Abstract
Previous papers have shown surgical neoangiogenesis to allow long-term bone allotransplant survival without immunosuppression. Whole joint composite tissue allotransplants (CTA) might be treated similarly. A novel rat knee CTA model is described for further study of the roles of neoangiogensis in joint allotransplant survival and adjustment of immunosuppression.
Microvascular knee CTA was performed in 9 rats across a major histocompatibility barrier with both pedicle repair and implantation of host-derived arteriovenous (‘a/v’) bundles. In the control group (N=3), the pedicle was ligated. Immunosuppression was given daily. Joint mobility, weight-bearing, pedicle patency, bone blood flow and sprouting from a/v bundles were assessed at three weeks.
All but the non-revascularized control knees had full passive motion and full weight bearing. One nutrient pedicle thrombosed prematurely. Blood flow was measurable in transplants with patent nutrient pedicles. Implanted a/v bundles produced new vascular networks on angiography.
This new rat microsurgical model permits further study of joint allotransplantation. Patency of both pedicles and implanted a/v bundles was maintained, laying a foundation for future studies.
INTRODUCTION
Patients requiring large joint reconstruction after trauma, tumor resection, infection or failed arthroplasty represent a significant surgical challenge. No current method offers an ideal solution. Osteoarticular allografts are prone to rapid joint destruction and arthritis, requiring salvage by arthroplasty within three years in up to 54% of reported cases1,2. Prosthetic replacement of joints may fail, loosen or produce periprosthetic fractures3–6. Tissue-engineered joint replacements remain only a theoretical possibility. Expendable vascularized bone autograft sources are limited. The fibular head has been used for distal radius articular replacement7,8. The only expendable whole joints readily available are toe phalangeal and metatarsal joints, used primarily for smaller joint defects in the hand and wrist9. The hip has been reconstructed with the ‘hankle’ procedure10, and the knee with the Van Nes rotationplasty11. In each case, the ankle becomes the hip or knee joint, requiring amputation of the leg either above the knee or below the knee, respectively. Titanium whole knee replacement arthroplasty is currently a widely performed procedure with low revision scores at long-term follow-up, and good quality of life outcomes12. However, when the extensor apparatus (patella, patellar ligament, quadriceps tendon) is absent, total knee arthroplasty is impossible, and the only alternatives to transplantation are above knee amputation or arthrodesis with shortening of the leg13.
An ideal replacement for large joint defects would be a living joint allogeneic transplant that exactly matches the dimensions and structural properties of the missing joint. This would also provide the superior healing properties of a vascularized autograft. However, few living joint transplantations have been performed. The long-term immunosuppression required to maintain circulation and viability are a significant drawback. Methods permitting vascularized joint allotransplantation without the health risks of immunosuppressive drugs or tolerance induction would be an important advance.
We propose a novel method by which this may be accomplished: combined microsurgical joint transfer and the development of a new osseous blood supply of host origin. Surgically implanted host arteriovenous (‘a/v’) bundles promote neoangiogenesis. As these new host-derived vessels are non-immunogenic, only short-term immunosuppression is necessary, allowing sufficient time for angiogenesis to occur. The current study describes a new surgical model of vascularized orthotopic joint allotransplantation in the rat with implantation of host-derived a/v bundles. Preservation of hind-limb neuromuscular function and weight bearing allow mechanical assessments to be made.
METHODS
Experimental design
Nine vascularized knee joints were transplanted across a strong major histocompatibility complex (MHC) barrier (Figure 1). The control group I consisted of three rats in which the vascular pedicles were ligated. Six animals in group II had patent vessels. Immunosuppression in the form of FK-506 (Fujisawa Pharmaceutical Co., Osaka, Japan) was administered daily for the duration of the study.
