Abstract
SPARC belongs to a class of extracellular matrix-associated proteins that have counteradhesive properties. The ability of SPARC to modulate cell-cell and cell-matrix interactions provides a strong rationale for studies designed to determine its expression in cancer. The objective of this study was to determine if SPARC expression was altered in cadmium (Cd+2) and arsenite (As+3) induced bladder cancer and if these alterations were present in archival specimens of human bladder cancer. The expression of SPARC was determined in human parental UROtsa cells, their Cd+2 and As+3 transformed counterparts and derived tumors, and in archival specimens of human bladder cancer using a combination of real time reverse transcriptase polymerase chain reaction, western blotting, immunofluoresence localization and immunohistochemical staining. It was demonstrated that SPARC expression was down-regulated in Cd+2 and As+3 transformed UROtsa cells. In addition, the malignant epithelial component of tumors derived from these cell lines were also down-regulated for SPARC expression, but the stromal cells recruited to these tumors was highly reactive for SPARC. This finding was shown to translate to specimens of human bladder cancer where tumor cells were SPARC negative, but stromal cells were positive. Acute exposure of UROtsa cells to both cadmium and arsenite reduced the expression of SPARC through a mechanism that did not involve changes in DNA methylation or histone acetylation. These studies suggest that environmental exposure to As+3 or Cd+2 can alter cell-cell and cell-matrix interactions in normal urothelial cells through a reduction in the expression of SPARC. The SPARC associated loss of cell-cell and cell-matrix contacts may participate in the multi-step process of bladder carcinogenesis.
Keywords: Arsenite, cadmium, SPARC, and bladder cancer
INTRODUCTION
SPARC (secreted protein acidic and rich in cysteine), also known as BM-40 and osteonectin, is a 43 kDa protein and a prototype for a class of extracellular matrix-associated proteins with counteradhesive properties (Hudson et al., 2005; Lane and Sage, 1994; Motamed et al., 1995; Sage and Bornstein, 1991). This property includes the ability to dismantle focal adhesions (Murphy-Ullrich et al., 1995, Murphy-Ullrich, 2001). In association with thombospondin-1 and tenascin C, this class comprises a non-homologous functional group of secreted matricellular proteins that interact with cell surface receptors, growth factors, and the extracellular matrix (Bornstein, 1995; Chiquet-Ehrismann, 1993; Crossin, 1996; Erickson, 1993; Hudson et al., 2005). The mechanism/s that control the expression of the SPARC gene in individual cells and tissues are not known. The ability of SPARC to modulate cell-cell and cell-matrix interactions and to have de-adhesive properties has led to many studies assessing its role in cancer (Tai and Tang, 2008). SPARC has been shown to be associated with highly aggressive tumors in some cancers, while in others it appears to function as a tumor suppressor. There have been limited studies of SPARC expression in human bladder cancer. The level of SPARC mRNA has been shown to correlate with increased histological grade, pathological stage, and poor prognosis (Yamanaka et al., 2001); however, the expression of SPARC protein has not been determined. In normal bladder, the SPARC protein has been localized to basal urothelial cells in mice as discrete 20–100 μm foci (Bassuk et al., 2000). In humans, SPARC has been shown to be expressed at the luminal surface of normal urothelium (Alpers et al., 2002). Primary cultures of human urothelial cells have been shown to express SPARC and secrete SPARC into the conditioned growth medium (Delostrinos et al., 2006; Hudson et al., 2005).
The development of bladder cancer is known to have a strong association with environmental exposures (Bischoff and Clark, 2009). This laboratory employs the human UROtsa cell line as a model to explore the relationship between As+3 and Cd+2 exposure and the development of urothelial cancer. The UROtsa cell line is an immortalized, but not tumorigenic model that retains features of transitional urothelium when propagated on a serum-free growth medium (Rossi et al., 2001). This cell line has been used to show that both Cd+2 and As+3 can cause the malignant transformation of human urothelial cells (Sens et al., 2004). The laboratory has subsequently isolated and characterized 6 additional Cd+2 transformed cell lines and 5 additional As+3 transformed cell lines (Cao et al., 2010; Somji et al., 2010). These cell lines were all shown to retain a morphology consistent with human urothelial cancer and to display phenotypic differences characteristic of tumor heterogeneity. The histology of subcutaneous tumor heterotransplants produced by these transformed isolates displayed histologic features of human urothelial carcinoma with areas of squamous differentiation. This observation is important since urothelial carcinoma is the most prominent type of bladder cancer in western countries and accounts for over 95% of all cases and is 5th in overall occurrence (Bischoff and Clark 2009). The association of bladder cancer with environmental exposure is particularly strong for arsenic and correlates to the same endemic areas of the world where populations were identified with arsenic-induced skin cancer (Cantor and Lubin, 2007; Chiou et al., 1995; Luster and Simeonova, 2004; Smith et al., 1998; Steinmaus et al., 2000; Tsuda et al., 1995). The association of Cd+2 with urothelial cancer is not as strong, but several epidemiological studies have implicated Cd+2 in the development of bladder cancer (Kellen et al., 2007; Siemiatycki et al., 1994; Waalkes, 2000). The high level of Cd+2 accumulation in individuals who smoke cigarettes, along with the strong association of bladder cancer and smoking, is the major factor indirectly implicating Cd+2 in the development of urothelial cancer (Satarug and Moore, 2004; Satarug et al., 2010).
