Abstract
The purpose of this study was to further characterize cell growth-inhibitory effects of a recently identified androgen receptor (AR) signaling inhibitor 6-amino-2-[2-(4-tert-butyl-pnenoxy)-ethylsulfanyl]-1H-pyrimidin-4-one (DL3)5 and antiandrogen bicalutamide (Bic). DL3 was more potent than Bic in induction of G1 arrest and reduction of G1-related cell cycle protein expression in AR-positive LNCaP cells. DL3, but not Bic, moderately inhibited growth of AR-negative PC-3 cells independent of G1 arrest. The data indicated that DL3 inhibit cell growth in both AR-dependent and -independent manners and is potentially a potent therapeutic agent for the management of advanced human prostate cancer.
Keywords: androgen receptor, antagonist, prostate cancer, cell cycle
1. Introduction
Prostate cancer is the most common cancer and the second most common cause of cancer death among men in the United States [1]. As detection techniques improve, more patients are diagnosed with localized disease and can be cured by either surgery or radiation therapy. Metastasis in many patients, however, still occurs prior to the initial diagnosis. Wildtype or mutant AR is expressed or often overexpressed in the majority of advanced prostate cancer. Hormonal therapies are the mainstay treatment for advanced diseases, which, however, are only palliative: delaying tumor progression by an average of less than 18 months [2]. Moreover, in many patients with hormone-refractory disease, discontinuation of an antiandrogen treatment often results in clinical improvement and a fall in serum PSA, i.e. antiandrogen withdrawal syndrome, which is partially caused by the intrinsic androgenic activity of the antiandrogens [3]. Therefore, much effort has been focused on development of novel and more potent AR signaling inhibitors and led to discovery of some AR signaling inhibitors [4–9] and identification of second-generation antiandrogens [10] and noncompetitive AR inhibitors [11].
Our previous study identified DL3, a novel synthetic small molecule compound with potent anti-AR signaling activities [12]. Specifically, DL3 reduces AR expression and inhibit androgen-stimulated PSA expression and growth of androgen-responsive human prostate cancer cells. Importantly, DL3 also blocks the androgenic activity of antiandrogens flutamide and nilutamide in LNCaP cells [12]. Thus DL3 is unique among antiandrogens in that it is a potent AR antagonist yet has no intrinsic androgenic activity in contrast to some currently approved antiandrogens. These data strongly suggested that DL3 may be a superior therapeutic agent for advanced prostate cancer. The purpose of this study was to determine mechanisms responsible for inhibition of cell growth by DL3. The data show that DL3- and Bic-mediated inhibition of growth correlates with G1 cell cycle arrest. In contrast to Bic, DL3 moderately inhibited growth of PC-3 cells, which, however, was mediated by mechanisms independent of G1 arrest.
2. Materials and Methods
2.1. Reagents
RPMI 1640 medium, Ca2+, Mg2+-free Hanks' balanced salt solution (HBSS), and fetal bovine serum (FBS) were purchased from M. A. Bioproducts (Walkersville, MD). Antibodies against cyclin D1, cyclin D3, CDK2, CDK4, CDK6, p21, p27, Rb, phospho-Rb (ser807/811), and phospho-(ser) CDKs substrate were purchased from Cell Signaling (Danvers, MA). An antibody to β-actin, propidium Iodide (PI), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 5α-dihydrotestosterone (DHT) were purchased from Sigma Chemical Co. (St. Louis, MO). Antibodies to AR and PSA were obtained from Eptomics, Inc (Burlingame, CA) and Dako North America, Inc. (Carpinteria, CA), respectively. The plasmid pARR2PB-EGFP was constructed by substitution of the CMV promoter in the pEGF-N1 (Clontech Laboratories, Inc., Mountain View, CA) with a composite rat probasin promoter (ARR2PB) that contains androgen response element [13] generously provided by Dr. Robert Matusik (Dept of Urologic Surgery, Vanderbilt University Medical Center).
2.2 Tumor Cells and Culture
The well characterized LNCaP (androgen-responsive) [14; 15] and AR-negative PC-3 cells [16] were obtained from ATCC (Manassas, VA). The cells were cultured in RPMI1640 medium supplemented with 10% FBS. LNCaP cells were stably transfected with pARR2PB-GFP to derive LNCaP-ARR2PB-GFP, in which GFP expression is driven by androgen stimulation. Cells in exponential growth phase were harvested by a 1–3 min treatment with a 0.25% trypsin - 0.02% EDTA solution. The flasks were tapped to detach the cells, RPMI 1640-10% FBS was added, and the cell suspension was gently agitated to produce a single-cell suspension. The cells were washed in Hanks’ balanced salt solution (HBSS) and resuspended growth medium. Only suspensions of single cells with viability exceeding 95% (ascertained by trypan blue exclusion) were used.
2.3. Cell Growth Assay
Cells were plated into 96-well plates at 2,000 cells/well. After an overnight incubation, the cells were treated as detailed in Results for 4 days and viable cells in the wells were stained using the MTT assay as described previously [17; 18]. During the final 2 hr, 0.4 mg/ml of MTT was added. After removing the medium, dark-blue formazan was dissolved in DMSO and the absorbance at 570 nm was measured with a FLUOstar Optima microplate reader (BMG LABTECH Inc., Durham, NC). Inhibition of cell growth was calculated by the formula: growth inhibition (%) = (1 − A570 of treated/A570 of control) × 100.
