Abstract
Previous studies from our laboratory demonstrated that mice treated with morphine pellets are sensitized to Salmonella enterica, serovar Typhimurium infection. However, the opioid receptor antagonist, naltrexone, only partially blocked the effect of morphine, raising the possibility that the opioid might have some of its effects through a nonopioid receptor. To further clarify whether sensitization to infection is an opioid receptor-dependent phenomenon, μ-opioid receptor knock-out (MORKO) mice were used in the present study. Wild type (WT) and MORKO mice were treated with morphine and their sensitivity to oral Salmonella infection assessed by mortality, bacterial burdens in gut associated lymphoid tissue and in blood and peritoneal fluid, and by levels of pro-inflammatory cytokines in plasma. MORKO animals treated with morphine were refractory to a sublethal dose of Salmonella, while similar treatment of WT animals resulted in 100% mortality. WT animals treated with morphine had high bacterial loads in all organs tested, while morphine-treated MORKO animals had no culturable Salmonella in any organs. Pro-inflammatory cytokine levels were elevated in morphine treated WT but not MORKO mice infected with Salmonella. These results provide definitive evidence that the morphine-mediated enhancement of oral Salmonella infection is dependent on the μ-opioid receptor.
Keywords: Opioids, Salmonella, μ-opioid receptor
1. Introduction
Morphine has been used as an analgesic for hundreds of years, and continues to be commonly prescribed for pain management. There is a substantial and compelling literature demonstrating that alkaloid opioids, such as morphine, and congeners such as heroin, have a wide of variety of immunosuppressive effects on the immune system [1, 2]. Cells of the immune system respond functionally to the drugs, as first demonstrated by Wybran in 1979 [3], and have been shown to express message for opioid receptors [4]. It is well documented that morphine depresses Natural Killer (NK) cell activity [5, 6], phagocytic cell function [7, 8], antibody formation [9, 10], responses to mitogens [11, 12], and delayed-type hypersensitivity [13]. In addition opioids depress production of reactive oxygen and nitrogen intermediates by phagocytes [8, 14]. The fact that opioids alter leukocyte function suggests a potential immunosuppressive role for this class of compounds in response to infecting microbes or microbial products. Opioid abusers have long been noted to have increased rates of infection [15–18]. Morphine has been found to potentiate a variety of experimental infections by pathogens including Streptococcus pneumoniae [19], Toxoplasma gondii [20], Klebsiella pneumoniae [21], Candida albicans [21], Plasmodium berghei [22], Listeria monocytogenes [23], and Leishmania donovani [24]. Our laboratory has previously demonstrated that morphine markedly sensitizes C3HeB/FeJ mice to oral infection with Salmonella enterica, serovar Typhimurium [25, 26]. However, the opioid antagonist, naltrexone, only partially blocked the effect of the drug in this infection model by prolonging mean time to death, without resulting in increased survivors compared to animals receiving morphine alone [25]. The current studies were undertaken to clarify the role of the μ-opioid receptor in mediating the effect of morphine on sensitization to oral Salmonella infection. C57BL/6 mice with a genetic lesion in the μ-opioid receptor, and wild-type (WT) animals of the same strain were used. It was found that in the absence of the μ-opioid receptor, morphine did not sensitize to oral Salmonella infection.
2. Results
2.1 Salmonella infection in MORKO mice given morphine
In order to assess the role of the MOR in oral Salmonella infection, groups of WT and MORKO mice were implanted with either a 25 mg slow-release morphine pellet or a placebo pellet and then orally challenged with a sublethal dose of Salmonella. This experiment was repeated twice and results were pooled. As shown in Figure 1, WT animals treated with a placebo pellet and challenged with a sublethal dose of Salmonella, showed no mortality. In accord with previous results [25], all of the wild-type animals receiving morphine, and challenged with the same dose of Salmonella as the placebo group, succumbed to infection, with 80% mortality in the first 3 days. In contrast, 100% of the MORKO animals receiving morphine survived Salmonella infection.
Figure 1.
