Abstract
The herbicide 2,6-dichlorobenzonitril (DCBN) is a potent and tissue-specific toxicant to the olfactory mucosa (OM). The toxicity of DCBN is mediated by cytochrome P450 (P450)-catalyzed bioactivation; however, it is not known whether target-tissue metabolic activation is essential for toxicity. CYP2A5, expressed abundantly in both liver and OM, was previously found to be one of the P450 enzymes active in DCBN bioactivation in vitro. The aims of this study were to determine the role of CYP2A5 in DCBN toxicity in vivo, by comparing the extents of DCBN toxicity between Cyp2a5-null and wild-type (WT) mice, and to determine whether hepatic microsomal P450 enzymes (including CYP2A5) are essential for the DCBN toxicity, by comparing the extents of DCBN toxicity between liver-Cpr-null (LCN) mice, which have little P450 activity in hepatocytes, and WT mice. We show that the loss of CYP2A5 expression did not alter systemic clearance of DCBN (at 25 mg/kg); but it did inhibit DCBN-induced non-protein thiol depletion and cytotoxicity in the OM. Thus, CYP2A5 plays an essential role in mediating DCBN toxicity in the OM. In contrast to the results seen in the Cyp2a5-null mice, the rates of systemic DCBN clearance were substantially reduced, while the extents of DCBN-induced nasal toxicity were increased, rather than decreased, in the LCN mice, compared to WT mice. Therefore, hepatic P450 enzymes, although essential for DCBN clearance, are not necessary for DCBN-induced OM toxicity. Our findings form the basis for a mechanism-based approach to assessing the potential risks of DCBN nasal toxicity in humans.
Keywords: Cytochrome P450, CYP2A5, gene knockout, nasal toxicity, dichlobenil, olfactory mucosa
Introduction
The herbicide 2,6-dichlorobenzonitrile (DCBN, see Fig. 1 for structure), which is widely used for weed control (Cox, 1997), is one of the most potent olfactory toxicants in rodents (Brandt et al., 1990). In mice, DCBN treatment results in necrosis of the Bowman’s glands, followed by degeneration and necrosis of the olfactory neuroepithelium. DCBN induces permanent loss of olfactory neurons in the dorsal medial part of the nasal cavity, accompanied by respiratory metaplasia in the damaged region (Bergman et al., 2002). Human exposure to DCBN occurs occupationally, through inhalation and dermal absorption, and in the environment, through ingestion of contaminated food and ground water.
Fig. 1. Structure of DCBN.
The involvement of P450-catalyzed metabolic activation in the toxicity of DCBN has long been proposed, based on the substantial inhibition of DCBN-induced in vivo formation of DCBN-protein adducts, as well as olfactory toxicity, by metyrapone, a P450 inhibitor (Brandt et al, 1990; Brittebo et al, 1995). The olfactory toxicity of DCBN is believed to derive from its reactive metabolites (2,3-oxo-DCBN and, to a lesser extent, 3,4-oxo-DCBN), produced through P450-catalyzed metabolic activation; these epoxides readily form conjugates with reduced glutathione (GSH) or attack sulfhydryl groups in cellular proteins, yielding protein adducts (Ding et al., 1996). P450 enzymes in both liver, the main metabolic organ for circulating xenobiotic compounds, and the nasal mucosa, the target organ for DCBN toxicity, can metabolize DCBN in vitro. The potential role of target tissue metabolic activation in DCBN toxicity has been suggested, mainly on the basis of the unique occurrence of abundant, tissue-selective P450 enzymes, CYP2A5 and CYP2G1, in the mouse OM; both enzymes could metabolize DCBN, with relatively low km values, into reactive intermediates that form protein adducts and GSH conjugates (Ding et al, 1996; Gu et al., 1998). However, till now, there has been no direct proof regarding the tissue source (liver versus OM) of the reactive intermediates that are responsible for the olfactory toxicity of DCBN, and it is not known whether CYP2A5 or CYP2G1 is more important for DCBN toxicity in vivo. The latter question is important for attempts to extrapolate rodent toxicity data to humans, for risk assessment. Notably, while human CYP2A6 and CYP2A13, orthologs of mouse CYP2A5, are expressed in human nasal mucosa, and both enzymes are active toward DCBN metabolic activation (Liu et al., 1996; Su et al., 2000), human CYP2G1 has become a pseudogene (Sheng et al., 2000).
The aims of this study were to identify the P450 enzyme responsible for DCBN metabolic activation in vivo, and to determine whether hepatic P450-generated DCBN metabolites play a significant role in DCBN toxicity in the OM. Two knockout mouse models were utilized: the Cyp2a5-null mouse (Zhou et al., 2010) and the liver-Cpr-null (LCN) mouse (Gu et al., 2003). CYP2A5 is expressed in many tissues, including OM, liver, kidney, and lung. In the Cyp2a5-null mouse, the Cyp2a5 gene was deleted in the germline, and CYP2A5 expression is no longer detected in the OM or elsewhere. This model has been used to demonstrate a critical role of CYP2A5 in the systemic clearance of nicotine and cotinine (Zhou et al., 2010). In the LCN mouse, the gene for the NADPH-cytochrome P450 reductase (CPR) was deleted in a tissue-specific fashion, in all hepatocytes, leading to inactivation of all microsomal P450 enzymes, including CYP2A5. The LCN mouse has been used to clarify the role of hepatic CPR/P450 in the systemic clearance of various xenobiotics (e.g., Gu et al., 2007; Weng et al, 2007; Zhang et al., 2007); they have also been used to identify in vivo contributions of hepatic P450s to xenobiotic toxicity in extrahepatic target organs, such as the renal toxicity induced by acetaminophen (Gu et al., 2005).