2.4. Cell Cycle Analysis
LNCaP and PC-3 cells were plated into 6-well plates at 2.5 × 105 and 1 × 105/well, respectively, and allowed to attach overnight. After desired treatments, cells were rinsed with HBSS, detached by trypsinization, suspended in RPMI1640-10% FBS, pelleted by centrifugation, and resuspended in PBS. The cell suspension was added into absolute ethanol to achieve a final concentration of 70% of ethanol. After an incubation on ice for 15 min (or over night at −20°C), the cells were pelleted, resuspended in PI staining solution (PBS containing 50 µg/ml PI, 100 ug/ml RNase A, and 0.05% Triton X-100), and incubated for 45 min at 37°C. After washing with PBS, the cells were resuspended in PBS for flow cytometry analysis in an Epics-MCL system (Beckman Coulter, Fullerton, CA). Twenty thousand cells at each time point were analyzed to determine their DNA content and to obtain two dimensional (X-axis fluorescence, Y-axis cell number) flow cytometric dot plot results. The 3 fractions (G0/G1, G2/M and S) were quantified by using the Beckton-Dickinson Lysis II and Cell Fit software with a computer equipped with the Epics-MCL system.
2.5. Western blot analysis
Cells (2 × 106/60-mm dish) were washed and scraped into a lysis buffer and analyzed by western blotting as described in our previous study [19] using desired antibodies. The immunoreactivity was revealed by using the ECL western blotting detection system (Michigan Diagnostic LLC, Troy, MI) and visualized in a KODAK Image Station IS4000MM Digital Imaging System (Eastman Kodak Co., Rochester, NY). Expression of target protein and β-actin were quantified in the linear range of exposure.
2.6. Immunoprecipitation
LNCaP or PC-3 cells (2 × 106/100 mm dish) were washed and scrapped into lysis buffer as described above. The lysates (200 ug protein in 200 ul lysis buffer) precleared with protein A/G plus agarose conjugate (25% slurry, v/v; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and then incubated with the antibody against Rb (2 ul/reaction) overnight at 4°C under constant agitation on a rocker platform. Twenty ul of protein A/G plus-agarose conjugate was added into each reaction and incubated for 2 hr at 4°C under constant agitation. The immunoprecipitates were collected by centrifugation at 1,000 × g for 30 seconds, washed 3 times in 1 ml of lysis buffer, resuspended in 20 ul 2× SDS-PAGE sample buffer, and analyzed by western blotting as described above.
2.7. Statistical analysis
All experiments were performed at least twice. Data shown are the mean ± S.E. Differences between means were compared using the two-tailed Student’s t test and were considered significantly different at the level of p < 0.05.
3. Results
3.1. Cell growth inhibitory effects of DL3 and Bic
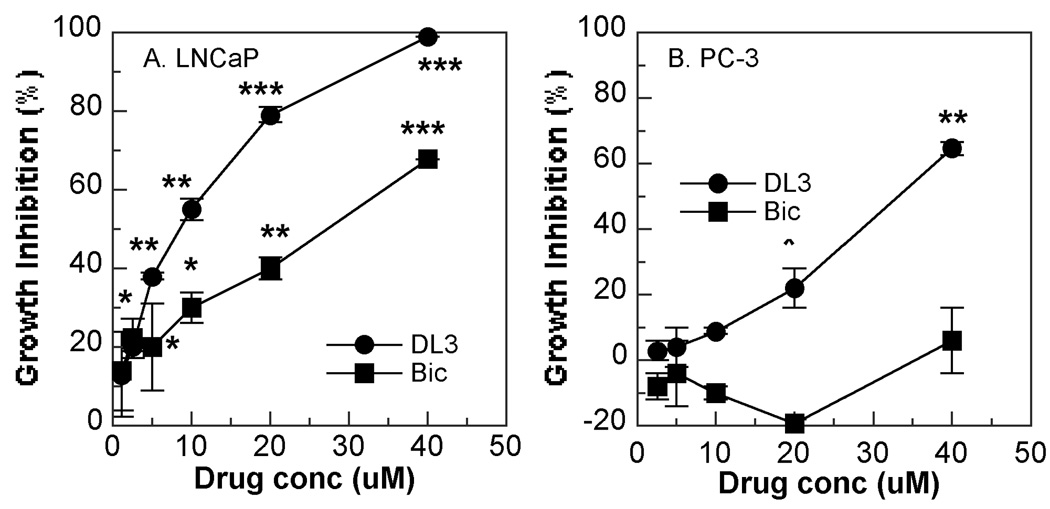
In the first set of experiments, effects of DL3 and Bic on cell growth were examined. As shown in Fig. 1A, both DL3 and Bic inhibited growth of AR+-androgen responsive LNCaP cells in a dose dependent manner. Consistent with our previous observation in LNCaP as well as 22Rv1 and LAPC-4 cells [12], the inhibitory effects of DL3 on LNCaP cell growth appeared much more potent than did Bic at all concentrations (2.5–40 uM) examined. Bic did not inhibit growth of AR-negative PC-3 cells at concentrations below 20 uM and had only marginal effect at 40 uM, indicating that Bic is indeed an AR specific inhibitor (Fig. 1B). In contrast, DL3 inhibited growth of PC-3 cells in a dose-dependent manner. It was noteworthy, however, the inhibitory effects of DL3 on PC-3 cells were much more moderate compared with those on LNCaP cells at any and all concentrations tested (Fig. 1B).
