many of the Tg T cells matured and were exported to the periphery, where they were found to have markedly reduced levels of CD8 or TCR, and this made them anergic [9]. To study the fate of these T cells, or the mechanisms involved in peripheral tolerance, Tg mice that express transgenic antigen exclusively in the periphery have been used. Although considerable evidence had been obtained for understanding central tolerance, the development of tolerance to antigens that are expressed only in the periphery has not been as well understood.
Early studies had approached this question of peripheral tolerance mechanisms by creating Tg mice that carry an allogeneic class I MHC transgene and the rat insulin promoter (RIP) as a tissue-specific promoter, in which the transgene is expressed exclusively in the pancreas [10,11]. This approach enabled the demonstration that peripheral tolerance to self antigen is mediated by a process of anergy rather than by clonal deletion [10,11]. Related experiments investigated the induction of tolerance in transgenic mice expressing non-MHC self antigen under the control of a rat insulin promoter. Unlike the transgenic MHC alloantigens, non-MHC antigens must be processed and presented for TCR recognition to occur, thus the mechanisms for tolerance induction were expected to be different. Transgenic mice expressing model self antigens such as lymphocytic choriomeningitis virus (LCMV) proteins [12,13], influenza virus hemagglutinin (HA) [14,15] or ovalbumin (OVA) [16,17] under the control of the insulin promoter have been utilized. Autoreactive T cells found in the periphery of these mice show complete ignorance of the target Ags [12,13] or are rendered unresponsive or are physically deleted after initial activation after encountering the self-Ag [14–17]. A similar approach has since been attempted for other antigens using various tissue-specific promoters and many different mechanisms have been described. Accumulated evidence has thus demonstrated that peripheral tolerance mechanisms include ignorance, anergy, T cell downregulation of TCR or accessory molecules, second signal depletion from APC, and control by regulatory T cells [18]. Thus, the immune system remarkably resists autoimmune disease by using many different safeguards [18].
3. Summary of K14- and K5-OVATg mouse models to study skin-associated disease and peripheral tolerance
As mentioned above, the generation of Tg mouse models expressing self-Ags under a tissue-specific promoter enhanced our understanding of the mechanisms underlying peripheral tolerance. Generally these Tg mice develop peripheral tolerance and do not exhibit tissue destruction after transfer of Tg T cells specific for self-Ags [16, 19–21]. In the case of mice expressing OVA under the control of RIP or an intestinal fatty acid binding promoter (iFABP), the encounter of transferred OT-I cells with OVA-expressing tissues resulted in proliferation of T cells followed by deletion, and tissue destruction was not observed [16,21]. In most cases, the mere existence of self-reactive cells is not sufficient to cause disease. Some additional, inflammatory insult is generally required [12–15, 21].
Recently, several Tg mice expressing epidermal self-Ags were generated using K14 or K5 promoters [5,22–27] and facilitated the study of immune responses to skin- and mucosal-associated Ags. Peripheral tolerance could then be studied after adoptive transfer of Ag-specific TCR Tg T cells. Hogquist and coworkers first reported Tg mice expressing skin-associated Ags. They generated Tg mice that express the OVA peptide under the control of a K14-promoter (K14-OVAp mice) [22–24]. When OT-I cells were transferred into K14-OVAp mice, they were activated and induced autoimmunity [24]. We and others have also utilized K14 or K5 promoters and developed K14-mOVA mice [5,26,27] and K5-mOVA mice [25]. In accord with the previous studies using RIP or iFAP promoters [15, 19–21], no significant inflammatory reaction was observed in K14-mOVA mice [26,27] or K5-mOVA mice [25] after adoptive transfer of OT-I cells except for our K14-mOVA mice [5].
In some mouse models, an additional inflammatory signal, either induced by sublethal irradiation [25] or tape stripping [27], is required to induce an inflammatory skin disease. However, our model in which self-antigen, membrane-bound chicken ovalbumin (OVA), is expressed under the control of a keratin 14 promoter, does not result in peripheral tolerance [5]. Interestingly, inflammatory skin reactions and tissue destruction were induced by simply transferring OT-I cells without any further activation in our K14-mOVA model. OT-I cells expanded and accumulated in skin-draining lymph nodes after intravenous injection into K14-mOVA mice and exhibited activation markers. Graft-versus-host disease-like (GVHD) skin lesions appeared in these K14-mOVA mice by day 7 after injection of OT-I cells (Fig1).
Figure 1. K14-mOVA (#6) mice develop graft-versus-host-like disease after adoptive transfer of OT-I cells.