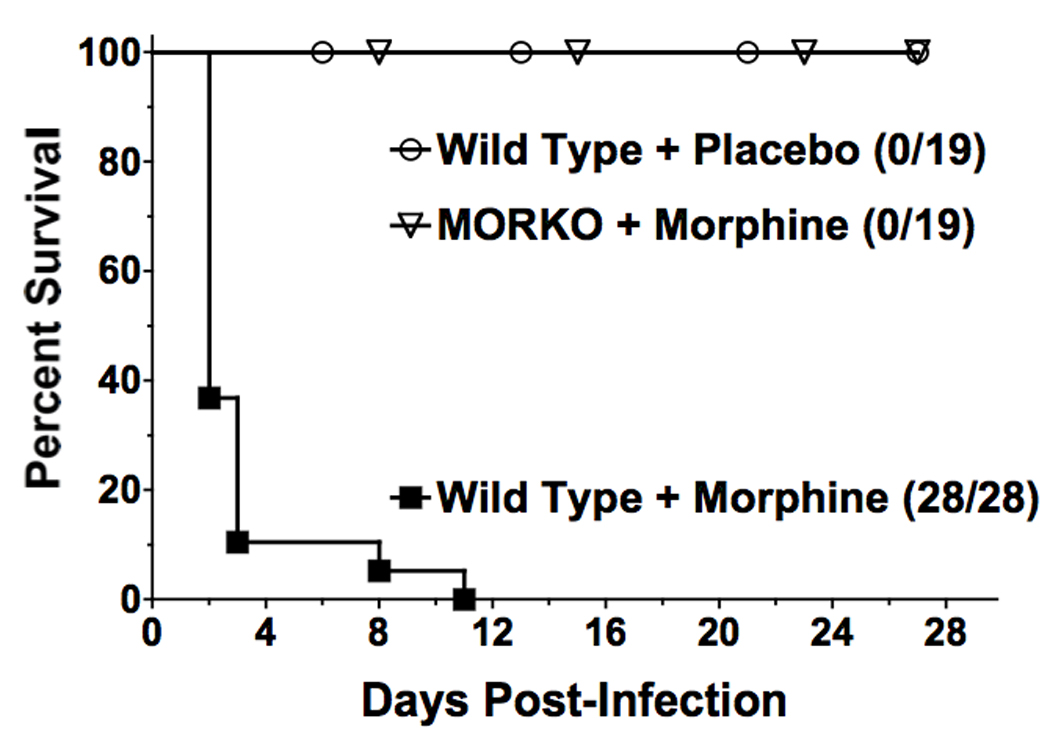
Absence of the μ opioid receptor blocks morphine induced sensitization to oral Salmonella infection as assessed by survival. Wild-type or MORKO C57BL/6J female mice implanted with a 25 mg morphine pellet or a placebo pellet were simultaneously orally challenged with either 3.8 × 103 or 3.5 × 103 Salmonella enterica serovar Typhimurium strain W118-2. Data are pooled results of two individual experiments. All groups versus WT plus morphine, p<0.0001.
These studies were extended to examine bacterial burdens in the organs of mice. WT and MORKO mice were implanted with morphine or placebo pellets and orally challenged with Salmonella. Figure 2 shows the bacterial numbers in Peyers patches (PP), mesenteric lymph nodes (MLN), blood, and peritoneal exudates of individual WT or MORKO mice treated with morphine or placebo, assayed 24 hrs after Salmonella challenge. Robust differences in Salmonella burden were observed in PP, blood, and MLN between the MORKO and WT mice, and a statistically significant difference, although less robust, was observed between these groups in the peritoneal exudate. Whereas most WT animals receiving morphine had measurable levels of Salmonella in all organs tested, none of the MORKO mice receiving the same treatment had any detectable colonies. Bacterial burdens in MORKO mice receiving morphine were not statistically different from those of wild-type animals receiving placebo. There are a few MORKO animals receiving placebo that had elevated counts in their mesenteric lymph nodes. The reason for these seemingly anomalous results in these animals is not known.
Figure 2.
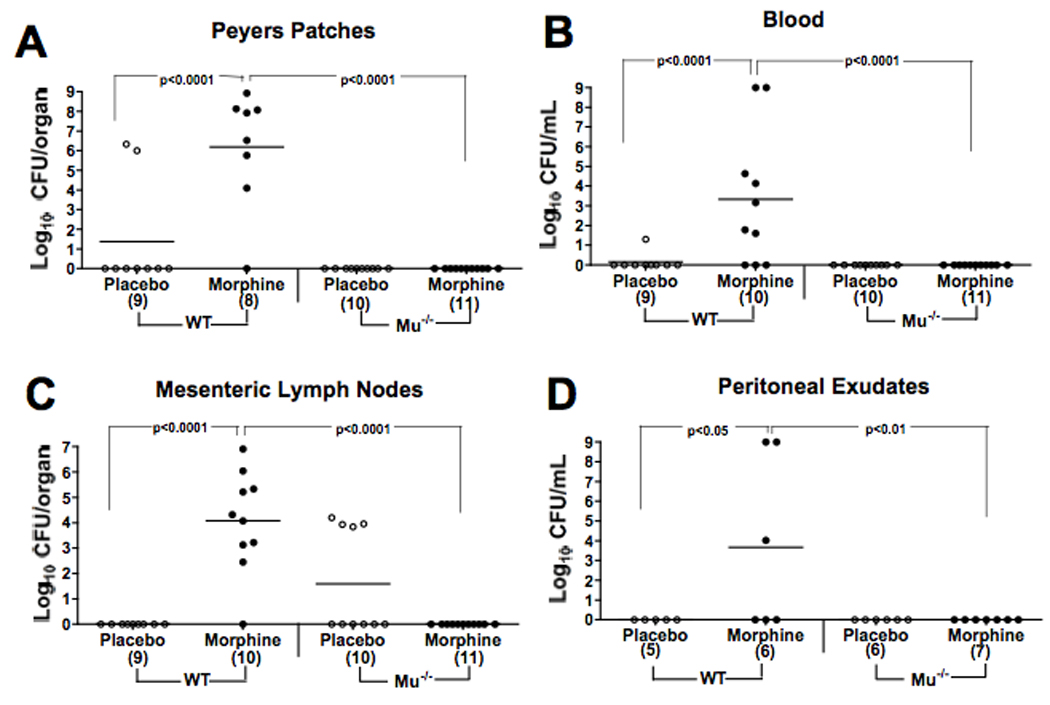
Absence of the μ-opioid receptor blocks morphine induced sensitization to oral Salmonella infection as assessed by bacterial burden. In two experiments, mice implanted with a 25 mg morphine or a placebo pellet were orally challenged at the same time as pellet implantation with either a 1.0 × 104 or 3.0 × 104 dose of W118-2. At 24 h post-infection, bacterial burdens in organs and fluids were determined by plate counts. Points represent bacterial counts of individual animals and are data from both experiments. Lines indicate the mean number of CFU/group. Numbers in parentheses on the X-axis represent the number of animals sampled in each group.