Fig. 1. Effects of DL3 and Bic on growth of LNCaP and PC-3 cells.
LNCaP (A) and PC-3 (B) cells (2000/well) were incubated for 4 days with various concentrations DL3 or Bic. Viable cells were stained with MTT and effects of treatments on cell growth were calculated. Data shown are mean ± SD of one representative experiment of three to five. *, **, and *** are p<0.05, p<0.01, and p<0.001, respectively, in comparison with the cultures in the absence of the drugs.
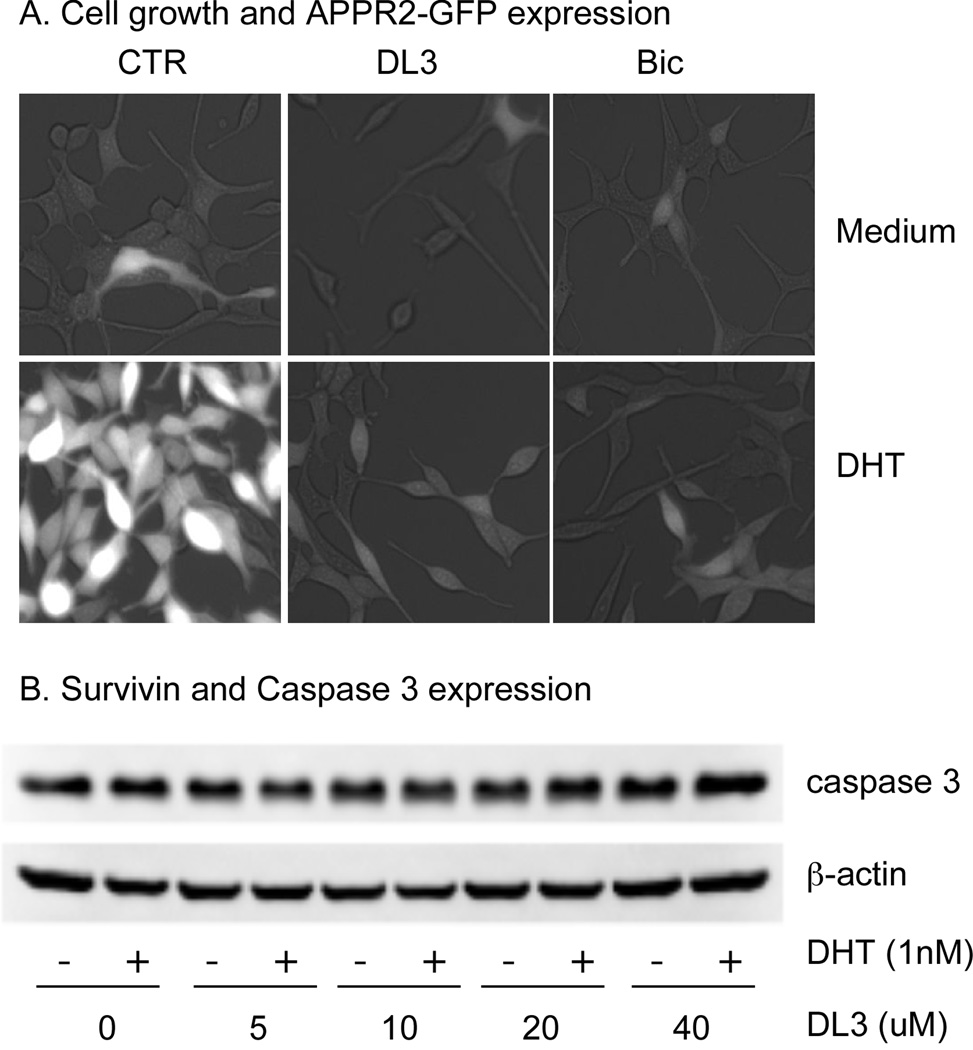
Next, effects of DL3 and Bic on growth of LNCaP cells were directly examined under a microscopy. LNCaP-ARR2PB-GFP cells, in which GFP expression is driven by androgen stimulation, were starved for 72 hr in phenol-free medium supplemented with 5% SFBS, treated with 20 uM of DL3 or Bic in the absence or presence of 1 nM DHT, and observed under a fluorescent microscope every 24 hr for up to 96 hr. In the absence of DHT, minimal cell growth and fluorescent signals were observed. DHT induced a significant cell growth and AR-dependent GFP expression. The treatment with DL3 and Bic reduced the basal GFP expression and attenuated DHT-induced cell growth and GFP expression (Fig. 2A). It was noteworthy that regardless of the absence or presence of DHT, neither DL3 nor Bic led to cytolysis or cell death through out the duration of the experiment (96 hr, Fig. 2A). To validate the morphological observation that DL3 did not induce apoptosis, we analyzed whether the treatment with DL3 altered activation of the proapoptotic enzyme caspases in LNCaP cells. Data in Fig. 2B showed that DL3 at concentrations up to 40 uM, regardless of the presence or absence of DHT did not induce cleavage/activation of caspase-3, a critical executioner of apoptosis that is either partially or totally responsible for the proteolytic cleavage of many key proteins in apoptosis. Similarly, the treatment with DL3 did not activate caspase-7, -8, and -9 in LNCaP cells (Data not shown). Taken together, these data suggested that DL3, as well as Bic, may inhibit cell growth by suppressing cell cycle progression.