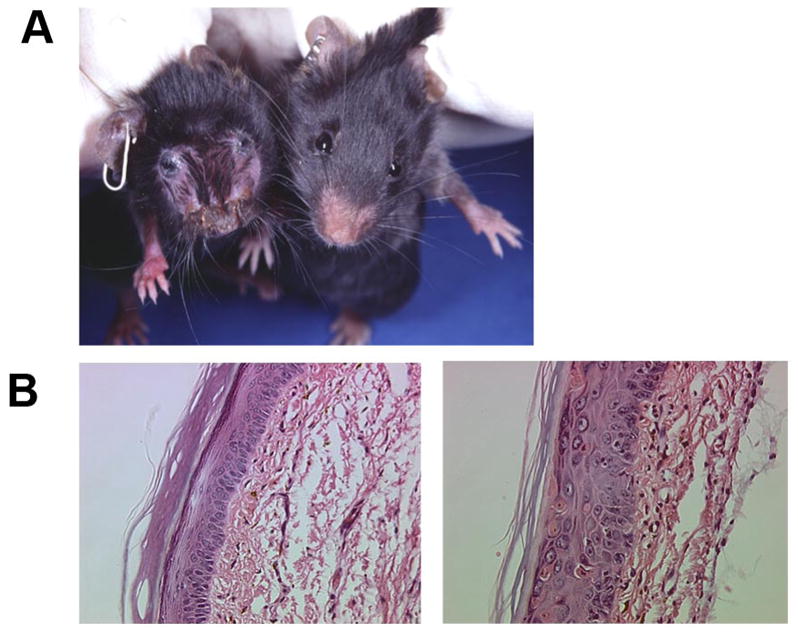
A) K14-mOVA (#6) mouse (left) shows scaly lesions and hair loss. Littermate control mouse (right) appears normal. B) Histology of the skin (footpad) of normal C57BL/6 mouse (left) and of K14-mOVA (#6) mouse (right) 14 days after injection of 5x106 OT-I cells. The K14-mOVA (#6) mouse shows a thickened epidermis, exocytosis, dyskeratotic keratinocytes and dermal inflammation.
Several factors may account for the discrepancy in results between our model and those used by others. The transgenic constructs used to generate mOVA fusion proteins differ in composition, resulting in structurally different expressed Ags. Our K14-mOVA mice express OVA fused to platelet-derived growth factor receptor, while the other mice express OVA fused to the transferrin receptor [25–27]. The level of transgene expression, the loci of integration, aberrant expression of transgenes, the difference in promoter (K14 vs K5), the form of Ags (membrane bound form of protein, soluble form of protein, peptide) may also affect the T cell responses. This will be discussed further in the following section.
4. Molecular and cell surface profile of our mice
We developed five strains of Tg mice, two of which express a membrane bound form of OVA (K14-mOVA mice; #3 and #6 mice) and three express a soluble form of OVA (K14-sOVA mice; #5, #15 and #17 mice) under the control of a K14- promoter. Following adoptive transfer of 5x106 naïve OT-I cells into two strains of K14-mOVA mice, K14-mOVA (#6) mice exhibited weight loss starting on day 4 and developed acute inflammatory skin lesions by day 7 [5,28], whereas K14-mOVA(#3) mice did not develop disease [28]. Histopathology of the skin from K14-mOVA (#6) mice showed a thickened epidermis, exocytosis, apoptotic epidermal cells, and dermal inflammation, consistent with GVHD (Fig.1). The other tissues including the esophagus and tongue showed no signs of inflammation. When 5x106 naive OT-I cells were transferred into the three strains of K14-sOVA mice, they induced weight loss and death in 4–8 days in K14-sOVA (#15) mice [29], however, no weight loss or signs of disease were observed in K14-sOVA (#5) and (#17) mice. K14-sOVA (#15) mice exhibited severe epithelial inflammation of the tongue and esophagus and died by day 9 but there were no pathological changes in the skin.
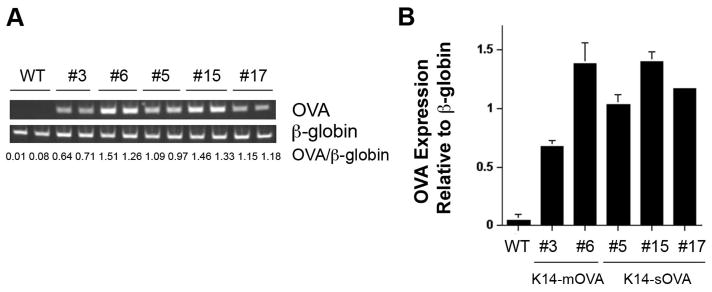
To characterize the five Tg OVA strains, we measured the expression level of the OVA transgene in genomic DNA. DNA was extracted from tails and the same amount of DNA was amplified by PCR. K14-mOVA (#6) mice express more OVA than K14-mOVA (#3) mice at the genomic DNA level (Fig.2) and this was confirmed by Southern blot (data not shown) since transgene copy number is usually determined by means of Sourthern blot analysis. Western blot analysis of the transgene of these two strains using anti-HA Ab also showed higher expression of OVA protein in the K14-mOVA(#6) mouse ear skin than that of the K14-mOVA (#3) mice [28]. In K14-sOVA Tg strains, K14-sOVA (#15) mice express the highest level of OVA followed by K14-sOVA (#17) mice and K14-sOVA (#5) mice in genomic DNA (Fig.2). We next determined the expression level of mRNA. Various tissues were prepared from the 5 strains and mRNA levels were determined by real-time PCR. In the K14-mOVA Tg mice, in contrast to the results mentioned above, K14-mOVA (#3) mice express higher levels of OVA mRNA than the K14-mOVA (#6) mice when RNA from single cell suspensions of the tissue was assessed (Fig. 3). The results varied depending on the methods we used and when RNA from whole tissue was used, K14-mOVA (#6) mice express higher levels of mRNA than the K14-mOVA (#3) mice [28]. In K14-sOVA Tg mice, the mRNA level is well correlated with the genomic DNA level when RNA from single suspensions of ear skin was used; K14-sOVA (#15) mice express the highest level followed by K14-sOVA (#17) mice and K14-sOVA (#5) mice (Fig.3). When RNA from whole tissue was used, K14-sOVA (#15) mice still express the highest level followed by K14-sOVA (#5) mice and K14-sOVA (#17) mice [30]. Finally, we determined the expression levels of the peptide-MHC class I (SIINFEKL-Kb) complex at the cell surface of epidermal cell suspension from ear skin using 25-D1.16 Ab [31] (Fig.4). Surprisingly, K14-mOVA (#3) mice express the highest level of peptide-MHC class I complexes followed by K14-sOVA (#15) mice.