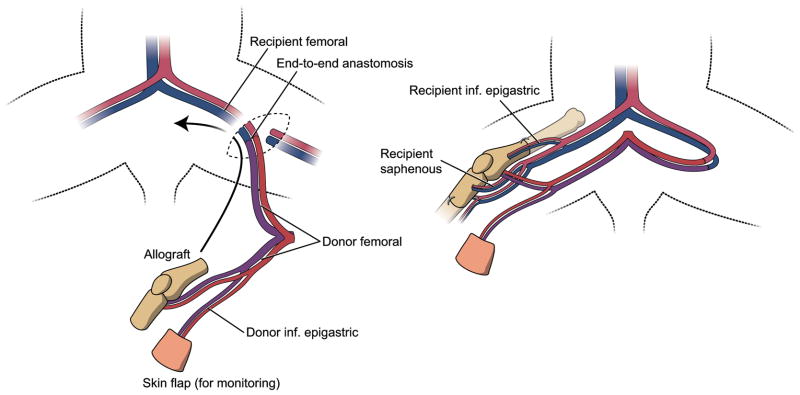
Figure 1.
Surgical procedure. The entire knee joint is transplanted, including tendons inserting at the knee, and a monitor or ‘bouy’ skin flap into a knee defect in a recipient animal. Vascular anastomoses are performed in the contralateral groin to preserve maximal flow in the recipient bed as well as the transplant. Fixation is performed with intramedullary needles serving as ‘rods’ (not shown) and interosseous wires.
Animals and anesthesia
Inbred female Dark Agouti rats (DA, genetic expression: RT1a) weighing 175–200g were used as donors and male Piebald Virol Glaxo rats (PVG, genetic expression: RT1c) weighing 200–250g were the recipient animals (Harlan Sprague Dawley, Madison, WI). One donor rat was required for each transplantation procedure (N=9). Donor rats were anesthetized with pentobarbital sodium at a dose of 35 mg/kg IP. Following graft harvest they were euthanized with an intracardiac injection of Beuthanasia (pentobarbital sodium) at dose of 200 mg/kg. Recipient rats were anesthetized with a combination of ketamine (90 mg/kg IM), and xylazine (10 mg/kg IM). If necessary, during the surgical procedure the recipient rat was re-dosed with 20 mg/kg injections of ketamine. Tribrissen (30 mg/kg) antibiotic prophylaxis was given preoperatively. Recipient rats received a subcutaneous injection of 10 units of Fragmin immediately pre-and postoperatively, followed by 20 units daily for five days to reduce the incidence of post-operative thrombosis14. Tacrolimus (FK-506) immunosuppression was given at a dose of 1mg/kg/day intramuscularly for the study duration. All experiments were performed according to established National Institute of Health (NIH) guidelines under the direction of the Mayo Clinic Institutional Animal Care and Use Committee (IACUC).
Harvest of vascularized knee joint allotransplant
The right knee joint was exposed through a longitudinal incision in a donor DA rat. A buoy skin flap (1 cm2) was preserved on the superficial inferior epigastric vessels and served as an external monitor of blood flow15 in the vascularized transplants. The vascular pedicle consisted of the bilateral femoral vessels, with the aortic bifurcation in between (Figure 1). Muscular branches of the femoral vessels (deep femoral, lateral femoral circumflex, external pudendal and saphenous) were ligated and divided. The anterior, medial and lateral tendinous insertions around the knee joint were preserved. The sural arteries and gastrocnemius muscles were divided and the posterior tibial artery was ligated distal to the superior nutrient vessel to the tibia. The anterior tibial muscle was dissected proximally to reveal the anterior tibial artery as it traversed the interosseous membrane. The anterior tibial artery was ligated distal to the origin of the recurrent tibial artery. All genicular branches were spared. The distal femur and the proximal tibia and fibula were osteotomized at 1.5cm from the joint with a hand-held circular saw and the fibular portion was removed from the transplant. The inferior aorta and vena cava were clamped proximal to the bifurcation, ligated and cut; the transplant was exsanguinated with heparinized saline through the contralateral femoral artery and chilled at 4°C during the preparation of the recipient site.