Fig. 2. DL3 and Bic did not induce apoptosis in LNCaP cells.
A. LNCaP-ARR2PB-GFP cells were incubated for 72 hr in phenol-free medium supplemented with 5% charcoal-dextran stripped FBS, followed by treatment with 20 uM of DL3 or Bic in the absence or presence of 1 nM of DHT for 96 hr. The cultures were observed under a fluorescent microscope and recorded. B. LNCaP cells were incubated for 72 hr in phenol-free medium supplemented with 5% FBS, followed by treatment with various concentrations of DL3 in the absence or presence of 1 nM of DHT for 48 hr. Cellular caspase 3 level was revealed by immunoblotting with β-actin as loading control. Data shown were one representative experiment of 3.
3.2. DL3 and Bic induced a G1 arrest in LNCaP cells
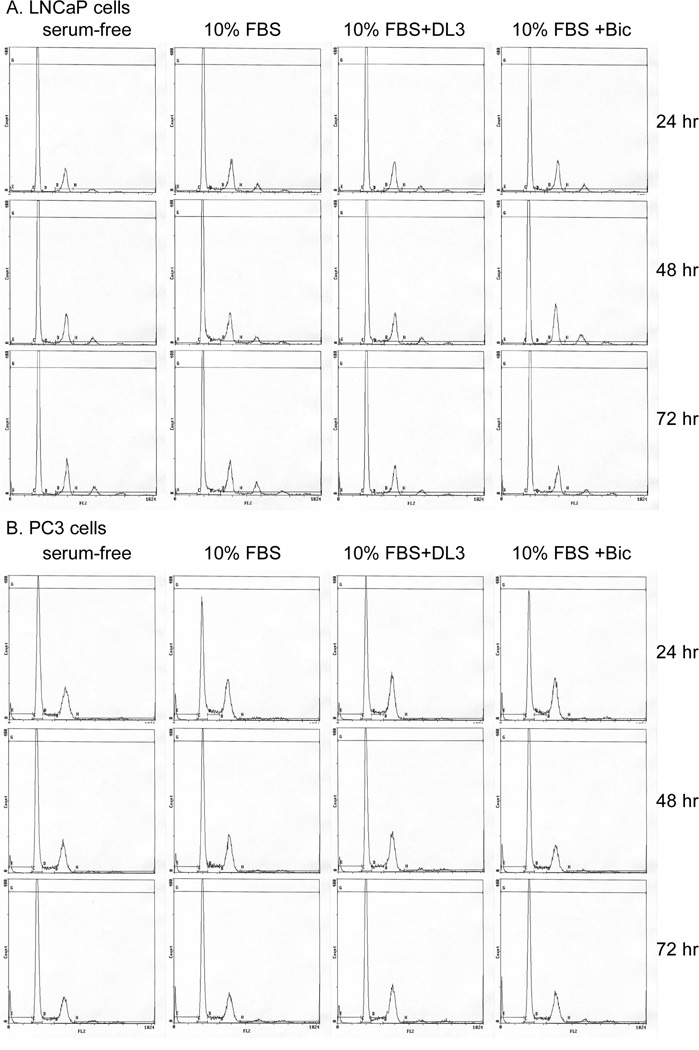
Flow cytometry analysis was performed to determine effects of DL3 and Bic on cell cycle distribution in LNCaP and PC-3 cells. Cells were starved in serum-free medium for 24 hr, followed by incubation with fresh medium supplemented with 10% FBS and 1 nM DHT in the absence or presence of 10 or 20 uM of DL3 or Bic for up to 72 hr. The cells were sampled every 24 hr for cell cycle distribution analysis (Fig. 3). In both LNCaP and PC-3 cells, the incubation with medium supplemented with FBS stimulated cell cycle progression, reflected in a reduction of the number of cells in G1 phase and an elevation of the number of cells in S and G2/M phases of the cell cycle (Fig. 3 and Table 1). Treatment with 10 or 20 uM of DL3 attenuated effects of FBS on cell cycle distribution in LNCaP cells and completely blocked effects of FBS at 20 uM at all times examined (Table 1). The treatment of LNCaP cells with Bic, although to a less extent, also reduced serum-stimulated cell cycle progression (Fig. 3 and Table 1). Although DL3 (20 uM) moderately inhibited growth of PC-3 cells (Fig. 1), it did not cause the accumulation of the cells in the G1 phase of the cell cycle (Fig. 3 and Table 1). In consistent with its effects on cell growth, Bic did not alter the cell cycle distribution in PC-3 cells (Fig. 3 and Table 1). In both cell lines, very few dead cells were detected in either control or DL3- or Bic-treated cells (Fig. 3). These data indicate that DL3 induces G1 phase arrest preferentially in AR-positive and androgen-responsive LNCaP cells and this effect of DL3 is more potent and more persistent than that of Bic.
Fig. 3. DL3 and Bic induced G1 phase arrest in LNCaP cells.
LNCaP and PC-3 cells in 6-well plates (5 × 105 and 2.5 × 105/well, respectively) were starved in serum-free medium for 24 hr, followed by stimulation with 10% FBS plus 1 nM DHT in the absence or presence of 10 or 20 uM of DL3 or Bic. The cells were sampled every 24 hr up to 72 hr for cell cycle analysis by FACS as detailed in Materials and Methods. Data shown were the cell cycle distribution pattern of cells treated for 48 hr. This is one representative experiment of 5.