2.2 Cytokine levels in Salmonella infected WT and MORKO mice
The levels of pro-inflammatory cytokines and chemokines were assessed in plasma of individual animals sacrificed at 36 hr after infection using a multiplex CBA assay. As shown in Fig. 3, WT infected mice given morphine had elevated levels of TNF-α, IL-6, IFN-γ, and IL-10, whereas infected MORKO animals did not.
Figure 3.
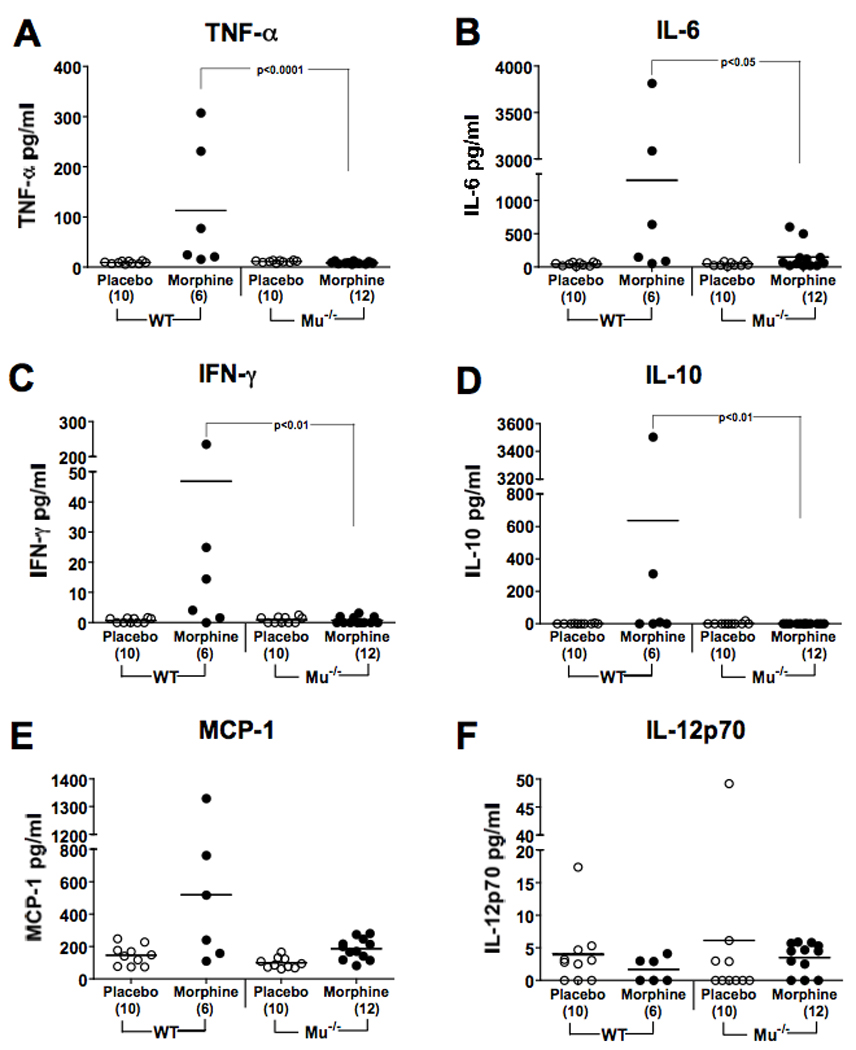
MORKO mice given morphine and orally infected with Salmonella do not respond with pro-inflammatory cytokines. WT or MORKO mice were implanted with 25 mg morphine or placebo pellets and simultaneously orally infected with 2.0 × 103 Salmonella. Thirty-six hr later, plasma was collected by cardiac puncture. Levels of cytokines and chemokines were determined by the multiplex CBA assay. Lines represent the mean cytokine concentration per group. Numbers in parentheses on the X-axis represent the number of animals sampled in each group. Levels of statistical significance between groups are indicated on the figure.