Preparation of recipient bed and transfer of vascularized knee joint and a/v bundles
In the recipient PVG rat, an anteromedial incision was made over the right thigh and knee joint extending to the ankle. The saphenous and inferior epigastric artery and vein were then dissected incorporating a portion of muscle fascia. The knee joint was removed as in the donor procedure, now preserving tendinous insertions on the musculature, as well as the neurovascular connections to the distal portion of the limb. The complete fibula was also preserved. The two knee joints were held side-by side and any adjustments to the length of the bones made with a file to create a properly-fitting transplant. Two 1mm diameter holes were drilled transversely through the metaphysis of the allotransplant femur and tibia for the passage of the a/v bundles. A combination of intramedullary and interosseous wire fixation was used. The femur was reamed with a 0.6mm needle; the tibia with a 0.5mm needle. Additional transverse holes were drilled for interosseous wire placement 5mm to either side of the femoral and tibial bone junctions. 1.2mm and 1mm needles were used as intramedullary rods, adjusting length to avoid obstruction of the a/v bundle holes. 26-gauge steel interosseous wires were passed through the previously-prepared holes and tightened. The fibular head was secured to the transplanted tibia with a 4-0 nonabsorbable suture. The epigastric and saphenous a/v bundles were passed through the femoral and tibial metaphyses, respectively, and fixed with 8-0 nylon sutures. The pedicle was then tunneled to the contralateral groin and the anastomosis was performed through a separate incision to the recipient femoral vessels using 11-0 nylon sutures under a surgical microscope (Zeiss Universal S3). Anastomoses of both artery and vein were initially performed end-to-end to the contralateral femoral vessels. Perioperative venous stasis occurred in one animal, due to compression of the pedicle. Moving the venous anastomosis site to the ipsilateral groin end-to-side resolved the problem. This technique was used in all subsequent animals. All recipient muscle groups were then attached to their corresponding donor tendons with nonabsorbable 6-0 monofilament suture and running fascicular sutures (Figure 2). Finally the wounds were closed using nylon sutures and surgical staples.
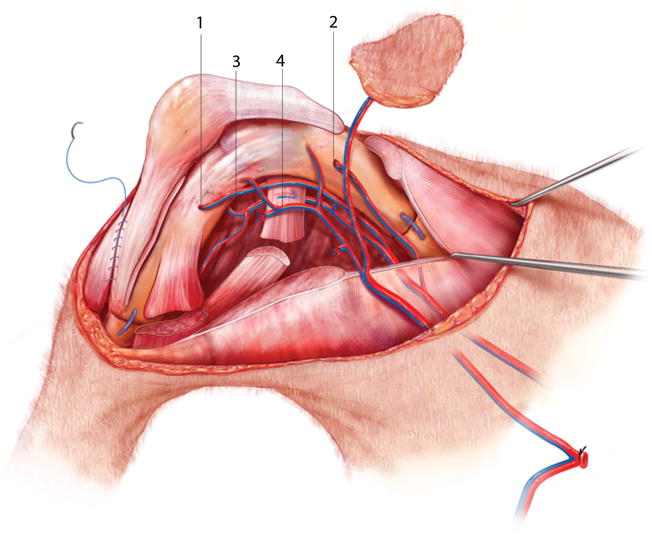
Figure 2.
Whole knee transplantation. Shown are the recipient saphenous (1) and epigastric (2) vessels introduced into the tibia and femur, respectively. The nutrient vessels to the lower leg (3) and the transplant pedicle (4) tunneled subcutaneously to the contralateral groin are also shown. The quadriceps and gastrocnemius tendons are directly sutured together. Mattress sutures (not shown) approximate the adductors and biceps femoris muscles. A running suture is used to approximate superficial muscle fascia.