Table 1.
Differential effects of DL3 and Bic on cell cycle distribution in LNCaP and PC-3 cells
| LNCaP | PC-3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (hr) |
Phase of cell cycle |
FBS (0%) |
10%FBS | FBS 0% |
10% FBS | ||||||||
| DL3 (uM) | Bic (uM) | DL3 (uM) | Bic (uM) | ||||||||||
| 0 | 10 | 20 | 10 | 20 | 0 | 10 | 20 | 10 | 20 | ||||
| G1 | 75 | 65 | 70 | 73 | 68 | 70 | 56 | 43 | N | 47 | N | 45 | |
| 24 | S | 1 | 4 | 5 | 2 | 4 | 3 | 7 | 10 | N | 10 | N | 10 |
| G2/M | 17 | 19 | 16 | 17 | 18 | 17 | 34 | 40 | N | 39 | N | 40 | |
| G1 | 70 | 61 | 66 | 66 | 61 | 64 | 60 | 49 | N | 50 | N | 54 | |
| 48 | S | 3 | 6 | 5 | 5 | 5 | 2 | 6 | 8 | N | 7 | N | 6 |
| G2/M | 17 | 19 | 18 | 18 | 20 | 20 | 29 | 37 | N | 38 | N | 35 | |
| G1 | 70 | 55 | 68 | 71 | 62 | 68 | 60 | 53 | N | 52 | N | 52 | |
| 72 | S | 2 | 6 | 5 | 3 | 4 | 4 | 6 | 7 | N | 5 | N | 6 |
| G2/M | 17 | 20 | 16 | 16 | 19 | 17 | 28 | 34 | N | 37 | N | 35 | |
LNCaP and PC-3 cells were starved in serum-free medium for 24 hr, followed by stimulation with fresh medium supplemented with 10% FBS and 1 nM DHT in the presence or absence of 10 or 20 uM of DL3 or Bic. The cells were sampled for FACS analysis on 24, 48, or 72 hr. Data shown were from one representative experiment of 7. N = not done.
3.3. Effects of DL3 on expression of proteins regulating G1 phase progression
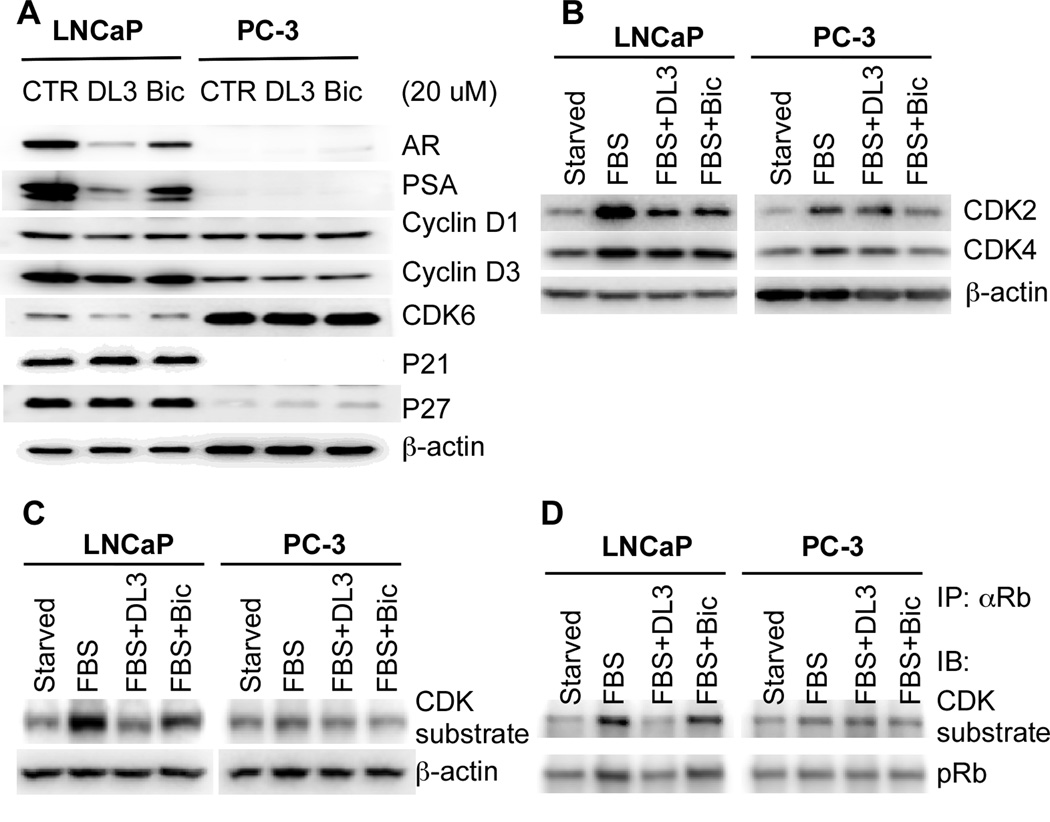
Expression of the cyclin D and cyclin-dependent protein kinase (CDK) 4/6 and phosphorylation of the retinoblastoma tumor suppressor protein (Rb) are required for cell progression through G1 phase of the cell cycle [20]. The progression of a cell through G1 phase of the cell cycle is also regulated by CDK inhibitors [20]. AR signaling regulates cell cycle protein expression. It has been shown that deprivation of androgen causes the reduction of cyclin D1/cyclin D3 expression and CDK4/6 activity, and an arrest of prostate cancer cells at the early G1 phase [21–23]. Moreover, it was shown that the D-type cyclin expression in prostate cancer cells is induced by androgens [21]. We therefore determined effects of DL3 and Bic on G1 phase-related cell cycle protein expression. Western blotting analysis showed that the LNCaP cells exposed to 20 uM of DL3 or Bic contained a lower level of cyclin D1, cyclin D3, and CDK6 proteins (Fig. 4A). The densitometry analysis revealed that the treatment with DL3 and Bic reduced the expression of these proteins by 40–50% and 10–20%, respectively (data not shown), which positively correlated with inhibitory effects of DL3 and Bic on cell growth (Fig. 1) and cell distribution (Fig. 3). In contrast, the expression levels of these G1 phase-regulating proteins in PC-3 cells were not significantly affected by DL3 or Bic (Fig. 4A). Moreover, data in Fig. 4B show that serum-induced expression of CDK2 and, to a lesser extent, CDK4 in LNCaP cells was also significantly reduced by DL3 and Bic. On the contrary, both DL3 and Bic had only modest effects on CDK2 and CDK4 expression in PC-3 cells (Fig. 4B). Furthermore, the treatment with DL3, and to a lesser extent with Bic, significantly reduced serum-induced phosphorylation of CDK substrates, particularly a species of 110 kD protein in LNCaP but not PC-3 cells (Fig. 4C), suggesting that DL3 and Bic selectively reduced CDK activity in LNCaP cells. Inactivation/phosphorylation of Rb, a 110 kD protein, is induced by androgen stimulation in androgen-responsive cells [23–25]. To determine whether the phosphorylated 110 kD protein differentially regulated by DL3 and Bic in LNCaP cells was Rb, serum-starved LNCaP and PC-3 cells were treated for 24 hr with serum in the absence or presence of 20 uM of DL3 or Bic. The lysates of treated cells were immunoprecipitated with an antibody to Rb and analyzed by western blotting