In this study, the Cyp2a5-null, LCN, and wild-type (WT) mice, all on a C57BL/6 (B6) genetic background, were treated with DCBN at doses established in previous studies to cause nasal toxicity in WT mice. The extent of DCBN-induced OM toxicity was assessed by histological analysis of the nasal cavity, and through measurements of tissue levels of non-protein thiol (NPSH). The impacts of the global loss of CYP2A5 expression, or the hepatocyte-specific loss of CPR expression, on rates of DCBN metabolic activation in vitro, and on the kinetics of DCBN clearance in vivo, were also examined. The results of our studies provide definitive evidence that CYP2A5 plays an essential role in mediating DCBN toxicity in the OM, and that hepatic P450 enzymes, although essential for DCBN clearance, are not necessary for DCBN-induced OM toxicity.
Materials and Methods
Chemicals and animal treatments
DCBN (97% pure), olive oil (highly refined, low acidity), and other chemicals were purchased from Sigma Aldrich (St. Louis, MO), unless stated otherwise. Procedures involving animals were approved by the Institutional Animal Care and Use Committee of the Wadsworth Center (Albany, NY). LCN (nearly congenic strain after backcrossing to the B6 strain for 10 generations), their corresponding WT littermates, Cyp2a5-null (on B6 background), and WT B6 mice were obtained from breeding stocks maintained at the Wadsworth Center. Animals were normally maintained at 22°C with a 12-h on, 12-h off light cycle and were allowed free access to water and a standard laboratory diet. Mice were treated with a single intraperitoneal dose of DCBN at 25 or 50 mg/kg (5 µl/g body weight); DCBN was dissolved in a mixture (1:4; v/v) of dimethyl sulfoxide (from Calbiochem) and olive oil. Control mice were injected with vehicle only. Animals were dosed at 10:00–11:00 AM, local time. Tail blood samples (10 or 20 µl each) were collected from individual mice at a series of time points after dosing (5, 30 min, and 1, 2, 4, 8, and 24 h) through the use of heparin-coated capillaries. Each blood sample was diluted with one volume of saline and then centrifuged, at 4°C and 9,000 g, for 10 min. The plasma was stored at −80° C until use. At 24 h after DCBN injection, the mice were euthanized by CO2 overdose, and noses were dissected for histopathological examination. For determination of tissue levels of NPSH, mice were fasted overnight, and liver and OM were dissected at 2 h following DCBN injection; tissue samples were stored frozen at −80°C before analysis.
Determination of catalytic activity in vitro
OM microsomes were prepared as described previously (Gu et al., 1997). Each microsomal sample was prepared from pooled OM of 6 – 8 mice. DCBN metabolism was assayed with the use of 2,6-[ring-14C]DCBN (20.1 Ci/mol, >99% pure, Sigma Chemical Co.), as reported before (Ding et al., 1996). The contents of reaction mixtures and the incubation conditions are indicated in the legends to figures. Formation of GS-DCBN was determined by radiometric HPLC as described previously (Ding et al., 1996).
Determination of tissue NPSH levels
NPSH was determined according to an established method (Tonge et al, 1998), with modifications in the homogenization step, in order to accommodate the small amount of nasal tissues available from a single mouse. Liver was homogenized on ice with a Polytron (model GT 10–35, Kinematica, Bohemia, NY), in 100 mM Tris-acetate buffer, pH 7.4, containing 1.0 mM EDTA and 150 mM potassium chloride (Buffer H), at a tissue-to-buffer ratio of ~1:10. OM (~20 mg wet weight) from individual mice was homogenized, with use of a Bullet Blender™ (Next Advance, Averill Park, NY), in an 1.5-ml Eppendorf centrifuge tube containing 500 µl of Buffer H and two stainless steel beads (0.5-mm diameter). The samples were homogenized in the blender at a speed setting of 4, continuously for 4 min, at 4° C. GSH was used as the standard; recovery was ~90%.
Histopathological examination
Tissue blocks containing the nasal passage were dissected and immersed in Bouin’s solution for two weeks, for fixation and decalcification, as described previously (Gu et al., 2005). Each tissue block was cut into smaller blocks, at four anterior-rostral levels, as described by Young (Young, 1981). Paraffin sections (4-µm thick) from levels 1–4 were stained with hematoxylin and eosin (H&E) for pathological examination. For semi-quantitative assessment of the extent of tissue toxicity, the severity of lesions in the OM was graded, as described previously (Gu et al., 2005). Tissue sectioning and staining were performed at the Wadsworth Center Pathology core facility. Images were obtained using a Nikon model 50i light microscope, fitted with a digital camera, at the Wadsworth Center Light Microscopy core.