3. Discussion
These studies conclusively demonstrate that the increased sensitivity of mice to oral Salmonella infection induced by morphine is dependent on the μ-opioid receptor. Animals genetically deficient in the receptor were not sensitized to this infection and behaved like the placebo treated group with respect to survival, bacterial burdens, and levels of pro-inflammatory cytokines.
The degree to which morphine sensitizes to oral Salmonella is indeed impressive, as the median bacterial burdens in WT animals given the drug is a million-fold higher than that observed in MORKO or WT-placebo treated animals. In our previous work using C3HeB/FeJ mice, the mechanism of the sensitization by morphine was partially explored. We demonstrated, using a strain of W118-2 carrying a temperature sensitive plasmid, that the increase in Salmonella burdens in the WT mice given morphine was due to multiplication of the challenge organisms in the intestinal tract [25]. The current studies also measured cytokines in the plasma of WT or MORKO mice given morphine and infected with Salmonella, with the result that TNF-α, IFN-γ, and IL-6 were shown to be elevated in WT, but not MORKO animals. This result is consonant with our previous finding that WT animals had an elevation of pro-inflammatory cytokines in the Peyer’s patches [25]. It is our interpretation that the higher levels of pro-inflammatory cytokines in the WT mice compared to the MORKO animals is a consequence of the higher bacterial burdens in the former.
It was established as long ago as 1917 that morphine applied to isolated guinea pig small intestine inhibits peristalsis [27]. It has subsequently been shown that morphine delays gastrointestinal (GI) transit in the mouse and the rat [28], as well as in humans [29,30]. We have shown that C3HeB/FeJ mice implanted with slow-release morphine pellets, or with osmotic mini-pumps dispensing morphine, decrease intestinal transit [26]. They also have an increased incidence of spontaneous sepsis [31]. Opioid receptors have been identified in the small intestine of animals and humans, which when activated lead to decreased peristalsis and increased muscle tone and spasms, resulting in consipation [32, 33]. Both μ and δ opioid receptors have been shown to mediate the delay in GI transit [28]. While morphine has higher affinity for the μ-opioid receptor, it can act on the κ and δ receptors. Naltrexone has activity at all three receptors. Therefore, our previous experiments, while demonstrating that morphine exacerbated Salmonella infection, did not definitively prove that the effect of the drug was through the μ-opioid receptor [25]. Further, the weak activity of naltrexone, previously demonstrated as only a prolongation of mean time to death, brought into question whether the effect of morphine was even through a classical opioid receptor [25]. The current experiments verify that the mu receptor is essential for the activity of morphine in this regard. This conclusion is supported by the prior work of Roy et al who reported that a different line of MORKO mice [34] failed to show inhibition of gastrointestinal transit in response to morphine. These experiments also solidify older reports in the literature by Takeuchi and Kent in Formal’s laboratory, in which opium was used in guinea pigs and shown to increase their sensitivity to Salmonella infection [35, 36].
Future studies will be needed to determine whether the mechanism by which morphine potentiates Salmonella infection is via intestinal stasis or via immunosuppression. Stasis might allow growth of Salmonella to higher numbers because of failure to flush them out of the intestinal lumen [37], or by allowing greater numbers of organisms to attach to and invade epithelial or M cells in the intestinal tract. In our previous work, we demonstrated that the increase in Salmonella burdens in the WT mice given morphine was due to multiplication of the challenge organisms in the intestinal tract [25]. However, greater growth of Salmonella could also be the result of immunosuppressive effects of the drug. Given the rapidity of mortality in morphine-treated, Salmonella infected animals, it is likely that the absence of the μ-opioid receptor would abrogate effects of morphine on the innate immune system. Morphine has been shown to inhibit phagocytic and microbicidal activity of polymorphonuclear leukocytes and macrophages [8, 38, 39]. Mu-opioid-deficient mice used by other investigators [40], containing different genetic lesions to those used in our studies [41], failed to exhibit morphine-mediated effects on macrophage phagocytosis and TNF-α secretion [42]. Thus, the hypothesis that, in addition to gastrointestinal stasis, morphine might sensitize to Salmonella infection by suppressing immune responses is tenable. These studies have potential implications for management of human infectious gastroenteritis or bacillary dysentery. The constipating effects of opioids in humans have been well documented [32], and are a major unwanted side effect of their use to control pain. Although their effect on the gastrointestinal tract is generally detrimental, leading to ileus, they can be used therapeutically to treat diarrhea. The experiments reported here on the sensitizing effect of morphine in mice to an oral Salmonella infection, raise the question as to the safety of using opioids to control diarrhea during bacterial infections. The literature on this question is extremely scant. Medical practice is largely based on a 1973 paper by DuPont and Hornick [43] which showed that in human volunteers, Lomotil®, which contains the opioid, diphenoxylate hydrochloride, prolonged excretion of experimentally administered Shigella flexneri. As studies in animals showed similar effects of opioids on Shigella and Salmonella oral infections, the package insert for Lomotil® contraindicates usage during Salmonella infection . The present experiments support a sensitizing effect of morphine acting via the μ-opioid receptor.