using antibodies against the CDK substrate and phospho-Rb. As shown in Fig. 4D, serum induced a phosphorylation of Rb in both LNCaP and PC-3 cells and pRb was indeed among the proteins detected by the CDK substrate antibody in Fig. 4C. The treatment with DL3, to a less extent with Bic, selectively reduced serum-induced phosphorylation of Rb in LNCaP cells (Fig. 4D). The treatment of DL3 or Bic did not significantly alter expression of the CDK inhibitors p21 and p27 in LNCaP cells (Fig. 4A). Expression of AR and PSA was used as positive controls for AR signaling in LNCaP cells. Indeed, Fig. 4A show that DL3 was more potent than was Bic in downregulating both AR and PSA expression, which is consistent with their inhibitory effects on LNCaP cell growth (Figs 1 and 3) and data reported in our previous study [12].
Fig. 4. Effects of DL3 and Bic on the expression of G1 phase-related cell cycle-regulating proteins.
A. LNCaP and PC-3 cells were treated for 48 hr with 20 uM of DL3 or Bic. Cell lysates were prepared and analyzed by immunoblotting with β-actin as loading control. B and C. LNCaP and PC-3 cells were starved in serum-free medium for 24 hr and then treated for 24 hr with 10% FBS in the absence or presence of 20 uM of DL3 or Bic. Cell lystates were analyzed by western blotting with β-actin as loading control. D. LNCaP and PC-3 cells were starved in serum-free medium for 24 hr and then treated for 24 hr with 10% FBS in the absence or presence of 20 uM of DL3 or Bic. Cell lystates were immunoprecipitated with an antibody to Rb and analyzed by western blotting with antibodies against CDK substrates and pRb (ser807/811). Data shown is from one representative experiment of 3.
4. Discussion
DL3 is a newly identified synthetic compound that downregulates AR, inhibits DHT-induced PSA expression, and suppresses growth of both parental LNCaP cells and their antiandrogen refractory variants [12]. Moreover, these effects of DL3 are more potent in comparison with those of the three antiandrogens, i.e. Bic, flutamide, and nilutamide [12]. The present study further investigated the growth inhibitory effects of DL3 in androgen responsive/AR expressing LNCaP cells and AR-negative PC-3 cells. As expected, we found that DL3 and Bic inhibit growth of LNCaP cells in a dose-dependent manner and the inhibitory effects of DL3 are more potent than those of Bic. The treatment with neither DL3 nor Bic induces cell death in LNCaP cells. Consistent with the finding reported by others [26], we observed that Bic, possibly by blocking the mitogenic effects of androgen, induces an accumulation of cells in G1 and a reduction of cells in S-phase. This G1 arrest effect is also observed in LNCaP cells treated with DL3. In correlation with their inhibition on cell growth, the G1 arrest induced by DL3 is more profound and persistent. Moreover, LNCaP cells exposed to DL3 or Bic contain lower levels of cyclin D1, cyclin D3, and CDK6. Serum-induced expression of CDK2 and, to a lesser extent, CDK4, and serum-induced phosphorylation of CDK substrate proteins, such as Rb, in LNCaP cells were also reduced. These effects are possibly due to a blockade of the mitogenic signaling by DL3 and Bic, and are very likely responsible for the G1 arrest and cell growth inhibition [21–23]. Together with the findings that DL3 inhibits PSA and AR expression, reduces DHT-stimulated cell growth and AR-driven GFP expression, and competes with DHT for binding to AR in LNCaP cells (data not shown), the data on cell growth and cell cycle distribution strongly suggest that DL3 is a novel antiandrogen that binds to AR and attenuates the mitogenic effects of androgen in AR expressing cells. We are currently carrying out further studies, including the binding of DL3 to purified AR, to test the hypothesis.