Figure 2. Expression levels of OVA in genomic DNA were quantified by PCR.
A) Genomic DNA was extracted from tails of the indicated mice and the same amount of DNA was amplified for OVA by PCR. Signal intensities of OVA and β-globin bands were determined by densitometry. B) The average of OVA/β-globin band intensity of two mice from each group in (A) is shown. WT: wild type mouse
Figure 3. OVA mRNA expression levels in epidermal cell suspension from ears of C57BL/6 and K14-OVA Tg mice.

Epidermal cell suspensions (ECs) were prepared from ears of indicated mice. RNA was extracted from the ECs. The OVA transgene mRNA expression levels were quantified by real-time PCR and mGAPDH was used as the housekeeping gene. The average of two mice from each group is shown.
Figure 4. OVA257-264 surface expression in the context of MHC-I in ear skin epidermal cell suspensions from C57BL/6 and K14-OVA Tg mice.

Epidermal cell suspensions (ECs) were prepared from ear skin from the indicated mice and stained with a biotinylated OVA257-264-H2Kb-specific antibody (25-D1.16) or with an isotype control (Rat IgG2b-Bio) followed streptavidin-APC. The mean fluorescence intensity (APC) was measured by flow cytometry (two mice per group, duplicates). The bars represent the average plus SD. Iso: isotype control
It has been reported that only a limited correlation is observed between the amounts of the MHC class I-associated peptides presented by cells and the relative expression of source proteins from which these peptides are derived [32]. A likely explanation for this discrepancy is that the MHC class I-associated peptide repertoire preferentially derives from defective ribosomal products (DRiPs) and short-lived proteins relative to slowly degraded proteins [33, 34]. It has also been reported that there is no clear correlation between mRNA levels and the density of MHC-peptide complexes on cells [35]. These reports may explain why K14-mOVA (#3) mice express higher levels of peptide-MHC class I complexes than any of the other strains and this does not correlate with genomic DNA, mRNA, and protein levels.
Furthermore, it has been reported that cell-associated ovalbumin is cross-presented much more efficintly than soluble ovalbumin in vivo. Cross-presentation of cell-associated OVA requires ~0.2 ng to activate OT-I cells and soluble OVA requires at least 10,000 ng of OVA for activation of OT-I cells [36]. This may explain why K14-sOVA (#5) mice and K14-sOVA (#17) mice do not develop disease even if the mRNA levels are similar to that of the K14-mOVA (#6) mice.
5. Strategy to break tolerance or induce tolerance in K14-OVA mice
Two parameters, antigen presenting cell (APC) maturation and self-antigen levels, critically control peripheral tolerance and autoimmunity [37, 38]. APCs, including dendritic cells (DC), capture self-antigens from other cells and present them to self-reactive T cells (cross-presentation) to induce tolerance or autoimmunity [37,38]. However, in the steady state, DC remain relatively immature, and cross-presentation of self-antigens by immature DC leads to tolerance induction rather than activation of self-reactive cells [39–42].