4. Materials and Methods
4.1 Animals
Pathogen-free, female, 6–8-week-old C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). The μ-opioid receptor knockout (MORKO) mice were developed by disruption of exon-1 of the MOR-1 gene through homologous recombination as described previously [41, 44]. The MOR-1 mutant (MORKO) C57BL/6J mice were derived following at least 15 generations of successive backcrossing to C57BL/6J mice. At Temple University, MORKO and WT mice were obtained from het:het matings. All MORKO and age-matched wild type (WT) littermates were bred and maintained in the Central Animal Facility of Temple University. All mice were allowed to acclimate for at least 1 week before use. Rodent chow (Purina, St. Louis) and fresh water were available ad libitum until just prior to the experiment when food and water were withheld 4 h prior to oral infection with Salmonella. All experiments were carried out with the approval of the Institutional Animal Care and Use Committee at Temple University and were housed in a barrier facility of the Central Animal Facility.
4.2 Bacterial strain
Salmonella enterica, serovar Typhimurium, strain W118-2, was used for experimental infection in this study. This strain has been used extensively by our laboratory [26, 45, 46]. For culture growth, a lyophil was rehydrated with 10 ml of brain heart infusion (BHI) broth, incubated overnight, and then streaked onto a tryptic soy agar (TSA) plate and grown overnight at 37°C. Typical colonies were picked and inoculated into 10 ml of Brain Heart Infusion (BHI) broth. The culture was incubated for 3.5 h at 37°C, then the top 5 ml of medium was transferred into 50 ml of BHI broth and incubated at 37°C for 1.5 h on an orbital shaker to produce late log-phase organisms. Bacteria were counted in a Petroff-Hauser counter and diluted to the desired concentration in sterile, pyrogen-free 0.9% saline. The actual number of organisms inoculated was verified later from duplicate spread plates on TSA.
4.3 Morphine and placebo treatment, and oral Salmonella infection
Mice were anesthetized by isoflurane inhalation, and an area of the back was shaved. A 1-cm incision was made in the skin of the back, and mice were implanted subcutaneously with a 25 mg slow-release morphine pellet or a placebo pellet (National Institute on Drug Abuse, Rockville, MD). Slow-release pellets are a preferred method of opioid delivery in studying chronic administration [47, 48], as the continuous presence of drug prevents opioid withdrawal [49]. Using this method, plasma levels of morphine are in the 0.6- to 2.0-µg/ml range [50]. Heroin, the prototypical opioid drug of abuse, is rapidly metabolized to morphine in the body. Immediately following pellet implantation, mice were orally inoculated with Salmonella in 0.2 ml saline, using a 21-gauge needle with a fine polished blunt end, as in previous work [25].
4.4 Morphine induced sensitivity to infection in WT and MORKO mice
Groups of MORKO or wild-type mice were implanted with either morphine or placebo pellets. All mice were orally inoculated with Salmonella. Sensitivity to infection was assessed by mortality, which was scored for 30 days, and by microbial burdens in various tissues and body fluids. At 48 h post morphine pellet-implantation and infection, bacterial burden in various organs was assessed. Mice were anesthetized with pentobarbital sodium injection intramuscularly (i.m.) and blood was collected via cardiac puncture into heparinized 3 ml syringes. Mice were euthanized via cervical dislocation and peritoneal exudates were collected by lavage. In addition, mesenteric lymph nodes (MLN), spleen, and Peyer’s patches (PP) were aseptically removed from individual animals. Tissues of individual animals were homogenized in 3 ml of ice-cold phosphate-buffered saline (PBS), using a Tekmar Tissuemizer® (Tekmar, Cincinnati, Ohio). Homogenates were spread onto Levine Eosin Methylene Blue (EMB) agar plates and incubated at 37°C overnight. Numbers of colony forming units (CFU) were counted and recorded. Typical individual bacterial colonies were identified as Salmonella enterica serovar Typhimurium by slide agglutination, using typing antiserum for O antigens 1, 4, 12 and 27 (Becton, Dickinson, and Company, Sparks, MD)
4.5 Cytokine levels in Salmonella infected WT and MORKO mice
Wild-type and MORKO mice were implanted with 25 mg morphine pellets or placebo pellets and infected with Salmonella. Thirty-six hrs later, mice were anesthetized using pentobarbital sodium injection intramuscularly (i.m.) and blood was collected via cardiac puncture into heparinized 3 ml syringes. Blood was spun in a microcentrifuge at 12,000 rpm at 4°C for 5 minutes, and plasma collected. Levels of pro-inflammatory cytokines and chemokines were determined using a multiplex Cytokine Bead Array assay (BD Biosciences, San Diego, CA). The assay was carried out according to the manufacturer’s instructions. Briefly, capture beads specific for the various cytokines were mixed together to form a bead suspension. Fifty µl aliquots of the bead suspension were then incubated with a 50 µl plasma sample collected from an individual mouse and 50 µl of a phycoerythrin-conjugated anti-murine detection reagent for 2 h at room temperature in the dark. One ml of wash buffer was then added to each assay tube and centrifuged at 200 × g for 6 min. Supernatants were discarded, and 200 µl of wash buffer was added to resuspend the bead pellet. The prepared tubes were analyzed on a BD FACS Calibur flow cytometer (BD PharMingen, San Diego, CA) calibrated with cytometer setup beads provided by the manufacturer. The phycoerythrin intensities of each sample were converted to concentration values of cytokines using computer software.