The first goal of the present study was to show that SPARC expression is altered when UROtsa cells are exposed to, or malignantly transformed, by As+3 or Cd+2. The second was to characterize SPARC expression in subcutaneous tumors generated from the Cd+2 and As+3 transformed cell lines. The third goal was to implicate or eliminate DNA methylation and histone acetylation as potential regulatory mechanisms for control of SPARC gene expression and the final goal was to show translation of the findings to human bladder cancer by characterizing SPARC expression in archival samples of human urothelial cancer.
MATERIALS AND METHODS
Cell Culture
The procedures for the culture of the parental UROtsa cell line and the Cd+2 and As+3 induced malignant transformants have been described previously (Cao et al., 2010; Sens et al., 2004; Somji et al., 2010). Briefly, stock cultures of the parental UROtsa cell line were maintained in 75 cm2 tissue culture flasks using Dulbecco’s modified Eagle’s medium (DMEM) containing 5% v/v fetal calf serum in a 37°C, 5% CO2: 95% air atmosphere (Rossi et al., 2001). The Cd+2 and As+3 transformed UROtsa cell lines were grown and maintained using identical conditions. Confluent flasks were subcultured at a 1:4 ratio using trypsin-EDTA (0.05%, 0.02%) and the cells were fed fresh growth medium every 3 days
Basal Expression of SPARC in UROtsa Cells and Tumor Heterotransplants
The preparation of total RNA and protein from the parental UROtsa cell line and from the Cd+2 and As+3 transformed cell lines and their subcutaneous heterotransplants have been described previously (Cao et al., 2010; Sens et al., 2004; Somji et al., 2010). Pre-existing samples from these studies were used to determine the basal expression of SPARC mRNA and protein in this study. The expression of SPARC mRNA was determined using real time RT-PCR and SPARC specific primers obtained from Qiagen (Valencia, CA). Briefly, 1 μg of purified RNA was subjected to complementary DNA (cDNA) synthesis using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) in a total volume of 20 μL. Real-time PCR was performed utilizing the SYBR Green kit (Bio-Rad Laboratories) with 2 μL of cDNA, 0.2 μM primers in a total volume of 20 μL in an iCycler iQ real-time detection system (Bio-Rad Laboratories). Amplification was monitored by SYBR Green fluorescence. The level of SPARC mRNA was determined relative to the UROtsa cells grown in serum-containing medium using serial dilutions of this sample as the standard curve. The resulting relative levels were then normalized to the fold change in β-actin expression assessed by the same assay using the primers, sense: CGACAACGGCTCCGGCATGT and antisense: TGCCGTGCTCGATGGGGTACT, giving a product size of 194 base pairs and with the cycling parameters of annealing/extension at 62°C for 45 sec and denaturation at 95°C for 15 sec.
The expression of SPARC protein was determined by Western blotting using 20 μg of total cellular protein. After blocking, the membranes were probed with mouse anti- human osteonectin primary antibody (5μg/mL; Haematologic Technologies Inc., Essex Junction, VT) in blocking buffer for 1 h at room temperature. After washing 3 times with Tris buffered saline (TBS) containing 0.1% Tween 20 (TBS-T), the membranes were incubated with the anti-mouse secondary antibody (1:2000) in antibody dilution buffer for 1 h. The blots were visualized using the Phototope-HP (horseradish peroxidase) Western blot detection system (Cell Signaling Technology, Beverly, MA).
Immunolocalilzation of SPARC in Parental UROtsa Cells
The UROtsa cell lines were grown in 24 well plates containing 12 mm glass coverslips at 37° C, 5% carbon dioxide. Cells at a subconfluent density were then fixed and stained according to published protocols (Cao et al., 2010; Somji et al., 2010). Briefly, cells were fixed in 3.7% buffered paraformaldehyde for 10 min at room temperature. Coverslips were then quenched of free aldehyde with 0.1 M ammonium chloride for 15 min, followed by permeabilization with 0.1% Igepal (NP-40) for 10 min. Cells were stained for SPARC by incubation for 45–60 min at 37° C with a 1:20 dilution of mouse anti-osteonectin antibody (Leica Microsystems Inc., Bannockburn, IL). Primary antibody was detected by incubating cells with 4.0 μg/mL of Alexa Fluor 594 goat anti-mouse IgG (Invitrogen, Carlsbad, CA) for 45–60 min at 37° C. Controls consisted of coverslips treated with the appropriate secondary antibody only. Coverslips were then mounted in ProLong Gold anti-fade reagent with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) for nuclear counter staining. Cells were observed and images captured using a Zeiss LSM 510 Meta Confocal Microscope with LSM 510 software (Carl Zeiss MicroImaging Inc.). Images were composed by capturing z-slices at a depth of 0.5 μm, stacking the z-slices together, and merging with the DAPI image of the same field so all cells in the field could be identified.
Immunohistochemical Localization of SPARC in Tumor Heterotransplants and Archival Specimens of Human Bladder Cancer
The production of subcutaneous nude mouse heterotransplants from the Cd+2 and As+3 transformed UROtsa cell lines has been previously described (Sens et al., 2004). Tumor tissues taken from these and related studies (Cao et al., 2010; Somji et al., 2010) were utilized in the present study to determine the localization and expression of SPARC in tumor heterotransplants. Tissue sections for the immunohistochemical analysis of SPARC expression in human bladder were obtained from archival paraffin blocks that originated from previously completed patient diagnostic procedures. These archival specimens contained no patient identifiers and use was approved by the University of North Dakota Internal Review Board. Prior to immunostaining, after routine deparaffinization and rehydration, sections were immersed in preheated 10 mM sodium citrate buffer (pH 6.0) and heated in a steamer for 20 min. The sections were allowed to cool to room temperature for 30 min and then immersed into TSB-T (Dako, Carpinteria, CA) for 5 min. Endogenous peroxidase was extinguished by incubating the sections in Peroxidase Blocking Reagent (Dako) for 10 min. SPARC was localized by incubating the slides with mouse monoclonal anti-osteonectin antibody (Leica Microsystems Inc.) for 30 min at room temperature. For archival human bladder specimens, the signal was detected using Dako EnVision + Dual Link System-HP (Dako). For tumor heterotransplants, Dakocytomation ARK (Animal Research Kit) was used to visualize the signal, following manufacturer’s instruction. For both human tissue and mouse tumor heterotransplants, liquid diaminobenzidine (Dako) was used as chromogen.