Therefore, one way to break tolerance and initiate autoimmune disease is to provide signals that promote APC maturation and thereby break tolerance [38]. For instance, co-transfer of CD4 T cells [43], injection of agonistic anti-CD40 antibodies [39], and stimulation via OX40 [44] provide decisive signals that can influence tolerance versus autoimmunity [37, 38]. However, in our K14-mOVA (#3) mice, co-transfer of OVA specific CD4 T cells (OT-II cells that have a TCR that recognizes OVA peptides in association with MHC class II) along with OT-I cells does not break tolerance. In addition, transfer of OT-II cells together with OT-I cells into K14-mOVA (#6) mice does not augment GVHD either, suggesting that CD4 T cell help does not play a role in our K14-mOVA model. On the other hand, injection of agonistic anti-CD40 mAb into K14-mOVA (#3) mice induced GVHD after OT-I transfer, indicating that immature APC in the steady state induce tolerance in K14-mOVA (#3) mice and that maturation of APC is indeed important in terms of affecting the differentiation of naïve CD8 T cells into effector CTL to break tolerance. Interestingly, in the RIP-HA model, in which activated HA-specific CD4 T cells prevent CD8 T cell tolerance induction and promote diabetes, activating anti-CD40 mAb cannot substitute for CD4 T cell help [45]. These findings suggest that mechanisms leading to the abrogation of tolerance are diverse amongst the various mouse models.
The induction of peripheral tolerance is also dependent upon the concentration of self antigen [19, 46]. In our three strains of K14-sOVA mice, there is a correlation between antigen dose and disease occurrence. Only 1x105 OT-I cells are needed to induce death in K14-sOVA (#15) mice which express the highest levels of OVA while even 5x106 OT-I cells do not induce any signs of disease in K14-sOVA (#5) and K14-sOVA (#17) mice.
In K14-mOVA mice, as discussed in the previous section, it appears that genomic DNA levels of the transgene and peptide-MHC class I expression level of the two strains are inversely correlated. As a result, K14-mOVA (#3) mice express more peptide on the epidermal cell surfaces and this may determine the final immune responses. If this is the case, high expression of peptide-MHC class I (K14-mOVA (#3) mice) leads to tolerance while low expression of peptide-MHC class I (K14-mOVA (#6) mice) enhances the development of GVHD after transfer of OT-I cells. Note that we defined K14-mOVA (#3) mice as low expressors of OVA and K14-mOVA (#6) mice as high expressors of the OVA based on OVA transgene level by PCR of genomic DNA, and Southern blot analysis [28]. It was reported that in the presence of persistent antigen, the fate of naïve CD8 T cells during peripheral tolerance may be determined by the strength of interaction between the TCR and peptide-MHC complex. It has been demonstrated that high doses of chronic antigen exposure promotes CD8 T cell anergy, whereas lower doses result in deletion [47–49]. If this is the case, OT-I cells transferred into K14-mOVA (#3) mice become anergic with downregulated TCR that occurs when T cells encounter robust signals [28]. However, ours is an acute exposure model and is different from that of chronic exposure to strong TCR signals previously mentioned [47–49]. We still do not understand the mechanisms whereby K14-mOVA (#3) mice develop tolerance and why this discrepancy between OVA transgene level and peptide-MHC occurs. As discussed in the previous section, we are currently considering that aberrant expression of OVA or that the integration site of the transgene may be involved. Indeed, K14-mOVA (#3) mice express OVA mRNA (endogenous OVA) in splenic DC and bone marrow.
Given that peripheral tolerance depends on antigen dose, one determinant that contributes to the reversal of tolerance could be merely quantitative. It has been demonstrated that there is a linear relationship between the number of autoreactive CD8 T cells and the time necessary to functionally eliminate them [19]. When the model is overwhelmed by transferring large numbers of autoreactive CD8 T cells recognizing self antigen, tolerizing mechanisms could be overcome and disease ensues [17].
K14-mOVA(#3), K14-sOVA(#5 and #17) mice, as previously discussed, do not develop any signs of disease after the adoptive transfer of naïve OT-I cells. However, GVHD can be induced in these K14-sOVA mice by simply increasing the number of transferred OT-I cells. Although adoptive transfer of 5x106 OT-I cells does not induce disease in K14-OVA (#5) mice and K14-mOVA (#17) mice, 1–1.5x107 OT-I cells causes GVHD and death. Interestingly, adoptive transfer of even 1.5x107 OT-I cells into K14-mOVA (#3) mice do not cause any disease.
In addition to the two parameters described above, recent studies suggest the requirement for cytokine signals such as IL-12 [50] and IFN-3 αβ [51] which act as signal 3 in determining the development of tolerance or autoimmunity. We administered several γc-using cytokines (e.g. IL-2, -7, -15 and -21) into K14-mOVA (#3) mice since these cytokines contribute to the homeostasis of CD8 T cells. In vivo administration of IL-15 converted peripheral tolerance to immunity in K14-mOVA (#3) mice that were adoptively transferred with OT-1 cells. IL-15 broke tolerance and caused GVHD-like skin lesions by altering the functional status of the adoptively-transferred OT-I cells. Similarly, injection of OT-I cells that had been pretreated with IL-15 ex vivo, into K14-mOVA (#3) mice caused disease. Furthermore, neutralizing IL-15 function by in vivo administration of an anti-IL-2/IL-15Rβ or anti-mouse IL-15 antibody effectively blocked the development of GVHD-like skin lesions in K14-mOVA (#6) mice, suggesting that the levels of IL-15 physiologically controls the onset of tolerance or disease in our experimental models. We also demonstrated the non-redundant relevance of IL-15 in our system because, other members of the γc-using cytokine, such as IL-7 and IL-21, failed to cause disease when injected into K14-mOVA(#3) mice with OT-I cells. Thus, our observations collectively demonstrate that in addition to antigen and costimulation, a cytokine, in particular IL-15, can be a critical co-factor in the determination of tolerance or autoimmunity [28].