Determination of DCBN in plasma
A GC-MS protocol was developed for DCBN determination. Each plasma sample was spiked with 50 ng of d10-phenanthrene, as an internal standard (as recommended by Mercer, 2005), and then extracted with 4 volumes of hexane. The organic phase was transferred to a new vial, and aliquots (2 µl) were analyzed on an Agilent Technologies (Santa Clara, CA) model 6890 GC interfaced to an Agilent 5973 mass spectrometer, operated in the electro ionization mode (with electron energy at +70 eV). An Rxi™ Capillary GC column (30 m × 0.25 mm I.D., with a 0.25-µm film thickness, Restec, Bellefonte, PA) was used. The inlet temperature of the GC was kept at 260 °C. The column was eluted at a helium flow rate of 1.0 ml/min, with an initial temperature of 70°C, for 2 min, and then a temperature gradient of 20°C/min to 270°C. Analytes were detected in the Selected Ion Monitoring mode, with use of m/z-171 values for DCBN quantitation, m/z-173 values for DCBN confirmation, and m/z-188 values for d10-phenanthrene quantitation, with a dwell time of 100 msec for each ion. The retention time for d10-phenanthrene was ~10.3 min, and that for DCBN was ~7.4 min. Calibration curves, prepared by spiking authentic DCBN (20 – 1000 ppb), as well as d10-phenanthrene, into blank mouse plasma at various concentrations, prior to extraction, were used for DCBN quantification. The recovery of DCBN from plasma samples was > 90%.
Other methods
Protein concentration was determined by the bicinchoninic acid method (Pierce Chemical, Rockford, IL). Pharmacokinetic parameters, including area under concentration-time curve (AUC), maximum plasma concentration (Cmax), time when Cmax is reached (Tmax), elimination half-time (t1/2), and clearance, were calculated using the WinNonlin software (Pharsight, Mountain View, CA). Statistical significance of differences between two groups in various parameters was examined using Student’s t-test, with use of the SigmaStat software (SPSS, Chicago, IL).
Results
CYP2A5 plays an essential role in mediating DCBN toxicity in the OM
The potential impact of the Cyp2a5 gene deletion on systemic clearance and OM toxicity of DCBN was studied by using an established single-dose nasal toxicity model. Following DCBN injection at 25 mg/kg (i.p.), plasma DCBN reached maximal concentration within 1 h, and then decreased quickly, in both WT B6 and Cyp2a5-null mice; by 24 h after the injection, plasma DCBN levels were undetectable in either group (data not shown). Plasma DCBN levels were not significantly different between the WT and the Cyp2a5-null mice, at any of the time points examined. There was also no significant difference between the WT and the Cyp2a5-null mice in any of the pharmacokinetic parameters calculated for plasma DCBN (Table 1). Thus, the loss of CYP2A5 expression does not alter systemic clearance of DCBN.
Table 1.
Pharmacokinetic parameters for DCBN clearance in WT and Cyp2a5-null mice
| Mouse strain | T1/2 (h) |
Tmax (h) |
Cmax (µg/ml) |
AUC0–24h (µg*h/ml) |
Clearance (ml/h) |
|---|---|---|---|---|---|
| WT | 6.6 ± 6.5 | 2.3 ± 1.6 | 0.6 ± 0.3 | 4.1 ± 0.9 | 170 ± 60 |
| Cyp2a5-null | 5.6 ± 3.7 | 2.2 ± 1.8 | 0.7 ± 0.3 | 4.8 ± 1.5 | 130 ± 30 |
Two- to three-month old, male, WT B6 and Cyp2a5-null mice were treated intraperitoneally with DCBN (at 25 mg/kg). Tail blood samples were collected from individual mice at various time points after dosing, for determination of plasma concentrations of DCBN, as described in Materials and Methods. Plasma levels of DCBN were used to calculate various pharmacokinetic parameters. Values represent means ± S.D. (n = 4–5 for each strain). There was no significant difference between the two mouse strains for any of the parameters (P>0.05, Student’s t-test)
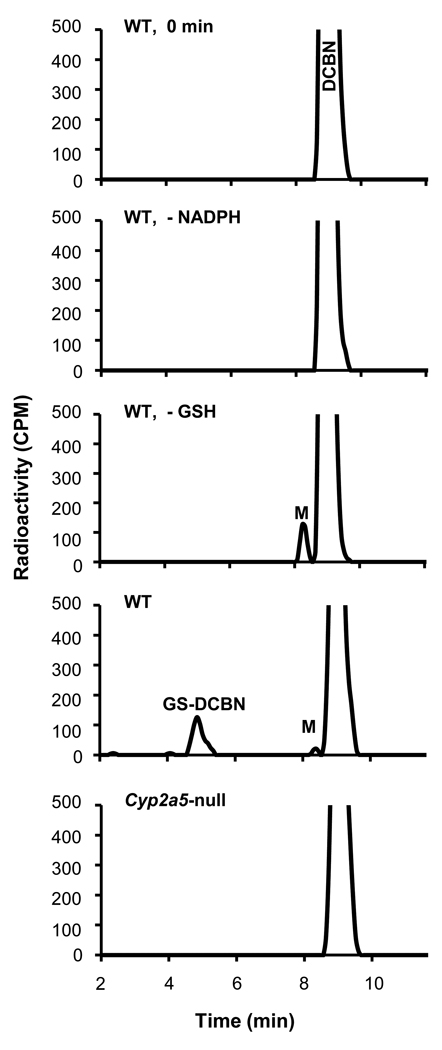
The ability of OM microsomes to convert DCBN to reactive intermediates was determined by measuring the rates of formation of DCBN-GSH conjugates (GS-DCBN). As shown in Figure 2, GS-DCBN was detected in reactions of DCBN (5 µM) with OM microsomes from WT mice, in an NADPH- and GSH-dependent manner, but not in reactions of DCBN with OM microsomes from Cyp2a5-null mice. A significant decrease (~60%; P<0.01) in the rates of OM microsomal GS-DCBN formation was also observed at a higher DCBN concentration (30 µM; with 0.5 mg/ml microsomal protein from 2-month-old male mice in the reactions mixtures), in the Cyp2a5-null mice (23.7 ± 9.7 pmol/min/mg protein, means ± S.D., n=3), compared to WT mice (56.5 ± 8.1 pmol/min/mg protein, n=3). These data indicate that the loss of CYP2A5 expression led to substantial decreases in rates of DCBN metabolic activation in OM microsomes.