Expression of SPARC in UROtsa Cells Exposed to Cd+2 and As+3
Preliminary experiments were performed to determine the conditions of exposure to Cd+2 and As+3 that were near to, but below, a level that produced cell death in confluent cultures of the parental UROtsa cells over a 10 day period of exposure. From these preliminary determinations, 3 concentrations of cadmium chloride (1.0, 2.0, and 4.0 μM) and sodium arsenite (1.0, 3.0 and 6.0 μM) were then chosen for experimental use such that over the 10-day time course, one concentration would result in minimal cell death and another that would result in appreciable cell death early in the time course. Cell viability, as an indicator of cytotoxicity, was determined by measuring the capacity of the UROtsa cells to reduce MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) to formazan (Sens et al., 2002). The determination of SPARC mRNA using real time RT-PCR and protein by western blotting was as described above.
Treatment of Cd+2 and As+3 Transformed UROtsa Cells with 5-Aza-2′-deoxycytidine (5-AZC) and Histone Deacetylase Inhibitor
The parental and transformed UROtsa cell lines were seeded at a ratio of 1:10 and the next day they were exposed to 0.5, 1.0 and 3.0 μM 5-AZC or the histone deacetylase inhibitor MS-275 at 0.5, 1.5, and 5.0 μM until the cells reached confluency (48 h). Cells were then harvested to determine SPARC mRNA expression.
Statistics
Statistical analysis consisted of ANOVA with Tukey post-hoc testing performed by Graphpad PRISM 4. All statistical significance is denoted at p < 0.05.
RESULTS
SPARC mRNA and Protein Expression in Parental UROtsa Cells and Cd+2 and As+3 Transformed Cell Lines
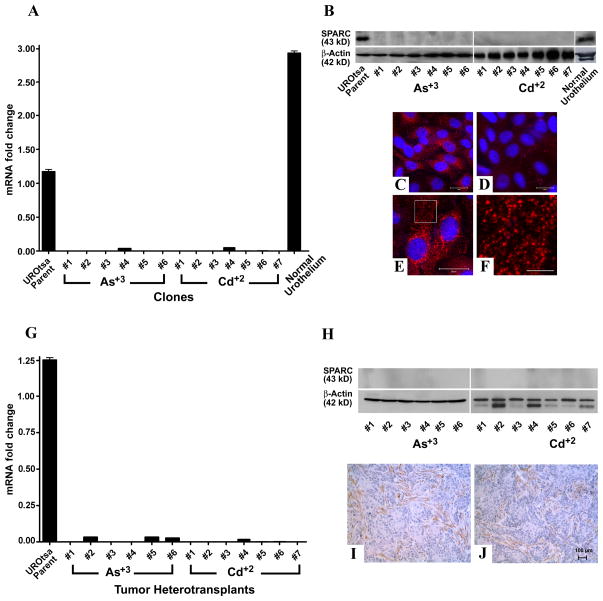
The expression and localization of SPARC was determined for the parental UROtsa cells and the 7 Cd+2 and 6 As+3 transformed cell lines. The parental UROtsa cells expressed a moderate amount of SPARC mRNA when compared to the common transcript, β-actin (Figure 1A). In contrast, SPARC mRNA expression was at the limit of detection in the UROtsa cell lines malignantly transformed by either Cd+2 or As+3 (Figure 1A). A corresponding analysis of SPARC protein expression by western blotting showed that only the parental UROtsa cell line had expression of the SPARC protein (Figure 1B). None of the 13 independent UROtsa cell lines transformed by either Cd+2 or As+3 showed any evidence of expression of the SPARC protein (Figure 1B). The localization of SPARC within the UROtsa cells was determined by immunofluoresence analysis. The analysis showed that the majority of the parental UROtsa cells showed intracellular expression of the SPARC protein, with only very infrequent cell profiles showing no SPARC immunoreactivity (Figure 1C). In contrast, none of the 13 independent UROtsa cell lines transformed by either Cd+2 or As+3 had cell profiles that were immunoreactive for the SPARC protein (Figure 1D). When present, SPARC was localized to the cytoplasm (Figure 1E) and appeared as distinct vesicles (Figure 1F).
Figure 1.