4.6 Statistical analysis
Survival data were analyzed using the Kaplan-Meier Product Limit Estimator followed by group comparisons using the log rank test. Numbers of bacteria in tissues and levels of cytokines were analyzed using analysis of variance for fixed effects (between groups and treatments). Data from multiple experiments (replications) were pooled after testing for differences. Data were transformed to normalized ranks prior to analysis due to failing the Wilk-Shapiro test of normality. Because of the exploratory nature of the experiments, no adjustments were made for multiple comparisons. In all cases statistical significance was based on p≤0.05. Data were analyzed using SAS V9.1 (Cary, NC).
Acknowledgements
This work was supported by National Institute on Drug Abuse grants DA13429, DA11134, and T32-DA07237
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Eisenstein TK, Rahim RT, Feng P, Thingalaya NK, Meissler JJ. Effects of opioid tolerance and withdrawal on the immune system. J.Neuroimm.Pharmacol. 2006;1:237–249. doi: 10.1007/s11481-006-9019-1. [DOI] [PubMed] [Google Scholar]
- 2.Roy S, Wang J, Kelschenbach J, Koodie L, Martin J. Modulation of immune function by morphine: implications for susceptibility to infection. J.Neuroimm.Pharmacol. 2006;1:77–89. doi: 10.1007/s11481-005-9009-8. [DOI] [PubMed] [Google Scholar]
- 3.Wybran J, Appelboom T, Famaey J-P, Govaerts A. Suggestive evidence for receptors for morphine and methionine-enkephalin on normal human blood T lympohcytes. J.Immunol. 1979;123:1068–1070. [PubMed] [Google Scholar]
- 4.McCarthy L, Wetzel M, Sliker J, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 2001;62:111–123. doi: 10.1016/s0376-8716(00)00181-2. [DOI] [PubMed] [Google Scholar]
- 5.Weber RJ, Pert A. The periaqueductal gray matter mediates opiate-induced immunosuppression. Science. 1989;245:188–190. doi: 10.1126/science.2749256. [DOI] [PubMed] [Google Scholar]
- 6.Yeager MP, Colacchio TA, Yu CT, Hildebrandt L, Howell AL, Weiss J, Guyre PM. Morphine inhibits spontaneous and cytokine-enhanced natural killer cell cytotoxicity in volunteers. Anesthesiology. 1995;83:500–508. doi: 10.1097/00000542-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Szabo I, Rojavin M, Bussiere JL, Eisenstein TK, Adler MW, Rogers TJ. Suppression of peritoneal macrophage phagocytosis of Candida albicans by opioids. J.Pharmacol.Exp.Ther. 1993;267:703–706. [PubMed] [Google Scholar]
- 8.Peterson PK, Sharp B, Gekker G, Brummitt C, Keane WF. Opioid-mediated suppression of cultured peripheral blood mononuclear cell respiratory burst activity. J.Immunol. 1987;138:3907–3912. [PubMed] [Google Scholar]
- 9.Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK. Differential effects of morphine and naltrexone on the antibody response in various mouse strains. Immunopharmacol.Immunotoxicol. 1992;14:657–673. doi: 10.3109/08923979209005416. [DOI] [PubMed] [Google Scholar]
- 10.Eisenstein TK, Meissler JJ, Jr, Rogers TJ, Geller EB, Adler MW. Mouse strain differences in immunosuppression by opioids in vitro. J.Pharmacol.Exp.Ther. 1995;275:1484–1489. [PubMed] [Google Scholar]
- 11.Bayer BM, Daussin S, Hernandez M, Irvin L. Morphine inhibition of lymphocyte activity is mediated by an opioid dependent mechanism. Neuropharmacology. 1990;29:369–374. doi: 10.1016/0028-3908(90)90096-a. [DOI] [PubMed] [Google Scholar]
- 12.Bryant HU, Bernton EW, Holaday JW. Immunosuppressive effects of chronic morphine treatment in mice. Life Sci. 1987;41:1731–1738. doi: 10.1016/0024-3205(87)90601-1. [DOI] [PubMed] [Google Scholar]
- 13.Molitor TW, Morilla A, Risdahl JM, Murtaugh MP, Chao CC, Peterson PK. Chronic morphine administration impairs cell-mediated immune responses in swine. J.Pharmacol.Exp.Ther. 1992;260:581–586. [PubMed] [Google Scholar]
- 14.Lysle DT, How T. Heroin modulates the expression of inducible nitric oxide synthase. Immunopharm. 2000;46:181–192. doi: 10.1016/s0162-3109(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 15.Ebright JR, Pieper B. Skin and soft tissue infections in injection drug users. Infect.Dis.Clinic.N.Amer. 2002;16:697–712. doi: 10.1016/s0891-5520(02)00017-x. [DOI] [PubMed] [Google Scholar]