In contrast to Bic, which selectively inhibits growth and affects the cell cycle distribution of LNCaP cells, DL3, particularly at higher concentrations, also displays moderate growth inhibitory effects on AR-negative PC-3 cells. The growth inhibition of AR-negative PC-3 cells by DL3 is, however, independent of the G1 arrest observed in LNCaP cells, suggesting that DL3 does not affect the mitogenic effects of growth factors in the serum that stimulate the cell cycle progression through G1 phase. Consistent with the differential effects of DL3 on cell cycle distribution in PC-3 and LNCaP cells, DL3 does not significantly alter expression levels of G1-regulating proteins in PC-3 cells. The mechanisms by which DL3 inhibits growth of PC-3 cells are, however, unclear and remain to be elucidated.
The lack of effective therapy for advanced prostate cancer, androgen-independent disease in particular, has led to much research effort to identify novel compounds that can interrupt AR signaling, either directly through downregulation of AR or indirectly through inhibition of Akt signaling or expression and function of molecular chaperones. This effort has led to the discovery of some compounds potentially useful for therapy against advanced PCa. For instance, resveratrol [4; 27] and indole-3-carbinol or its derivative 3,3-diindolylmethane [28–31] that inhibit AR expression and PI3K activity; quercetin [5; 32; 33] that downregulates AR; emodin that induces AR degradation [6]; selenium compounds that downregulate AR expression [7; 8]; and geldanamycin or 17AAG that inhibits ATPase activity and the chaperone function of hsp90 [34; 35]. Moreover, more recent studies have led the discoveries of the second-generation antiandrogens [10] and noncompetitive AR inhibitors [11]. Our research has identified DL3, a compound that can inhibit growth of prostate cancer cells by attenuating the mitogenic effects of AR signaling and by mechanisms independent of AR. These unique properties confer DL3 great potentials in management of advanced prostate cancer.
Acknowledgments
The authors would like to thank Dr. Robert Franco and Mrs. Mary for their technical support in FACS machine operation and Dr. Franco for FACS data interpretation and Dr. Robert Matusik (Dept of Urologic Surgery, Vanderbilt University Medical Center) for providing us pARR2PB promoter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported in parts by grants from University of Cincinnati Cancer Center (to SL3 and ZD) and Grant NIH-NCI 1 R01 CA131137-01A1.
The abbreviations used are: AR, androgen receptor; Bic, bicalutamide; DL3, 6-amino-2-[2-(4-tert-butyl-pnenoxy)-ethylsulfanyl]-1H-pyrimidin-4-one; FBS, fetal bovine serum; FACS, fluorescence-activated cell sorting, MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PSA, prostate-specific antigen.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J. Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Crawford ED. Challenges in the management of prostate cancer. Br. J. Urol. 1992;70 Suppl 1:33–38. doi: 10.1111/j.1464-410x.1992.tb15865.x. [DOI] [PubMed] [Google Scholar]
- 3.Miyamoto H, Rahman MM, Chang C. Molecular basis for the antiandrogen withdrawal syndrome. J. Cell. Biochem. 2004;91:3–12. doi: 10.1002/jcb.10757. [DOI] [PubMed] [Google Scholar]
- 4.Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3'-kinase/Akt pathway and Bcl-2 family proteins. Mol. Cancer Ther. 2006;5:1335–1341. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 5.Yuan H, Gong A, Young CY. Involvement of transcription factor Sp1 in quercetin-mediated inhibitory effect on the androgen receptor in human prostate cancer cells. Carcinogenesis. 2005;26:793–801. doi: 10.1093/carcin/bgi021. [DOI] [PubMed] [Google Scholar]
- 6.Cha TL, Qiu L, Chen CT, Wen Y, Hung MC. Emodin downregulates androgen receptor and inhibits prostate cancer cell growth. Cancer Res. 2005;65:2287–2295. doi: 10.1158/0008-5472.CAN-04-3250. [DOI] [PubMed] [Google Scholar]
- 7.Chun JY, Nadiminty N, Lee SO, Onate SA, Lou W, Gao AC. Mechanisms of selenium down-regulation of androgen receptor signaling in prostate cancer. Mol. Cancer Ther. 2006;5:913–918. doi: 10.1158/1535-7163.MCT-05-0389. [DOI] [PubMed] [Google Scholar]
- 8.Husbeck B, Bhattacharyya RS, Feldman D, Knox SJ. Inhibition of androgen receptor signaling by selenite and methylseleninic acid in prostate cancer cells: two distinct mechanisms of action. Mol. Cancer Ther. 2006;5:2078–2085. doi: 10.1158/1535-7163.MCT-06-0056. [DOI] [PubMed] [Google Scholar]
- 9.Bhuiyan MM, Li Y, Banerjee S, Ahmed F, Wang Z, Ali S, Sarkar FH. Down-regulation of androgen receptor by 3,3'-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B prostate cancer cells. Cancer Res. 2006;66:10064–10072. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- 10.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones JO, Bolton EC, Huang Y, Feau C, Guy RK, Yamamoto KR, Hann B, Diamond MI. Non-competitive androgen receptor inhibition in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7233–7238. doi: 10.1073/pnas.0807282106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu S, Wang A, Lu S, Dong Z. A novel synthetic compound that interrupts androgen receptor signaling in human prostate cancer cells. Mol. Cancer Ther. 2007;6:2057–2064. doi: 10.1158/1535-7163.MCT-06-0735. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Thomas TZ, Kasper S, Matusik RJ. A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinol. 2000;141:4698–4710. doi: 10.1210/endo.141.12.7837. [DOI] [PubMed] [Google Scholar]