Expression of SPARC mRNA and protein. (A & G). Real time RT-PCR analysis of SPARC expression in parental UROtsa cells, UROtsa cells transformed by Cd+2 and As+3 and normal human urothelium (A) and in tumor heterotransplants (G). Real time data is plotted as the mean+/−SEM of triplicate determinations. (B & H). Western analysis of SPARC protein in parental UROtsa cells, UROtsa cells transformed by Cd+2 and As+3 and normal human urothelium (B) and in tumor heterotransplants (H). (C–F). Immunofluorescent staining for SPARC. (C). SPARC (red) staining in the parent UROtsa cells. (D). Staining for SPARC in UROtsa cells transformed by As+3. (E). SPARC staining in UROtsa parental cells localized to small punctate structures throughout the cytoplasm. (F). Higher magnification image from the boxed area in panel E showing SPARC localized to structures that resemble vesicles. The DAPI counterstain (blue) was used to identify all the cells in the fields. Bars in C–E = 20 μm, the bar in F = 5 μm. (I & J). Immunohistochemical analysis of SPARC protein in Cd+2 or As+3 tumor heterotransplants respectively. The brown color indicates SPARC positive cells. The tumors were generated from the Cd+2 #1 and the As+3 #1 cell lines. Images are taken at the magnification of X200. Bar = 100 μM.
SPARC mRNA and Protein Expression in Tumor Heterotransplants Produced From Cd+2 and As+3 Transformed UROtsa Cell Lines
The expression of SPARC mRNA and protein was determined on extracts prepared from the subcutaneous tumors generated from the 7 Cd+2 and 6 As+3 transformed cell lines (Figure 1G, H). For all the isolates, the expression of SPARC mRNA was at the limit of detection and western blotting failed to demonstrate any expression of the SPARC protein. The immunohistochemical analysis of SPARC expression in the heterotransplants showed no staining of SPARC in the urothelial cancer cells from any of the 7 Cd+2 and 6 As+3 transformed cell lines. In contrast, the stromal components of the urothelial tumors generated from the cell lines were positive for the expression of the SPARC protein. An example of this immunostaining pattern of SPARC is illustrated for one tumor generated from a Cd+2 transformed cell line and one from a As+3 transformed cell line (Figure 1I, J).
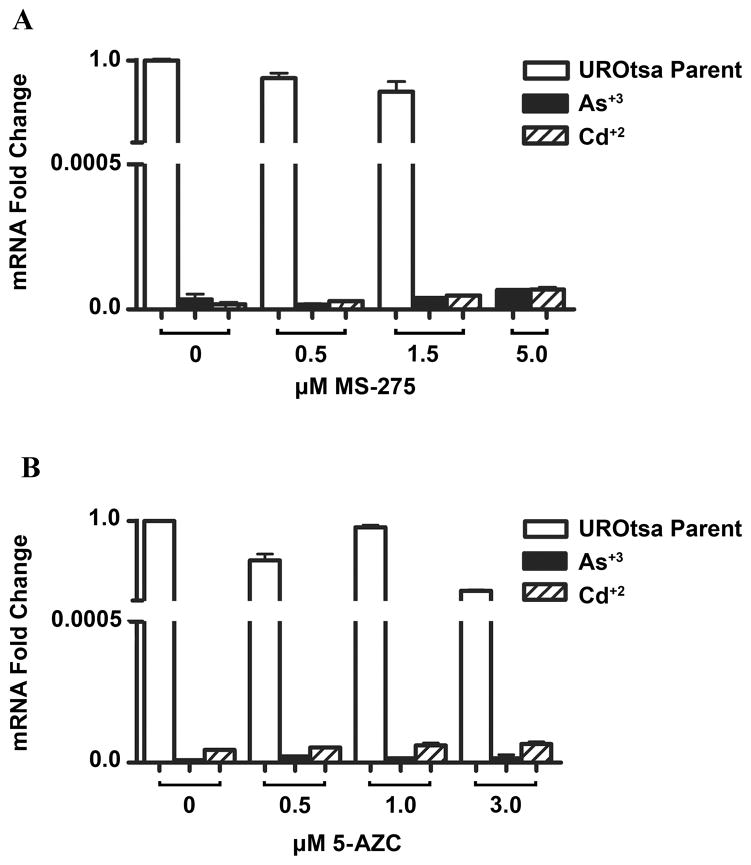
SPARC mRNA Expression in Parental and As+3 and Cd+2 Transformed UROtsa Cells Following Treatment with Inhibitors of DNA Methyation and Acetylation
The parental cell line and single isolates of the As+3 and Cd+2 transformed UROtsa cells were treated with the histone deacetylase inhibitor, MS-275, and the methylation inhibitor, 5-AZC, to determine the possible role of epigenetic modifications on SPARC mRNA expression. This analysis demonstrated that none of the cell lines, parental or transformed, treated with MS-275 or 5-AZC expressed increased levels of SPARC mRNA compared to the untreated controls (Figure 2). Additional experiments were performed where treatment of the cells with both drugs was increased to 72 h with no change in the results (data not shown). The treatment of the three cell lines with a combination of the two drugs also had no effect on SPARC mRNA expression (data not shown). An identical protocol has been used by the laboratory to show that treatment of MCF-10 human breast cells with 5-AZC and MS-275 induces the expression of MT-3 mRNA (Somji et al., 2010).
Figure 2.
Real-time RT-PCR analysis of SPARC mRNA levels in parental UROtsa cells, and UROtsa cells transformed by Cd+2 and As+3 treated with epigenetic regulators. The Cd+2 #1 and the As+3 #1 transformed cell lines were used in these experiments. (A). Expression of SPARC mRNA after treatment with MS-275 for 48 h. (B). Expression of SPARC mRNA after treatment with 5-AZC for 48 h. The level of SPARC mRNA was determined relative to the UROtsa cells using serial dilutions of this sample as the standard curve. The resulting relative levels were then normalized to the fold change in β-actin. Real time data is plotted as the mean+/−SEM of triplicate determinations.