6. Phenotype of K14- and K5-OVATg/OT-I double Tg mouse models
Double transgenic mice K14-OVAp/OT-1 were generated by Hogquist and coworkers to assess central tolerance [22]. Since this promoter drives gene expression in thymic epithelial cells as well as in the basal layer of the skin, tongue and esophagus, these K14-OVAp/OT-I double Tg mice were predicted to exhibit clonal deletion and elimination of self-reactive OT-I cells. However, they did not delete OT-I cells; instead OT-I cells were detected in the periphery of the double transgenic mice [22]. K14-OVAp/OT-I DTg mice exhibited a lethal disease between 2 and 6 wks of age with 20% of animals showing no signs of disease. An inflammatory infiltrate was observed in skin and esophagus and around bile ducts of the liver. In addition, peripheral OT-I cells were activated. The phenotype included hair loss, skin lesions, weight loss, and a hunched appearance [23]. They reported that the breakdown of tolerance does not correlate with the extent of clonal deletion or receptor editing in the thymus [22]. They also demonstrated that dual-reactive T cells, which were shown to have autoimmune potential and were found in OT-I/K14-OVAp DTg, are not required for disease. This is because OT-I/OVAp animals on a RAG background still experienced disease although the average age of onset was delayed by about 4 weeks. They concluded that breakdown of tolerance may reflect a breakdown in an as-yet-unidentified peripheral tolerance process [23].
We crossed our K14-sOVA(#15) mice with OT-I mice and these DTg mice exhibited a similar phenotype to that observed by Hogquist [21]. 80–85% of K14-sOVA (#15)/OT-I mice die between day 12 and 20 of life, while 15–20% of the pups survive beyond day 21 of life [29]. Dying pups are characterized by inflammatory infiltrates in multiple organs (tongue, esophagus, liver, thymus, lung, plexus choroideus). This pattern of inflammation coincides well with the expression pattern of the sOVA transgene. Increased disease severity is characterized by increased percentages of activated OT-I cells in skin draining lymph nodes of K14-sOVA (#15)/OT-I mice. Mice that survive the first 3 weeks of life then develop a GVHD-like disease with skin lesions (erythema, erosions, and erythematous scaly plaques), weight loss, hair loss and hepatitis. Affected mice live on for a number of weeks, but finally die from disease progression [29].
In contrast to soluble OVA, DTg mice using K14-mOVA parents show different phenotypes. We generated K14-mOVA (#6)/OT-I DTg and K14-mOVA (#3)/OT-I DTg mice and to our surprise, neither DTg developed spontaneous disease. 20% of the lymphocyte populations express theVα2Vβ5 TCR in lymph nodes and 60–80% of them are CD4-CD8- double negative, suggesting that OT-I cells downregulate the CD8 molecule. Only a few OT-I cells (Vα2Vβ5CD8+) were found in the periphery.
Similarly, K5-mOVA/OT-I DTg mice showed thymic deletion of OT-I cells, had few of these cells in the periphery, and never developed skin changes [52]. However, when DTg mice were generated on an athymic nude mouse background, they spontaneously developed a toxic epidermal necrolysis-like skin disease at 8–12 weeks of age. OT-I cells isolated from skin-draining lymph nodes of athymic DTg mice expressed surface markers of activated T cells. The authors concluded that the underlying mechanism of tolerance in euthymic DTg mice was via regulatory T cells since development of skin autoimmunity was completely inhibited by transferring regulatory T cells from euthymic DTg mice [52].
7. Strategy to attenuate autoimmunity in double Tg mice
Systemic administration of peptides is one strategy to induce antigen-specific T cell tolerance. This can be an ideal approach since peptides selectively target the pathologic T cells, leaving the remainder of the immune system intact [53]. However, it is well known that peptides are able to induce immunity as well as tolerance. The route of administration and the dose appear to be critical factors in determining autoimmunity versus tolerance [53,54]. It was demonstrated that a single local subcutaneous injection of 50–500 μg peptide emulsified in incomplete Freund’s’adjuvant induced priming of LCMV-specific CTL, whereas repetitive and systemic intraperitoneal injection of the same dose caused tolerance [54]. The efficacy of peptide treatment was shown in several CD8 T cell-mediated autoimmunity disease models. Transgenic mice expressing LCMV glycoprotein (GP) under the control of a RIP promoter (RIP-LCMV GP mice) develop CD8 T cell-mediated diabetes after LCMV infection. Three i.p. injections of LCMV GP33 peptide (3x500 μg or 3x100 μg) induced tolerance in these RIP-LCMV GP mice and prevented autoimmune destruction of β islet cells and diabetes [55]. In RIP-HAxCL4-TCR double Tg mice, which develop spontaneous autoimmune diabetes, injection of HA peptide also proved effective. In this case, three i.v. injections of 30 μg of peptide significantly prolonged the survival of the double transgenic pups and eliminated the CD8 T cell pancreatic infiltrate [56]. Activation-induced apoptosis of the CD8 T cells has been implicated as a mechanism of tolerance in their mice [56].