Fig. 2. DCBN metabolic activation by OM microsomes from WT and Cyp2a5-null mice.
Complete reaction mixtures contained 50 mM phosphate buffer, pH 7.4, 5 µM 2,6-[ring-14C]DCBN, added in 1 µl methanol, 0.1 mg/ml OM microsomal protein from 2-month-old male mice, 3 mM GSH, and 1 mM NADPH, in a final volume of 0.1 ml. The reaction was carried out at 37°C for 10 min. In control incubations, either GSH or NADPH was omitted, or else the reaction was quenched prior to addition of NADPH (0 min). DCBN metabolite (M) and DCBN conjugate with GSH (GS-DCBN) were detected using radiometric HPLC, as descried in Materials and Methods. The rates of GS-DCBN formation by OM microsomes from WT mice were ~170 pmol/min/mg protein. Typical results are shown.
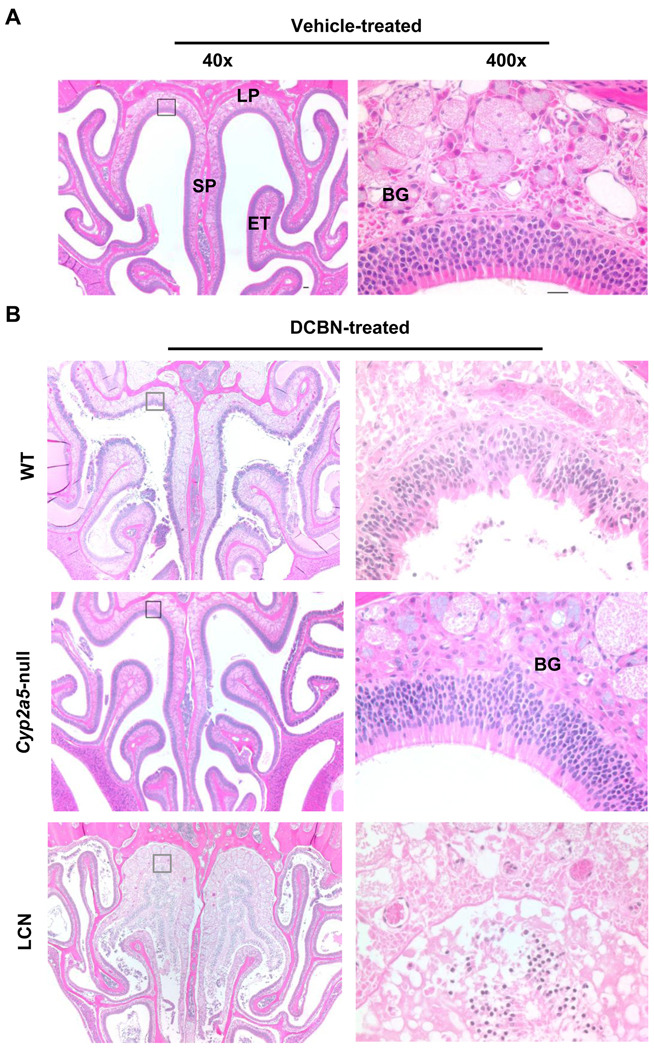
DCBN has been found to induce observable pathological changes in the mouse OM at doses ranging from 12 mg/kg to 50 mg/kg, with the manifestation of tissue damage clearly visible at 24 to 48 h following a single i.p. injection (Brandt et al., 1990). Thus, the extents of DCBN toxicity in the OM of WT B6 and Cyp2a5-null mice were examined at 24 h following a single intraperitoneal dose of DCBN at 25 mg/kg. As exemplified in Figure 3, while no signs of toxicity were observed in any vehicle-treated mice (panel A), obvious pathological changes were observed in both olfactory epithelium and submucosa, especially in the dorsal medial meatus, of DCBN-treated WT mice (panel B). Cells of the submucosal glands showed features of coagulative necrosis, and early stage disintegration of nuclei. The lesions in the epithelium were mainly deformation and partly detachment. Remarkably, no lesion was observed in any of the DCBN-treated Cyp2a5-null mice examined (n=5). This result strongly supports the hypothesis that CYP2A5 plays a critical role in mediating the cytotoxicity of DCBN in the OM.