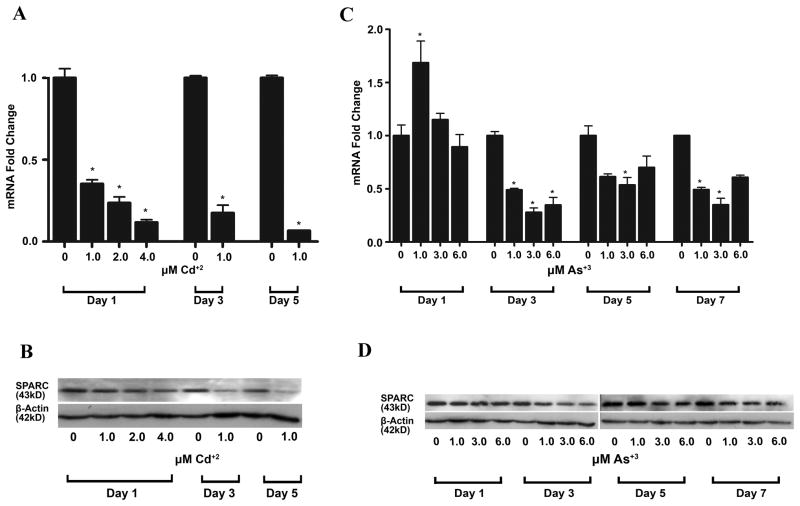
SPARC Expression in Parental UROtsa Cells Exposed to Cd+2 and As+3
The expression of SPARC was determined in parental UROtsa cells following exposure to Cd+2 and As+3. The results showed that the expression of SPARC mRNA was significantly reduced following a 24 h exposure to as little as 1 μM of Cd+2 (Figure 3A). The expression of SPARC mRNA showed further reductions when either the level of Cd+2 was increased during a 24 h period of exposure or when the period of exposure to 1 μM Cd+2 was extended to 5 days. The expression of SPARC protein was also reduced significantly following Cd+2 exposure and in general followed the pattern of SPARC mRNA, when an expected slower rate of SPARC protein degradation is taken into account (Figure 3B). The expression of SPARC was also shown to be reduced in parental UROtsa cells following exposure to As+3 (Figure 3C, D). The level of SPARC mRNA and protein was not reduced following a 24 h exposure of the cells to 1, 3 or 6 μM As+3. In contrast, a reduction in SPARC mRNA and protein did occur when the parental UROtsa cells were exposed to 1, 3 or 6 μM As+3 for 3, 5 and 7 days of exposure.
Figure 3.
Expression of SPARC mRNA and protein in parental UROtsa cells exposed to Cd+2 and As+3. (A & C). Real time RT-PCR analysis of SPARC. The level of SPARC mRNA was determined relative to the UROtsa cells using serial dilutions of this sample as the standard curve. The resulting relative levels were then normalized to the fold change in β-actin. * denotes a significant difference from untreated UROtsa cells (p < 0.05). Real time data is plotted as the mean+/−SEM of triplicate determinations. (B & D). Western blot analysis of SPARC protein.
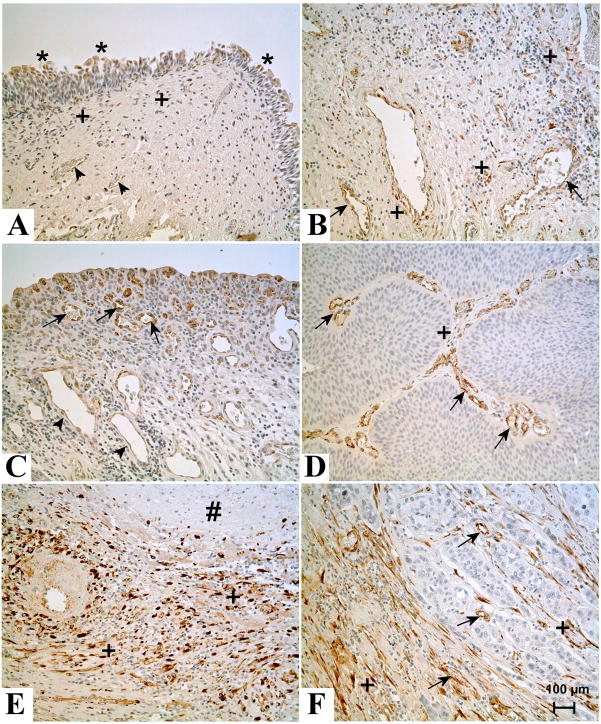
Immunohistochemical staining of SPARC in normal human bladder, cystitis, noninvasive and invasive urothelial carcinoma
The immunohistochemical staining of SPARC was determined on 4 samples of non-cancerous “normal” urothelium, 5 cases of low grade urothelial cancer and 6 cases of high grade urothelial cancer. Two cases of normal urothelium had no evidence of cystitis or inflammation. In these two cases, SPARC was moderately expressed in the upper superficial cells of the urothelium (Figure 4A). In addition, SPARC was also expressed in a few small stromal cells located in the superficial lamina propria just beneath the urotheluim. There was no SPARC staining of the blood vessels in the normal lamina propria. One case of archived normal urothelium was accompanied by frozen tissue and total RNA and protein were prepared and shown to contain SPARC mRNA and protein (Figure 1A, B).. The other 2 cases of normal urothelium were non-cancerous, but did have prominent inflammation (cystitis). The expression of SPARC in the urothelium of these two samples was identical to that of the above samples, with SPARC expression localized to the superficial layer of the urothelium. However, in these samples with inflammation, there were more frequent profiles of SPARC expression localized to stromal cells and also for the endothelium of the blood vessels located in the lamina propria (Figure 4B). The stromal cells that express SPARC were usually spindle-shaped and could be located anywhere in the bladder where inflammation was present; not being limited in expression to the superficial laminar propria as that found in normal bladder specimens with no inflammation. In an area with granulation formation, the newly formed blood vessels with plump endothelial cells were strongly positive for SPARC, while the flat endothelial cells in mature blood vessels located deep in the lamina propria were only weakly positive or negative for SPARC (Figure 4C).