We have also shown that prophylactic OVA257–264 peptide ip injections attenuate the course of disease in our K14-sOVA(#15)/OT-I DTg mice [29]. Two consecutive (10μg) OVA257–264 injections on day 5 and 9 led to survival of 60% by day 21. Increasing the dose to 100μg on days 5 and 9 improved survival by day 21 from 17% to 98.5% although mice that survived the first 3 weeks of life developed a GVHD-like phenotype in the subsequent weeks to months. We also demonstrated that therapeutic OVA257–264 peptide injections are also effective. When acutely ill sOVA/OT-I DTg pups were treated on day 10 of life with 100 μg OVA257–264 by i.p. injection, 66% of treated, as opposed to 0% of untreated, pups survived the first 3 weeks of life but later developed a GVHD-like phenotype.
Analysis of tolerance mechanisms that mediate survival of self-peptide treatment revealed a number of tolerance mechanisms in healthy peptide-treated sOVA/OT-I mice as well as spontaneous survivors. They exhibited reductions in peripheral CD8 T cells, and in CD8 coreceptor and Vα2 expression. Furthermore, CD8 T cells from healthy survivors were anergic and could not be activated by exogenous IL-2. A block in IL-2/IL-7 signaling via the STAT5 pathway provided the basis for low surface expression of the CD8 coreceptor and failure of IL-2 to break CD8 T cell anergy [29].
8. Epilogue and dedication
Studying the above-described Tg mice in which model antigens are used has provided us with new insights into mechanisms involved in the generation of autoimmunity and specific immunological tolerance. Many of these mice have been shared with others in the U.S., Europe, and Japan, in the spirit of advancing this knowledge base.
This paper is dedicated to Professor H. Moutsopoulos, as part of the journal series that recognizes outstanding autoimmunologists [57–60]. Professor Moutsopoulos is a long-time colleague and dear friend of one of us (SIK). We spent our early career together at the NIH and his enthusiasm for science still pervades the halls of the NIH. Professor Moutsopoulos has dedicated his career to studying mechanisms involved in Sjogren’s syndrome and lupus erythematosus and other autoimmune and rheumatic diseases. It is with great admiration of his life’s work and to his commitment to mentoring that we dedicate this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2(1):11–9. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 2.Lohr J, Knoechel B, Nagabhushanam V, Abbas AK. T-cell tolerance and autoimmunity to systemic and tissue-restricted self-antigens. Immunol Rev. 2005;204:116–27. doi: 10.1111/j.0105-2896.2005.00241.x. [DOI] [PubMed] [Google Scholar]
- 3.Abbas AK, Lohr J, Knoechel B, Nagabhushanam V. T cell tolerance and autoimmunity. Autoimmun Rev. 2004;3(7–8):471–5. doi: 10.1016/j.autrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Miller JF, Flavell RA. T-cell tolerance and autoimmunity in transgenic models of central and peripheral tolerance. Curr Opin Immunol. 1994;6(6):892–9. doi: 10.1016/0952-7915(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 5.Shibaki A, Sato A, Vogel JC, Miyagawa F, Katz SI. Induction of GVHD-like skin disease by passively transferred CD8(+) T-cell receptor transgenic T cells into keratin 14-ovalbumin transgenic mice. J Invest Dermatol. 2004;123(1):109–15. doi: 10.1111/j.0022-202X.2004.22701.x. [DOI] [PubMed] [Google Scholar]
- 6.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49(2):273–80. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 7.Kappler JW, Staerz U, White J, Marrack PC. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald HR, Schneider R, Lees RK, Howe RC, Acha-Orbea H, Festenstein H, et al. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988;332(6159):40–5. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- 9.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333(6175):742–6. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 10.Morahan G, Allison J, Miller JF. Tolerance of class I histocompatibility antigens expressed extrathymically. Nature. 1989;339(6226):622–4. doi: 10.1038/339622a0. [DOI] [PubMed] [Google Scholar]
- 11.Miller JF, Allison J, Morahan G, Cox KO, Mulebacher A, Blanden RV. Expression of class I histocompatibility antigens in defined tissues: effects on T cell function. Semin Immunol. 1989;1(2):137–46. [PubMed] [Google Scholar]
- 12.Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, et al. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65(2):305–17. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 13.Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65(2):319–31. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 14.Roman LM, Simons LF, Hammer RE, Sambrook JF, Gething MJ. The expression of influenza virus hemagglutinin in the pancreatic beta cells of transgenic mice results in autoimmune diabetes. Cell. 1990;61(3):383–96. doi: 10.1016/0092-8674(90)90521-f. [DOI] [PubMed] [Google Scholar]