- 16.Binswanger IA, Kral AH, Blumenthal RN, Rybold DJ, Edlin BR. High prevalence of abscesses and cellulitis among community-recruited injection drug users in San Francisco. Clin.Infect.Dis. 2000;30:579–581. doi: 10.1086/313703. [DOI] [PubMed] [Google Scholar]
- 17.Haverkos HW, Lange RW. Serious infections other than human immunodeficiency virus among intravenous drug users. J.Infect.Dis. 1990;161:894–902. doi: 10.1093/infdis/161.5.894. [DOI] [PubMed] [Google Scholar]
- 18.Risdahl JM, Peterson PK, Molitor TW. Opiates, infection and immunity. In: Friedman H, Klein TW, Specter S, editors. Drugs of Abuse, Immunity, and Infections. Boca Raton: CRC Press Inc.; 1996. pp. 1–42. [Google Scholar]
- 19.Wang J, Barke RA, Charboneau R, Roy S. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J.Immunol. 2005;174:426–434. doi: 10.4049/jimmunol.174.1.426. [DOI] [PubMed] [Google Scholar]
- 20.Chao CC, Sharp BM, Pomeroy C, Filice GA, Peterson PK. Lethality of morphine in mice infected with Toxoplasma gondii. J.Pharmacol.Exp.Ther. 1990;252:605–609. [PubMed] [Google Scholar]
- 21.Tubaro E, Borelli G, Croce C, Cavallo G, Santiangeli C. Effect of morphine on resistance to infection. J.Infec.Dis. 1983;148:656–666. doi: 10.1093/infdis/148.4.656. [DOI] [PubMed] [Google Scholar]
- 22.Singh PP, Singh S, Dutta GP, Srimal RC. Immunomodulation by morphine in Plasmodium berghei-infected mice. Life Sci. 1993;54:331–339. doi: 10.1016/0024-3205(94)00789-6. [DOI] [PubMed] [Google Scholar]
- 23.Asakura H, Kawamoto K, Igimi S, Yamamoto S, Makino S. Enhancement of mice susceptibility to infection with Listeria monocytogenes by the treatment of morphine. Microbiol.Immunol. 2006;50:543–547. doi: 10.1111/j.1348-0421.2006.tb03824.x. [DOI] [PubMed] [Google Scholar]
- 24.Singh PP, Singal P. Morphine-induced neuroimmunomodulation in murine visceral leishmaniasis: the role(s) of cytokines and nitric oxide. J.Neuroimm.Pharmacol. 2007;2:338–351. doi: 10.1007/s11481-007-9094-y. [DOI] [PubMed] [Google Scholar]
- 25.MacFarlane AS, Peng X, Meissler JJ, Jr, Rogers TJ, Geller EB, Adler MW, Eisenstein TK. Morphine increases susceptibility to oral Salmonella typhimurium infection. J.Infect.Dis. 2000;181:1350–1358. doi: 10.1086/315403. [DOI] [PubMed] [Google Scholar]
- 26.Feng P, Rahim RT, Cowan A, Liu-Chen L-Y, Peng X, Gaughan J, Meissler JJ, Jr, Adler MW, Eisenstein TK. Effects of mu, kappa or delta opioids administered by pellet or pump on oral Salmonella infection and gastrointestinal transit. Eur.J.Pharmacol. 2006;534:250–257. doi: 10.1016/j.ejphar.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 27.Trendelenburg P. Physiological and pharmacological experiments about the small intestine peristaltic. Naunyn-Schmiedebergs Arch.Exp.Pathol.Pharmakol. 1917;81:55–129. [Google Scholar]
- 28.Burks TF, Fox DA, Hirning LD, Shook JE, Porreca F. Regulation of gastrointestinal function by multiple opioid receptors. Life Sci. 1988;43:2177–2181. doi: 10.1016/0024-3205(88)90410-9. [DOI] [PubMed] [Google Scholar]
- 29.Yukioka H, Rosen M, Evans KT, Leach KG, Hayward MW, Saggu GS. Gastric emptying and small bowel transit times in volunteers after introvenous morphine and nalbuphine. Anaesth. 1987;42:704–710. doi: 10.1111/j.1365-2044.1987.tb05314.x. [DOI] [PubMed] [Google Scholar]
- 30.Schleifer KW, Mansfield JM. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J.Immunol. 1993;151:5492–5503. [PubMed] [Google Scholar]
- 31.Hilburger ME, Adler MW, Truant AL, Meissler JJ, Jr, Satishchandran V, Rogers TJ, Eisenstein TK. Morphine induces sepsis in mice. J.Infect.Dis. 1997;176:183–188. doi: 10.1086/514021. [DOI] [PubMed] [Google Scholar]
- 32.Kromer W. Endogenous and exogenous opioids in the control of gastrointestinal motility and secretion. Pharmacol.Rev. 1988;40:121–162. [PubMed] [Google Scholar]
- 33.De Luca A, Coupar IM. Insights into opioid action in the intestinal tract. Pharmacol.Therapeut. 1996;69:103–115. doi: 10.1016/0163-7258(95)02053-5. [DOI] [PubMed] [Google Scholar]