Figure 4.
Immunohistochemical staining of SPARC in normal human bladder, cystitis, invasive and noninvasive urothelial carcinoma. (A). Localization of SPARC in normal human bladder tissue sample. * indicates SPARC staining in the normal urothelium. + indicates staining in the stromal cells. Arrow heads indicate lack of staining in blood vessels. (B). Localization of SPARC in a bladder tissue sample obtained from a patient with cystitis. + indicates frequent staining of stromal cells. Arrows indicate moderate staining in blood vessels. (C). Localization of SPARC in blood vessels of bladder tissue obtained from a patient with cystitis. Arrows indicate strong staining in the newly formed blood vessels, whereas mature blood vessels in deep lamina propria are weakly positive or negative for SPARC (arrow heads). (D). Localization of SPARC in low grade urothelial papillary carcinoma. The tumor cells did not stain for SPARC whereas few stromal cells in the papillary core (+) stained for SPARC. Arrow indicates strong staining in small blood vessels. (E). Localization of SPARC in low grade urothelial papillary carcinoma containing areas of necrosis. # indicates an area of necrosis, around which a large number of SPARC positive (+) stromal cells are present. (F). Localization of SPARC in high grade invasive bladder cancer. The tumor cells did not stain for SPARC whereas the desmoplastic stromal cells both in and around the invasive carcinoma stained strongly (+). Arrows indicate positive staining of the endothelial cells of the blood vessels. All images are at a magnification of X200. Bar = 100 μm.
In contrast to normal urothelium, all 5 cases of low grade bladder cancer were found to have no expression of SPARC in the tumor urothelium (Figure 4D). In cases where there was no tumor-associated inflammation or necrosis, only a few SPARC positive stromal cells were present in the papillary core, while more stromal cells expressing SPARC could be found in the basal area of the tumor, where the tumor connects to the wall of the bladder (data not shown). The endothelial cells of the blood vessels in the papillary cores of the tumors were usually strongly stained for SPARC (Figure 4D). In some of the cases of low grade carcinoma, a band of inflammatory reaction could be identified in the interface between tumor and bladder wall; and in these instances there was an increase in the number of SPARC reactive stromal cells (data not shown). In areas of necrosis, whether in the tumor or in a tumor free area, there was a large number of SPARC positive stromal cells that surrounded the area of necrosis (Figure 4E).
Identical to that found in low grade urothelial cancer, the tumor cells of high grade, invasive urothelial cancer were found to have no expression of SPARC protein (Figure 4F). The high grade urothelial cancers did have a prominent desmoplastic stromal reaction that was present in and around the invasive carcinoma. These desmoplastic stromal cells were prominently stained for SPARC as were the endothelial cells of the blood vessels in or adjacent to the invasive carcinoma (Figure 4F). The stromal cells that stained for SPARC were more prominent in areas surrounding the invasive carcinoma than the stromal cells located within the invasive carcinoma itself. In areas of the invasive carcinomas that did not exhibit a prominent stromal reaction there were fewer SPARC reactive stromal cells, but the associated blood vessels, when present, had endothelium that was strongly positive for SPARC (data not shown). The expression of SPARC in the stromal cells of the tumor free areas from the cases of invasive carcinoma was similar to that noted above for normal bladder, depending on the degree of inflammation (data not shown).
DISCUSSION
The present study is the first to show that the heavy metals, Cd+2 and As+3, may down-regulate the expression of SPARC during the development and progression of bladder cancer. It is known from previous studies that SPARC is expressed in normal urothelium and urothelial cell cultures (Alpers et al., 2002; Bassuk et al., 2000; Delostrinos et al., 2006; Hudson et al., 2005). The present study shows that SPARC is also expressed in the parental UROtsa cells and that SPARC expression is reduced to the limit of detection when the cells are malignantly transformed by both Cd+2 and As+3. Immunohistochemical analysis showed that the expression of SPARC was also down-regulated to background levels in the epithelial component of the tumors produced from cells injected subcutaneously into nude mice. In contrast, the stromal component of these tumors showed strong immunoreactivity for the SPARC protein. The stromal component originates from the murine host and is likely recruited to the tumor site by secretions from the tumor cells. The murine origin of the stromal component caused some minor difficulty in the interpretation of SPARC expression since the real time PCR primers and antibody used to assess human SPARC expression were chosen to perform optimally on human cells and tissue. While the human SPARC antibody was effective in the immunohistochemical localization of SPARC in murine stroma, it performed very poorly when used for western blotting. The sequence of the primers used for real time analysis of SPARC mRNA in humans is not preserved in the murine sequence. Despite these technical limitations, the results clearly show that SPARC expression is down-regulated when UROtsa cells are transformed by Cd+2 or As+3 and that the stroma recruited to tumors produced by the SPARC negative epithelial cells are strongly immunoreactive for SPARC.