- 15.Lo D, Freedman J, Hesse S, Palmiter RD, Brinster RL, Sherman LA. Peripheral tolerance to an islet cell-specific hemagglutinin transgene affects both CD4+ and CD8+ T cells. Eur J Immunol. 1992;22(4):1013–22. doi: 10.1002/eji.1830220421. [DOI] [PubMed] [Google Scholar]
- 16.Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184(3):923–30. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997;186(2):239–45. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyton RJ, Altmann DM. Transgenic models of autoimmune disease. Clin Exp Immunol. 2002;127(1):4–11. doi: 10.1046/j.1365-2249.2002.01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan DJ, Kreuwel HT, Sherman LA. Antigen concentration and precursor frequency determine the rate of CD8+ T cell tolerance to peripherally expressed antigens. J Immunol. 1999;163(2):723–7. [PubMed] [Google Scholar]
- 20.Morgan DJ, Kurts C, Kreuwel HT, Holst KL, Heath WR, Sherman LA. Ontogeny of T cell tolerance to peripherally expressed antigens. Proc Natl Acad Sci U S A. 1999;96(7):3854–8. doi: 10.1073/pnas.96.7.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vezys V, Olson S, Lefrancois L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 2000;12(5):505–14. doi: 10.1016/s1074-7613(00)80202-2. [DOI] [PubMed] [Google Scholar]
- 22.McGargill MA, Derbinski JM, Hogquist KA. Receptor editing in developing T cells. Nat Immunol. 2000;1(4):336–41. doi: 10.1038/79790. [DOI] [PubMed] [Google Scholar]
- 23.McGargill MA, Mayerova D, Stefanski HE, Koehn B, Parke EA, Jameson SC, et al. A spontaneous CD8 T cell-dependent autoimmune disease to an antigen expressed under the human keratin 14 promoter. J Immunol. 2002;169(4):2141–7. doi: 10.4049/jimmunol.169.4.2141. [DOI] [PubMed] [Google Scholar]
- 24.Mayerova D, Parke EA, Bursch LS, Odumade OA, Hogquist KA. Langerhans cells activate naive self-antigen-specific CD8 T cells in the steady state. Immunity. 2004;21(3):391–400. doi: 10.1016/j.immuni.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Azukizawa H, Kosaka H, Sano S, Heath WR, Takahashi I, Gao XH, et al. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur J Immunol. 2003;33(7):1879–88. doi: 10.1002/eji.200323630. [DOI] [PubMed] [Google Scholar]
- 26.Bursch LS, Rich BE, Hogquist KA. Langerhans cells are not required for the CD8 T cell response to epidermal self-antigens. J Immunol. 2009;182(8):4657–64. doi: 10.4049/jimmunol.0803656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bianchi T, Pincus LB, Wurbel MA, Rich BE, Kupper TS, Fuhlbrigge RC, et al. Maintenance of peripheral tolerance through controlled tissue homing of antigen-specific T cells in K14-mOVA mice. J Immunol. 2009;182(8):4665–74. doi: 10.4049/jimmunol.0803628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyagawa F, Tagaya Y, Kim BS, Patel HJ, Ishida K, Ohteki T, et al. IL-15 serves as a costimulator in determining the activity of autoreactive CD8 T cells in an experimental mouse model of graft-versus-host-like disease. J Immunol. 2008;181(2):1109–19. doi: 10.4049/jimmunol.181.2.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutermuth J, Nograles KE, Miyagawa F, Nelson E, Cho YH, Katz SI. Self-peptides prolong survival in murine autoimmunity via reduced IL-2/IL-7-mediated STAT5 signaling, CD8 coreceptor, and V alpha 2 down-regulation. J Immunol. 2009;183(5):3130–8. doi: 10.4049/jimmunol.0900793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim BS, Miyagawa F, Cho YH, Bennett CL, Clausen BE, Katz SI. Keratinocytes function as accessory cells for presentation of endogenous antigen expressed in the epidermis. J Invest Dermatol. 2009;129(12):2805–17. doi: 10.1038/jid.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6(6):715–26. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 32.Milner E, Barnea E, Beer I, Admon A. The turnover kinetics of major histocompatibility complex peptides of human cancer cells. Mol Cell Proteomics. 2006;5(2):357–65. doi: 10.1074/mcp.M500241-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Caron E, Charbonneau R, Huppe G, Brochu S, Perreault C. The structure and location of SIMP/STT3B account for its prominent imprint on the MHC I immunopeptidome. Int Immunol. 2005;17(12):1583–96. doi: 10.1093/intimm/dxh336. [DOI] [PubMed] [Google Scholar]