Fig. 3. Histopathological analysis of nasal mucosal damages caused by DCBN treatment in WT, Cyp2a5-null, and LCN mice.
Two- to three-month old male mice were treated with a single i.p. injection of DCBN (at 25 mg/kg), or with the vehicle alone. Tissues were obtained for histopathological examination at 24 h after the treatment. Representative H&E-stained cross sections of the nasal cavity, obtained at the level 3 of Young et al. (1981), are shown. A, Vehicle-treated mice had normal ethmoidal turbinates (ET) and nasal septum (SP) lined by a thick, pseudostratified olfactory neuroepithelium in the dorsomedial part, and a clear nasal passage. B, DCBN-treated WT B6 mice showed extensive injury to the OM, evident as epithelial necrosis, detachment, sloughing, and ulceration; DCBN-treated Cyp2a5-null mice showed essentially normal olfactory epithelium and Bowman’s glands (BG); DCBN-treated LCN mice showed sever tissue damage in the nasal cavity, with the extent of damage even greater than that seen in DCBN-treated WT mice. LP, lamina propria. Boxed areas, at the dorsal medial meatus, in the left panel (40×) are shown at 400× on the right. Scale bar: 50 µm (left) and 20 µm (right).
Nasal toxicants often induce rapid depletion of NPSH in the OM, with maximal depletion seen usually within 2 to 4 h after an intraperitoneal injection (Brittebo et al., 1992; Bergstrom et al., 2003). Thus, we examined the potential impact of DCBN treatment on NPSH levels in the OM of the Cyp2a5-null and WT mice (male, 2–3 month old), at 2 h following DCBN treatment. A significant decrease in OM NPSH levels was detected in WT mice, treated with DCBN at 25 mg/kg, compared to vehicle-treated WT mice (~23%, n=6, p<0.05, Student’s t-test); however, at this dose, a DCBN-induced decrease in OM NPSH levels was not detected in Cyp2a5-null mice. When mice were treated with DCBN at 50 mg/kg, the extent of DCBN-induced decrease in OM NPSH levels was ~60% in WT mice (P<0.01, compared to vehicle-treated group), while the extent of decrease in Cyp2a5-null mice was significantly lower, at ~30% (P<0.05, compared to DCBN-treated WT group, Student’s t-test; n=3 for each group). These results further support the notion that CYP2A5 plays a critical role in mediating the cytotoxicity of DCBN in the OM.
Hepatic P450 enzymes are essential for DCBN clearance, but not for DCBN-induced OM toxicity
In contrast to the results seen in the Cyp2a5-null mice, the rates of systemic DCBN clearance were substantially reduced in the LCN mice, compared to WT littermates. As shown in Table 2, clearance of plasma DCBN was significantly lower (by 7–8 folds) in the LCN mice than in WT mice, when DCBN was given at either 25 mg/kg or 50 mg/kg. The decreases in elimination rates were accompanied by significant increases in t1/2 and AUC values, in the LCN mice, compared to WT mice. Thus, tissues of the LCN mice were exposed to much greater amounts of DCBN than did tissues of the WT mice. In other experiments, DCBN clearance was also found to be much slower in LCN mice than in WT mice, when DCBN was administered at 10 mg/kg (data not shown).
Table 2.
Pharmacokinetic parameters for DCBN clearance in LCN mice and WT littermates
| DCBN dose |
Mouse Strain |
T1/2 (h) |
Tmax (h) |
Cmax (µg/ml) |
AUC0–24h (µg*h/ml) |
Clearance (ml/h) |
|---|---|---|---|---|---|---|
| 25 mg/kg | WT | 1.8±0.4 | 0.5±0 | 1.2±0.2 | 3.4±0.2 | 200±20 |
| LCN | 21±30a | 3.9±3.4 | 1.7±1.0 | 29±22b | 28±20c | |
| 50 mg/kg | WT | 2.5±0.6 | 1.7±1.4 | 1.8±0.8 | 11±2.3 | 120 ± 20 |
| LCN | 45±49c | 5.6±2.2a | 1.9±0.3 | 36±7c | 15±12c |
Two- to three-month old, male, LCN mice and WT littermates were treated intraperitoneally with DCBN (at 25 mg/kg or 50 mg/kg). Tail blood samples were collected at various time points after dosing, for determination of plasma concentrations of DCBN. Plasma levels of DCBN were used to calculate various pharmacokinetic parameters. Values represent means ± S.D. (n = 4–5 for each strain).
aP<0.05; bP=0.05; cP<0.01; compared to WT in the same treatment group, Student’s t-test
On the contrary to the essential absence of any pathological changes in the OM of the Cyp2a5-null mice treated with DCBN at 25 mg/kg, the extent of OM toxicity in the LCN mice was actually greater, rather than lesser, than the extents seen in WT mice. As shown in Figure 3B, at 24 h after DCBN treatment, OM from the LCN mouse displayed severe pathological changes, including disappearance of Bowman’s glands in the lamina propria (the submucosa), accompanied by congestion and edema; presence of fragmented and hyperbasophilic (pyknotic) nuclei in the detached olfactory epithelium, an indication of degeneration and necrosis; and signs of early inflammation, as indicated by the accumulation of protein-rich fluid in the space between the submucosal connective tissues and the detached epithelium.