- 14.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 15.van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–225. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 16.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest. Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 17.Zhang F, Lee J, Lu S, Pettaway CA, Dong Z. Blockade of transforming growth factor-beta signaling suppresses progression of androgen-independent human prostate cancer in nude mice. Clin Cancer Res. 2005;11:4512–4520. doi: 10.1158/1078-0432.CCR-04-2571. [DOI] [PubMed] [Google Scholar]
- 18.Lu S, Dong Z. Characterization of TGF-beta-regulated interleukin-8 expression in human prostate cancer cells. Prostate. 2006;66:996–1004. doi: 10.1002/pros.20424. [DOI] [PubMed] [Google Scholar]
- 19.Dong Z, Liu Y, Lu S, Wang A, Lee K, Wang LH, Revelo M, Lu S. Vav3 oncogene is overexpressed and regulates cell growth and androgen receptor activity in human prostate cancer. Mol. Endocrinol. 2006;20:2315–2325. doi: 10.1210/me.2006-0048. [DOI] [PubMed] [Google Scholar]
- 20.Weinberg RA. pRb and control of the cell cycle clock, The Biology of Cancer. New York: Garland Science; 2007. pp. 255–306. 2007. [Google Scholar]
- 21.Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–7792. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- 22.Knudsen KE, Arden KC, Cavenee WK. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J. Biol. Chem. 1998;273:20213–20222. doi: 10.1074/jbc.273.32.20213. [DOI] [PubMed] [Google Scholar]
- 23.Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl. Recept. Signal. 2008;6:e001. doi: 10.1621/nrs.06001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knudsen K, Fribourg AF, Petre C, Wetherill Y. Androgen mediated regulation of the G1-S transition in prostate cancer. In: Burnstein KL, editor. Steroid hormones and cell cycle regulation. Boston: Kluwer Academic Publishers; 2002. pp. 91–110. 2002. [Google Scholar]
- 25.Comstock CE, Knudsen KE. The complex role of AR signaling after cytotoxic insult: implications for cell-cycle-based chemotherapeutics. Cell Cycle. 2007;6:1307–1313. doi: 10.4161/cc.6.11.4353. [DOI] [PubMed] [Google Scholar]
- 26.Rigas AC, Robson CN, Curtin NJ. Therapeutic potential of CDK inhibitor NU2058 in androgen-independent prostate cancer. Oncogene. 2007;26:7611–7619. doi: 10.1038/sj.onc.1210586. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell SH, Zhu W, Young CY. Resveratrol inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Cancer Res. 1999;59:5892–5895. [PubMed] [Google Scholar]
- 28.Li Y, Chinni SR, Sarkar FH. Selective growth regulatory and proapoptotic effects of DIM is mediated by AKT and NF-kappaB pathways in prostate cancer cells. Front. Biosci. 2005;10:236–243. doi: 10.2741/1523. [DOI] [PubMed] [Google Scholar]
- 29.Chinni SR, Sarkar FH. Akt inactivation is a key event in indole-3-carbinol-induced apoptosis in PC-3 cells. Clin. Cancer Res. 2002;8:1228–1236. [PubMed] [Google Scholar]
- 30.Hsu JC, Zhang J, Dev A, Wing A, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibition of androgen receptor expression and downregulation of androgen responsiveness in human prostate cancer cells. Carcinogenesis. 2005;26:1896–1904. doi: 10.1093/carcin/bgi155. [DOI] [PubMed] [Google Scholar]
- 31.Le HT, Schaldach CM, Firestone GL, Bjeldanes LF. Plant-derived 3,3'-Diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J. Biol. Chem. 2003;278:21136–21145. doi: 10.1074/jbc.M300588200. [DOI] [PubMed] [Google Scholar]
- 32.Maggiolini M, Vivacqua A, Carpino A, Bonofiglio D, Fasanella G, Salerno M, Picard D, Ando S. The mutant androgen receptor T877A mediates the proliferative but not the cytotoxic dose-dependent effects of genistein and quercetin on human LNCaP prostate cancer cells. Mol. Pharmacol. 2002;62:1027–1035. doi: 10.1124/mol.62.5.1027. [DOI] [PubMed] [Google Scholar]
- 33.Hansen RK, Oesterreich S, Lemieux P, Sarge KD, Fuqua SA. Quercetin inhibits heat shock protein induction but not heat shock factor DNA-binding in human breast carcinoma cells. Biochem. Biophys. Res. Commun. 1997;239:851–856. doi: 10.1006/bbrc.1997.7572. [DOI] [PubMed] [Google Scholar]
- 34.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell. Stress. Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanaja DK, Mitchell SH, Toft DO, Young CY. Effect of geldanamycin on androgen receptor function and stability. Cell. Stress. Chaperones. 2002;7:55–64. doi: 10.1379/1466-1268(2002)007<0055:eogoar>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]