Another significant finding is that the above alterations in SPARC expression found in tumors from Cd+2 and As+3 transformed UROtsa cells translates to human urothelial cancer. The present study confirmed that SPARC is expressed in normal urothelium. A new finding in this study using archival specimens of human bladder cancer was that SPARC expression is absent in the malignant urothelial cells comprising human urothelial cancer, but is highly expressed in the stromal component of these tumors. The expression of SPARC was also noted in endothelial cells in areas of inflammation and in inflammatory cells at these sites. These findings impact on the previous report that SPARC expression is increased in human urothelial cancer (Yamanaka et al., 2001). In this study, SPARC expression was determined only at the level of mRNA expression with no determination of the SPARC protein by immunohistochemical localization. It is highly likely that this study noted increased expression of SPARC in urothelial cancer due to SPARC expression in the tumor recruited stroma and not in the malignant urothelial cells themselves. Using this interpretation, the previous study would have actually correlated to increased expression of SPARC in the stromal component of urothelial cancer, and not to the cancer cells themselves, with increased histological grade, pathological stage, and poor prognosis. A future retrospective study will need to be performed to determine if it is the amount of stroma that expresses SPARC or the level of SPARC expression in the stroma, or both, that correlated to these important clinical parameters.
It was also determined that acute exposure of the parental UROtsa cells to both Cd+2 and As+3 resulted in a reduction in the expression of SPARC mRNA and protein. The reduction was especially pronounced for Cd+2, but reductions by both agents occurred at concentrations routinely used to mimic the effects of environmental exposure to these pollutants. To our knowledge, this is the first indication that exposure to Cd+2 or As+3 might cause a reduction in the expression of SPARC in human cells. The study also showed that SPARC expression was not changed in the normal or transformed cells by treatment of the cells with either a histone deacetylase inhibitor or a demethylating agent. The possibility that SPARC expression might be influenced by histone modification or DNA methylation was suggested by studies showing aberrant methylation of the SPARC gene in human lung and ovarian cancers (Socha et al., 2009; Suzuki et al., 2005). The finding that both Cd+2 and As+3 had similar effects on SPARC expression before and following malignant transformation suggests a similar mechanism of action once the agents are fully elaborated inside the cell. The laboratory employs both agents in environmental bladder cancer research since each has distinctly different modes of cellular uptake and processing once inside the cell. This is especially pronounced for cellular processing since Cd+2 remains chemically unaltered inside the cell and As+3 requires methyation to become active. The present study implicates both Cd+2 and As+3 as agents affecting SPARC expression in bladder cancer.
Acknowledgments
This work was supported by grant number R01 ES015100 from the National Institute of Environmental Health Sciences, NIH. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer Larson, Email: jlarson@medicine.nodak.edu.
Tahmina Yasmin, Email: tyasmin@medicine.nodak.edu.
Donald A. Sens, Email: dsens@medicine.nodak.edu.
Xu Dong Zhou, Email: zxudong@medicine.nodak.edu.
Mary Ann Sens, Email: msens@medicine.nodak.edu.
Scott H. Garrett, Email: sgarrett@medicine.nodak.edu.
Jane R. Dunlevy, Email: jdunlevy@medicine.nodak.edu.
Ling Cao, Email: lcao@medicine.nodak.edu.
References
- Alpers CE, Hudkins KL, Segerer S, Sage EH, Pichler R, Couser WG, Johnson RJ, Bassuk JA. Localization of SPARC in developing, mature, and chonically injured human allograft kidneys. Kidney Int. 2002;62:2073–2086. doi: 10.1046/j.1523-1755.2002.00680.x. [DOI] [PubMed] [Google Scholar]
- Bassuk JA, Grady R, Mitchell M. Review Article: The molecular era of bladder research. Transgenic mice as experimental tools in the study of outlet obstruction. J Urol. 2000;164:170–179. [PubMed] [Google Scholar]
- Bischoff CJ, Clark PE. Bladder Cancer. Curr Opin Oncol. 2009;21:272–277. doi: 10.1097/cco.0b013e328329f184. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteins: An appraisal of thombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor KP, Lubin JH. Arsenic, internal cancers, and issues in inference from studies at low-level exposures in human populations. Toxicol Appl Pharmacol. 2007;222:252–257. doi: 10.1016/j.taap.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Zhou XD, Sens MA, Garrett SH, Zheng Y, Dunlevy JR, Sens DA, Somji S. Keratin 6 expression correlates to areas of squamous differentiation in multiple independent isolates of As+3- induced bladder cancer. J Appl Toxicol. 2010 Feb 22; doi: 10.1002/jat.1513. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou HY, Hsueh Y, Liaw KF, Hong SF, Chiang MH, Pu YS, Lin JS, Huang CH, Chen CJ. Incidence of internal cancers and ingested inorganic arsenic: a 7-year follow-up study in Taiwan. Cancer Res. 1995;55:1296–1300. [PubMed] [Google Scholar]
- Chiquet-Ehismann R. Tenascin and other adhesion-modulating proteins in cancer. Semin Cancer Biol. 1993;4:301–310. [PubMed] [Google Scholar]