- 34.Roy S, Liu H, Loh HH. μ-opioid receptor-knockout mice: the role of μ-opioid receptor in gastrointestinal transit. Mol.Brain Res. 1998;56:281–283. doi: 10.1016/s0169-328x(98)00051-5. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Amer.J.Path. 1967;50:109–139. [PMC free article] [PubMed] [Google Scholar]
- 36.Kent TH, Formal SB, Labrec EH. Acute enteritis due to Salmonella typhimurium in opium-treated guinea pigs. Arch.Path. 1966;81:501–508. [PubMed] [Google Scholar]
- 37.Formal SB, Abrams GD, Schneider H, Sprinz H. Experimental Shigella infections. VI. Role of the small intestine in an experimental infection in guinea pigs. J.Bacteriol. 1963;85:119–125. doi: 10.1128/jb.85.1.119-125.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tubaro E, Santiangeli C, Belogi L, Borelli G, Cavallo G, Croce C. Methadone vs. morphine: comparison of their effect on phagocytic functions. Int.J.Immunopharmacol. 1987;9:79–88. doi: 10.1016/0192-0561(87)90113-5. [DOI] [PubMed] [Google Scholar]
- 39.Rojavin M, Szabo I, Bussiere JL, Rogers TJ, Adler MW, Eisenstein TK. Morphine treatment in vitro or in vivo decreases phagocytic functions of murine macrophages. Life Sci. 1993;53:997–1006. doi: 10.1016/0024-3205(93)90122-j. [DOI] [PubMed] [Google Scholar]
- 40.Roy S, Charboneau RG, Barke RA, Loh HH. Role of mu-opioid receptors in immune function. In: Friedman H, Klein TW, Madden JJ, editors. Neuroimmune Circuits, Drugs of Abuse, and Infectious Diseases. New York: Kluwer: Academic/Plenum Publishers; 2001. pp. 117–126. [Google Scholar]
- 41.Schuller AGP, King MA, Zhang J, Bolan E, Pan Y-X, Morgan DJ, Chang A, Czick ME, Unterwald E, Pasternak GW, Pintar JE. Retention of heroin and morphine-6-beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nature Neurosci. 1999;2:151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- 42.Roy S, Barke RA, Loh HH. Mu-opioid receptor-knockout mice: role of μ-opioid receptor in morphine mediated immune functions. Mol.Brain Res. 1998;61:190–194. doi: 10.1016/s0169-328x(98)00212-5. [DOI] [PubMed] [Google Scholar]
- 43.DuPont HL, Hornick RB. Adverse effect of Lomotil therapy in Shigellosis. JAMA. 1973;226:1525–1528. [PubMed] [Google Scholar]
- 44.Hummel M, Ansonoff MA, Pintar JE, Unterwald EM. Genetic and pharmacological manipulation of μ opioid receptors in mice reveals a differential effect on behavioral sensitization to cocaine. Neurosci. 2004;125:211–220. doi: 10.1016/j.neuroscience.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 45.Feng P, Wilson QM, Meissler JJ, Jr, Adler MW, Eisenstein TK. Increased sensitivity to Salmonella enterica serovar Typhimurium infection in mice undergoing withdrawal from morphine is associated with suppression of interleukin-12. Infect.Immun. 2005;73:7953–7959. doi: 10.1128/IAI.73.12.7953-7959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng P, Truant AL, Meissler JJ, Jr, Gaughan JP, Adler MW, Eisenstein TK. Morphine withdrawal lowers host defense to enteric bacteria: spontaneous sepsis and increased sensitivity to oral Salmonella enterica serovar Typhimurium infection. Infect.Immun. 2006;74:5221–5226. doi: 10.1128/IAI.00208-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bryant HU, Bernton EW, Holaday JW. Morphine pellet-induced immunomodulation in mice: temporal relationships. J.Pharmacol.Exp.Ther. 1988;245:913–920. [PubMed] [Google Scholar]
- 48.Cheney DL, Goldstein A. Tolerance to opioid narcotics: time course and reversibility of physical dependence in mice. Nature. 1971;232:477–478. doi: 10.1038/232477a0. [DOI] [PubMed] [Google Scholar]
- 49.Cerletti C, Keinath SH, Reidenberg MM, Adler MW. Chronic morphine administration: Plasma levels and withdrawal syndrome in rats. Pharmacol.Biochem.Behav. 1976;4:323–327. doi: 10.1016/0091-3057(76)90249-5. [DOI] [PubMed] [Google Scholar]
- 50.Bryant HU, Yoburn BC, Inturrisi CE, Bernton EW, Holaday JW. Morphine-induced immunomodulation is not related to serum morphine concentrations. Eur.J.Pharmacol. 1988;149:165–169. doi: 10.1016/0014-2999(88)90057-x. [DOI] [PubMed] [Google Scholar]