Consistent with the severe pathological changes observed at 24 h after dosing, OM NPSH levels were decreased by >40% in the LCN mice, at 2 h following DCBN treatment (at 25 mg/kg, i.p.; data not shown). In additional experiments not shown, a subtle increase in the extent of OM toxicity was also noted in LCN mice, compared to WT littermates, at 24 h after treatment with DCBN at 50 mg/kg (i.p.), a dose that caused much greater damage to the OM than the 25 mg/kg dose did, in both LCN and WT mice. Therefore, we conclude that hepatic P450-mediated DCBN metabolism does not play a noticeable role, if any, in mediating DCBN toxicity in the OM. The observation of the greater toxicity in the LCN OM can be explained by the much increased circulating levels of DCBN in the LCN mice than in the WT mice.
Discussion
Through the utility of two recently developed knockout mouse models, the Cyp2a5-null mouse (Zhou et al., 2010) and the LCN mouse (Gu et al., 2003), we have obtained definitive in vivo evidence that hepatic metabolic activation is not necessary for DCBN-induced tissue-specific toxicity in the OM, and that CYP2A5, one of the most predominant P450 enzymes expressed in the OM, is responsible for target-tissue metabolic activation and toxicity of this widely used herbicide. Our findings provide validation for an important part of the previously proposed mechanisms for the tissue-specific toxicity of DCBN in the OM, that OM P450s play an important role in the metabolic activation and the resultant toxicity of DCBN in the OM. Our data also show that, although multiple P450 enzymes are active toward DCBN metabolic activation in vitro, it is CYP2A5 that plays the critical role in vivo in mediating the remarkable tissue-specific toxicity of DCBN in the OM.
Several studies from this and other laboratories have been reported previously, which attempted to address the question of which P450(s) are responsible for DCBN toxicity in the OM. Among the major nasal P450 enzymes, CYP1A2 and CYP2E1 have been suggested to be nonessential in the metabolic activation and toxicity of DCBN (Eriksson and Brittebo, 1995; Gu et al., 1998). A potentially significant role by CYP2G1, which is uniquely expressed in the OM (Hua et al., 1997), and was ~30% less efficient than CYP2A5 was in converting DCBN to GS-DCBN in vitro (Gu et al. 1998), has also been ruled out by our finding that a Cyp2g1-null mouse (Zhou et al., unpublished) was not protected from DCBN-induced nasal toxicity (data not shown). Results from these previous studies are consistent with the present finding that CYP2A5 plays a significant role in DCBN metabolism and toxicity in the OM.
Notably, while the LCN mouse has been used in several studies for determination of the hepatic contributions to xenobiotic toxicity in extrahepatic tissues, the Cyp2a5-null mouse has not been used previously for studies on the role of CYP2A5 in chemical toxicity in vivo. Considering the large number of toxicants and chemical carcinogens already identified as CYP2A5 substrates (for reviews, see Su and Ding., 2004; Raunio et al., 2008), the expression of CYP2A5 in multiple tissues, and the known or potential overlap between CYP2A5 and other P450 enzymes in the Cyp2 gene family, we anticipate that the Cyp2a5-null mouse model will be valuable for numerous studies in mechanistic toxicology, as well as for risk assessment.
Given the potent nasal toxicity of DCBN found in a number of animal species, ranging from rodents to amphibians (Brandt et al., 1990; Genter et al., 1995; Delaleu and Sicard, 1995), it is important to assess the potential toxicity of DCBN in humans. As far as we know, the ability of DCBN to induce nasal mucosal toxicity in humans has not been examined directly, neither have the actual levels of DCBN exposure in humans been reported. As a widely applied herbicide, DCBN can be easily contacted by humans, in an occupational setting (workers who prepare, load, and apply the herbicide), through ingestion of drift-contaminated berries or vegetables, or through skin contact with treated vegetation or contaminated water (WSDOT Roadside Vegetation Management Herbicide Fact Sheet, 2006). Both CYP2A6 and CYP2A13, orthologs of mouse CYP2A5, are expressed in the human respiratory tract, including the OM, and both are active in the metabolic activation of DCBN in vitro (Su et al., 2000; Gu et al., 1998). Thus, it is conceivable that nasal toxicity also occurs in humans upon DCBN exposure.
In the present study, DCBN was administered by the intraperitoneal route, one most commonly used for studies of nasal toxicity of this compound. The intraperitoneal route also provides the greatest opportunity to reveal any hepatic contribution to OM toxicity. In human exposure scenarios, inhalation, dermal absorption, and oral intake are all likely exposure routes. Our finding, that hepatic P450 enzymes are essential for the systemic clearance of DCBN, suggests that DCBN nasal toxicity could be exacerbated in people with compromised hepatic P450 enzyme activity, particularly following oral exposure; the greater bioavailability of DCBN in these people would lead to the formation of greater amounts of reactive intermediates, and consequently greater extents of tissue damage, in the nasal passage. In addition, we expect that individuals would be more sensitive to DCBN toxicity following inhalation or dermal exposure, than they are following oral exposure, due to the avoidance of gastrointestinal/hepatic first-pass metabolism in the first two routes.