- Crossin KL. Tenascin: A multifunctional extracellular matrix protein with a restricted distribution in development and disease. J Cell Biochem. 1996;61:592–598. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C592::AID-JCB13%3E3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Delostrinos CF, Hudson AE, Feng WC, Kosman J, Bassuk JA. The C-terminal Ca2+-binding domain of SPARC confers anti-spreading activity to human urothelial cells. J Cell Physiol. 2006;206:211–220. doi: 10.1002/jcp.20462. [DOI] [PubMed] [Google Scholar]
- Erickson HP. Tenascin-C, tenascin-R, and tenascin-X: A family of talented proteins in search of functions. Curr Opin Biol. 1993;5:869–876. doi: 10.1016/0955-0674(93)90037-q. [DOI] [PubMed] [Google Scholar]
- Hudson AE, Feng WC, Delostrinos CF, Carmean N, Bassuk J. Spreading of embryologically distinct urothelial cells is inhibited by SPARC. J Cell Physiol. 2005;202:453–463. doi: 10.1002/jcp.20140. [DOI] [PubMed] [Google Scholar]
- Kellen E, Zeegers M, Hond ED, Buntinx F. Blood cadmium may be associated with bladder carcinogenesis; the Belgian case control study on bladder cancer. Cancer Detect Prev. 2007;31:77–82. doi: 10.1016/j.cdp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Lane TF, Sage EH. The biology of SPARC: A protein that modulates cell-matrix interactions. FASEB J. 1994;8:163–173. [PubMed] [Google Scholar]
- Luster MI, Simeonova PP. Arsenic and urinary bladder cell proliferation. Toxicol Appl Pharmacol. 2004;198:419–423. doi: 10.1016/j.taap.2003.07.017. [DOI] [PubMed] [Google Scholar]
- Motamed K, Bassuk JA, Sage EH. Anti-adhesive properties of SPARC: Structural and functional correlates. In: Crossin KL, editor. Tenascin and other counteradhesive molecules of the extracellular matrix. Harwood Academic Press; Amsterdam: 1995. pp. 111–131. [Google Scholar]
- Murphy-Ullrich JE, Lane TF, Pallero MA, Sage EH. SPARC mediates focal adhesion disassembly in endothelial cells though a follistatin-like region and the Ca+2-binding EF-hand. J Cell Biochem. 1995;57:341–350. doi: 10.1002/jcb.240570218. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: Is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MR, Masters JRW, Park S, Todd JH, Garrett SH, Sens MA, Somji S, Nath J, Sens DA. The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environ Health Perspect. 2001;109:801–808. doi: 10.1289/ehp.01109801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions: SPARC, tenascin, and thombospondin. J Biol Chem. 1991;266:14831–14834. [PubMed] [Google Scholar]
- Satarug S, Moore MR. Adverse health effects of chonic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect. 2004;112:1099–1103. doi: 10.1289/ehp.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure and heath outcomes. Environ Health Perspect. 2010;118:182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socha MJ, Said N, Dai YS, Kwong J, Ramalingan P, Trieu V, Desai N, Mok C, Motamed K. Abberant promoter methylation of Sparc in ovarian cancer. Neoplasia. 2009;11:126–135. doi: 10.1593/neo.81146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sens DA, Park S, Gurel V, Sens MA, Garrett SH, Somji S. Inorganic cadmium- and arsenite-induced malignant transformation of human bladder urothelial cells. Toxicol Sci. 2004;79:56–63. doi: 10.1093/toxsci/kfh086. [DOI] [PubMed] [Google Scholar]
- Siemiatycki J, Dewar R, Nadon L, Gerin M. Occupational risk factors for bladder cancer: Results from a case control study in Montreal, Quebec, Canada. Am J Epidemiol. 1994;140:1061–1080. doi: 10.1093/oxfordjournals.aje.a117207. [DOI] [PubMed] [Google Scholar]
- Smith AH, Goycolea M, Haque R, Biggs ML. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998;147:660–669. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- Somji S, Garrett SH, Zhou XD, Zheng Y, Sens DA, Sens MA. Absence of metallothionein 3 expression in breast cancer is a rare, but favorable marker of outcome that is under epigenetic control. Toxicol Environ Chem. 2010 doi: 10.1080/02772241003711274. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somji S, Zhou XD, Mehus A, Sens MA, Garrett SH, Lutz KL, Dunlevy JR, Zheng Y, Sens DA. Variation of keratin 7 expression and other phenotypic characteristics of independent isolates of cadmium transformed human urothelial cells (UROtsa) Chem Res Toxicol. 2010;23:348–356. doi: 10.1021/tx900346q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Moore L, Hopenhayn-Rich C, Biggs ML, Smith AH. Arsenic in drinking water and bladder cancer. Cancer Invest. 2000;18:174–182. doi: 10.3109/07357900009038249. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hao C, Takahashi T, Shigematsu H, Shivapurkar N, Sathyanarayaria UG, Lizasa T, Fujisawa T, Hiroshima K, Gazdar AF. Abberant methylation of SPARC in human lung cancer. Br J Cancer. 92:942–948. doi: 10.1038/sj.bjc.6602376. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tai IT, Tang MJ. SPARC in cancer biology: Its role in cancer progression and potential for therapy. Drug Resistance Updates. 2008;11:231–246. doi: 10.1016/j.drup.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Babazono A, Yamamoto E, Kurumatini N, Mino Y, Ogawa T, Kishi Y, Aoyama H. Ingested arsenic and internal cancer: a historical cohort study followed for 33 years. Am J Epidemiol. 1995;141:198–209. doi: 10.1093/oxfordjournals.aje.a117421. [DOI] [PubMed] [Google Scholar]
- Waalkes MP. Cadmium carcinogenesis in review. J Inorg Biochem. 2000;79:241–244. doi: 10.1016/s0162-0134(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Yamanaka M, Kanda K, Li N-C, Fukumori T, Oka N, Kanayama H-O, Kagawa S. Analysis of the gene expression of SPARC and its prognostic value for bladder cancer. J Urol. 2001;166:2495–2499. [PubMed] [Google Scholar]