- 34.Yewdell JW, Nicchitta CV. The DRiP hypothesis decennial: support, controversy, refinement and extension. Trends Immunol. 2006;27(8):368–73. doi: 10.1016/j.it.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Weinzierl AO, Lemmel C, Schoor O, Muller M, Kruger T, Wernet D, et al. Distorted relation between mRNA copy number and corresponding major histocompatibility complex ligand density on the cell surface. Mol Cell Proteomics. 2007;6(1):102–13. doi: 10.1074/mcp.M600310-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Li M, Davey GM, Sutherland RM, Kurts C, Lew AM, Hirst C, et al. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol. 2001;166(10):6099–103. doi: 10.4049/jimmunol.166.10.6099. [DOI] [PubMed] [Google Scholar]
- 37.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 38.Ohashi PS, DeFranco AL. Making and breaking tolerance. Curr Opin Immunol. 2002;14(6):744–59. doi: 10.1016/s0952-7915(02)00406-5. [DOI] [PubMed] [Google Scholar]
- 39.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194(6):769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196(8):1091–7. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheinecker C, McHugh R, Shevach EM, Germain RN. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J Exp Med. 2002;196(8):1079–90. doi: 10.1084/jem.20020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196(12):1627–38. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurts C, Carbone FR, Barnden M, Blanas E, Allison J, Heath WR, et al. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J Exp Med. 1997;186(12):2057–62. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bansal-Pakala P, Jember AG, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med. 2001;7(8):907–12. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez J, Aung S, Marquardt K, Sherman LA. Uncoupling of proliferative potential and gain of effector function by CD8(+) T cells responding to self-antigens. J Exp Med. 2002;196(3):323–33. doi: 10.1084/jem.20011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurts C, Sutherland RM, Davey G, Li M, Lew AM, Blanas E, et al. CD8 T cell ignorance or tolerance to islet antigens depends on antigen dose. Proc Natl Acad Sci U S A. 1999;96(22):12703–7. doi: 10.1073/pnas.96.22.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rocha B, Grandien A, Freitas AA. Anergy and exhaustion are independent mechanisms of peripheral T cell tolerance. J Exp Med. 1995;181(3):993–1003. doi: 10.1084/jem.181.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redmond WL, Marincek BC, Sherman LA. Distinct requirements for deletion versus anergy during CD8 T cell peripheral tolerance in vivo. J Immunol. 2005;174(4):2046–53. doi: 10.4049/jimmunol.174.4.2046. [DOI] [PubMed] [Google Scholar]
- 49.Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22(3):275–84. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197(9):1141–51. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174(8):4465–9. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 52.Azukizawa H, Sano S, Kosaka H, Sumikawa Y, Itami S. Prevention of toxic epidermal necrolysis by regulatory T cells. Eur J Immunol. 2005;35(6):1722–30. doi: 10.1002/eji.200425773. [DOI] [PubMed] [Google Scholar]
- 53.Liblau R, Tisch R, Bercovici N, McDevitt HO. Systemic antigen in the treatment of T-cell-mediated autoimmune diseases. Immunol Today. 1997;18(12):599–604. doi: 10.1016/s0167-5699(97)01171-7. [DOI] [PubMed] [Google Scholar]
- 54.Aichele P, Brduscha-Riem K, Zinkernagel RM, Hengartner H, Pircher H. T cell priming versus T cell tolerance induced by synthetic peptides. J Exp Med. 1995;182(1):261–6. doi: 10.1084/jem.182.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aichele P, Kyburz D, Ohashi PS, Odermatt B, Zinkernagel RM, Hengartner H, et al. Peptide-induced T-cell tolerance to prevent autoimmune diabetes in a transgenic mouse model. Proc Natl Acad Sci U S A. 1994;91(2):444–8. doi: 10.1073/pnas.91.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bercovici N, Heurtier A, Vizler C, Pardigon N, Cambouris C, Desreumaux P, et al. Systemic administration of agonist peptide blocks the progression of spontaneous CD8-mediated autoimmune diabetes in transgenic mice without bystander damage. J Immunol. 2000;165(1):202–10. doi: 10.4049/jimmunol.165.1.202. [DOI] [PubMed] [Google Scholar]
- 57.Ansari AA, Gershwin ME. Navigating the passage between Charybdis and Scylla: Recognizing the achievements of Noel Rose. J Autoimmun. 2009;33:165–9. doi: 10.1016/j.jaut.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 58.Whittingham S, Rowley MJ, Gershwin ME. A tribute to an outstanding immunologist - Ian Reay Mackay. J Autoimmun. 2008;31:197–200. doi: 10.1016/j.jaut.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Gershwin ME. Bone marrow transplantation, refractory autoimmunity and the contributions of Susumu Ikehara. J Autoimmun. 2008;30:105–7. doi: 10.1016/j.jaut.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 60.Gershwin ME. The mosaic of autoimmunity. Autoimmun Rev. 2008;7:161–3. doi: 10.1016/j.autrev.2007.11.021. [DOI] [PubMed] [Google Scholar]