We believe that the conclusions from the present study, that, while hepatic P450-catalyzed metabolism is essential for the systemic clearance of DCBN, nasal CYP2A5-catalyzed metabolic activation is responsible for DCBN-induced OM toxicity in mice, form the basis for a mechanism-based approach to assessing the potential risks of DCBN nasal toxicity in humans. We envision that this approach would involve determination of the capabilities of human nasal mucosa to activate DCBN in vitro; development of relevant and practical biomarkers for monitoring not only human exposure to DCBN, but also in vivo DCBN metabolic activation in exposed individuals; and identification of potential relationships between genetic polymorphisms in human nasal CYPs (such as CYP2A13) that are capable of DCBN metabolic activation and incidences of nasal toxicity in exposed individuals. Further studies along these lines are underway.
Acknowledgments
We gratefully acknowledge the use of the services of the Pathology Core and the Advanced Light Microscopy and Image Analysis Core Facilities of the Wadsworth Center. We thank Ms. Weizhu Yang for assistance with mouse breeding. This study is supported in part by grant ES007462 from the National Institute of Environmental Health Sciences, NIH.
ABBREVIATIONS
- P450 or CYP
cytochrome P450
- CPR
NADPH-cytochrome P450 reductase
- GC
gas chromatography
- MS
mass spectrometry
- HPLC
high-performance liquid chromatography
- AUC
area under the concentration-time curve
- Cmax
maximum plasma concentration
- Tmax
time when Cmax is reached
- t1/2
elimination half-time
- OM
olfactory mucosa
- GSH
reduced glutathione
- DCBN
2,6-dichlorobenzonitrile (or dichlobenil)
- GS-DCBN
glutathione conjugate with DCBN
- LCN
liver-Cpr-null
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Bergman U, Ostergren A, Gustafson AL, Brittebo B. Differential effects of olfactory toxicants on olfactory regeneration. Arch.Toxicol. 2002;76:104–112. doi: 10.1007/s00204-002-0321-2. [DOI] [PubMed] [Google Scholar]
- Bergström U, Giovanetti A, Piras E, Brittebo EB. Methimazole-induced damage in the olfactory mucosa: effects on ultrastructure and glutathione levels. Toxicol Pathol. 2003;31:379–387. doi: 10.1080/01926230390201101. [DOI] [PubMed] [Google Scholar]
- Brandt I, Brittebo EB, Feil VJ, Bakke JE. Irreversible binding and toxicity of the herbicide dichlobenil (2,6-dichlorobenzonitrile) in the olfactory mucosa of mice. Toxicol.Appl.Pharmacol. 1990;103:491–501. doi: 10.1016/0041-008x(90)90322-l. [DOI] [PubMed] [Google Scholar]
- Brittebo EB, Eriksson C, Brandt I. Effects of glutathione-modulating agents on the covalent binding and toxicity of dichlobenil in the mouse olfactory mucosa. Toxicol.Appl.Pharmacol. 1992;114:31–40. doi: 10.1016/0041-008x(92)90093-8. [DOI] [PubMed] [Google Scholar]
- Brittebo EB. Metabolism-dependent toxicity of methimazole in the olfactory nasal mucosa. Pharmacol.Toxicol. 1995;76:76–79. doi: 10.1111/j.1600-0773.1995.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Cox C. Dichlobenil. Journal of Pesticide Reform. 1997;17:14–20. [Google Scholar]
- Delaleu JC, Sicard G. Physiological and histological recovery of the olfactory mucosa of the frog after a dichlobenil injection. Chem.Senses. 1995;20:433–440. doi: 10.1093/chemse/20.4.433. [DOI] [PubMed] [Google Scholar]
- Ding X, Spink DC, Bhama JK, Sheng JJ, Vaz AD, Coon MJ. Metabolic activation of 2,6-dichlorobenzonitrile, an olfactory-specific toxicant, by rat, rabbit, and human cytochromes P450. Mol.Pharmacol. 1996;49:1113–1121. [PubMed] [Google Scholar]
- Eriksson C, Brittebo EB. Metabolic activation of the olfactory toxicant, dichlobenil, in rat olfactory microsomes: comparative studies with p-nitrophenol. Chem.Biol.Interact. 1995;94:183–196. doi: 10.1016/0009-2797(94)03333-4. [DOI] [PubMed] [Google Scholar]
- Genter MB, Owens DM, Deamer NJ. Distribution of microsomal epoxide hydrolase and glutathione S-transferase in the rat olfactory mucosa: relevance to distribution of lesions caused by systemically-administered olfactory toxicants. Chem.Senses. 1995;20:385–392. doi: 10.1093/chemse/20.4.385. [DOI] [PubMed] [Google Scholar]
- Gu J, Walker VE, Lipinskas TW, Walker DM, Ding X. Intraperitoneal administration of coumarin causes tissue-selective depletion of cytochromes P450 and cytotoxicity in the olfactory mucosa. Toxicol.Appl.Pharmacol. 1997;146:134–143. doi: 10.1006/taap.1997.8238. [DOI] [PubMed] [Google Scholar]
- Gu J, Zhang QY, Genter MB, Lipinskas TW, Negishi M, Nebert DW, Ding X. Purification and characterization of heterologously expressed mouse CYP2A5 and CYP2G1: role in metabolic activation of acetaminophen and 2,6-dichlorobenzonitrile in mouse olfactory mucosal microsomes. J.Pharmacol.Exp.Ther. 1998;285:1287–1295. [PubMed] [Google Scholar]
- Gu J, Weng Y, Zhang QY, Cui H, Behr M, Wu L, Yang W, Zhang L, Ding X. Liver-specific deletion of the NADPH-cytochrome P450 reductase gene: impact on plasma cholesterol homeostasis and the function and regulation of microsomal cytochrome P450 and heme oxygenase. J.Biol.Chem. 2003;278:25895–25901. doi: 10.1074/jbc.M303125200. [DOI] [PubMed] [Google Scholar]
- Gu J, Cui H, Behr M, Zhang L, Zhang QY, Yang W, Hinson JA, Ding X. In vivo mechanisms of tissue-selective drug toxicity: effects of liver-specific knockout of the NADPH-cytochrome P450 reductase gene on acetaminophen toxicity in kidney, lung, and nasal mucosa. Mol.Pharmacol. 2005;67:623–630. doi: 10.1124/mol.104.007898. [DOI] [PubMed] [Google Scholar]
- Gu J, Chen CS, Wei Y, Fang C, Xie F, Kannan K, Yang W, Waxman DJ, Ding X. A mouse model with liver-specific deletion and global suppression of the NADPH-cytochrome P450 reductase gene: characterization and utility for in vivo studies of cyclophosphamide disposition. J.Pharmacol.Exp.Ther. 2007;321:9–17. doi: 10.1124/jpet.106.118240. [DOI] [PubMed] [Google Scholar]
- Hua Z, Zhang QY, Su T, Lipinskas TW, Ding X. cDNA cloning, heterologous expression, and characterization of mouse CYP2G1, an olfactory-specific steroid hydroxylase. Arch.Biochem.Biophys. 1997;340:208–214. doi: 10.1006/abbi.1997.9899. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhuo X, Gonzalez FJ, Ding X. Baculovirus-mediated expression and characterization of rat CYP2A3 and human CYP2a6: role in metabolic activation of nasal toxicants. Mol.Pharmacol. 1996;50:781–788. [PubMed] [Google Scholar]
- Mercer GE. Determination of 112 halogenated pesticides using gas chromatography/mass spectrometry with selected ion monitoring. Journal of Aoac International. 2005;88:1452–1462. [PubMed] [Google Scholar]
- Raunio H, Hakkola J, Pelkonen O. The CYP2A Subfamily. In: Ioannides C, editor. Cytochrome P450: Role in the Metabolism and Toxicity of Drugs and Other Xenobiotics. Cambridge, UK: RSC Publishing; 2008. pp. 150–178. [Google Scholar]
- Sheng J, Guo J, Hua Z, Caggana M, Ding X. Characterization of human CYP2G genes: widespread loss-of-function mutations and genetic polymorphism. Pharmacogenetics. 2000;10:667–678. doi: 10.1097/00008571-200011000-00001. [DOI] [PubMed] [Google Scholar]
- Su T, Bao Z, Zhang QY, Smith TJ, Hong JY, Ding X. Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 2000;60:5074–5079. [PubMed] [Google Scholar]
- Su T, Ding X. Regulation of the Cytochrome P450 2A Genes. Toxicol. Appl. Pharmacol. 2004;199:285–294. doi: 10.1016/j.taap.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Tonge RP, Kelly EJ, Bruschi SA, Kalhorn T, Eaton DL, Nebert DW, Nelson SD. Role of CYP1A2 in the hepatotoxicity of acetaminophen: investigations using Cyp1a2 null mice. Toxicol.Appl.Pharmacol. 1998;153:102–108. doi: 10.1006/taap.1998.8543. [DOI] [PubMed] [Google Scholar]
- Weng Y, Fang C, Turesky RJ, Behr M, Kaminsky LS, Ding X. Determination of the role of target tissue metabolism in lung carcinogenesis using conditional cytochrome P450 reductase-null mice. Cancer Res. 2007;67:7825–7832. doi: 10.1158/0008-5472.CAN-07-1006. [DOI] [PubMed] [Google Scholar]
- Young JT. Histopathologic examination of the rat nasal cavity. Fundam.Appl.Toxicol. 1981;1:309–312. doi: 10.1016/s0272-0590(81)80037-1. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Kaminsky LS, Dunbar D, Zhang J, Ding X. Role of small intestinal cytochromes p450 in the bioavailability of oral nifedipine. Drug Metab Dispos. 2007;35:1617–1623. doi: 10.1124/dmd.107.016543. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhuo X, Xie F, Kluetzman K, Shu YZ, Humphreys WG, Ding X. Role of CYP2A5 in the clearance of nicotine and cotinine: insights from studies on a Cyp2a5-null mouse model. J.Pharmacol.Exp.Ther. 2010;332:578–587. doi: 10.1124/jpet.109.162610. [DOI] [PMC free article] [PubMed] [Google Scholar]