In vivo assessment
Skin flaps were assessed for signs of necrosis (color change, decreased suppleness) daily, and were scored pre-harvest as either necrotic or viable. Ambulation and joint mobility were assessed immediately prior to anesthesia at three weeks on a subjective basis and qualified as none, little or full. Full weight bearing and joint range of motion implied no difference in observed ambulation and manual movement of the joint when compared to the contralateral knee. Rats with little mobility and weight bearing were obviously limping and the joint was less mobile than the contralateral side. No weight bearing and very little or no joint mobility was the third category in this assessment.
Assessment of pedicle patency, bone blood flow and capillary sprouting
After three weeks’ survival, patency and bone blood flow were measured in a second non-survival anesthetic procedure. The transplant was exposed under the operating microscope and the pedicles and a/v bundles were carefully tested for patency by downstream occlusion and release. Bone blood flow was next measured using the quantitative hydrogen washout method previously detailed16,17. The aorta and vena cava were cannulated and the rat was euthanized. The lower body was flushed with heparinized saline and microangiography performed, infusing a synthetic endovascular polymer (Microfil®, Flow Tech, Carver MA) at physiologic pressures. After polymerization for 24 hours at 4 degrees C, grafts were fixed in 10% formalin for 48 hours, and surplus soft tissue removed. The transplants were divided into portions for histology assessment of the joint space and cortical bone, and for assessment of capillary sprouting from the a/v bundles. The intraosseous vasculature was visualized using modified Spalteholz methyl salicylate optical bone clearing18,19.
Histological assessment of viability and inflammation
Histologic sections were then taken from the femur, tibia and joint space. A previously described system20–22 was used on hematoxylin-eosin stained specimens to grade bone inflammation (none: normal bone; mild: periosteal infiltrate; moderate: irregular cortical bone and nonviable woven bone; severe: edema, necrosis, hemorrhage). Assessments of cartilage structure were made by looking for any signs of chondrocyte and matrix degeneration.
RESULTS
All animals survived for the duration of the study. Average weight at surgery was 246.3g. Results are shown in Table 1.
Table 1.
Results. Skin was graded as viable and supple or necrotic and dry; weight bearing at 3 weeks was deemed none, little, some, or full; downstream patency was tested under the surgical microscope; bone rejection and joint inflammation were based on criteria in Table 1. EB = epigastric a/v bundle, F = femur, T = tibia.
| Animal | Pedicle patency: Initial (3 week result) | AV bundle patency: Initial (3 week result) | Bone blood flow (ml/min/100g) F: femur T: tibia |
Inflammation | Ambulation at 21 days |
|---|---|---|---|---|---|
| Group I: Control (nonviable) knee allotransplant | |||||
| 1 (control) | Ligated (thrombosed) | Patent (thrombosed) | F: none T: none |
Severe | Little |
| 2 (control) | Ligated (thrombosed) | Patent (patent) | F: none T: none |
Severe | Little |
| 3 (control) | Ligated (thrombosed) | Patent (thrombosed) | F: none T: none |
Severe | None |
| Group II: viable allotransplanted knee | |||||
| 4 | Patent (thrombosed) | Patent (EB thrombosed) | F: none T: none |
Moderate | Full |
| 5 | Patent (patent) | Patent (patent) | F: 0.15 T: 0.11 |
Mild | Full |
| 6 | Patent (patent) | Patent (patent) | F: 0.21 T: 0.17 |
None | Full |
| 7 | Patent (patent) | Patent (patent) | F: 0.14 T: 0.09 |
None | Full |
| 8 | Patent (patent) | Patent (patent) | F: 0.27 T: 0.16 |
None | Full |
| 9 | Patent (patent) | Patent (patent) | F: 0.18 T: 0.14 |
None | Full |
In control animals, all transplants were necrotic with histologic evidence of bone resorption and joint destruction by gross leukocyte infiltration (Figure 3). Both a/v bundles were thrombosed in 2/3 controls, whereas both a/v bundles remained patent in the third control. The use of the hind limb was characterized by little weight bearing after 21 days, with a stiffer joint than the contralateral side. No blood flow was measurable in either the femur or tibia in control rats.
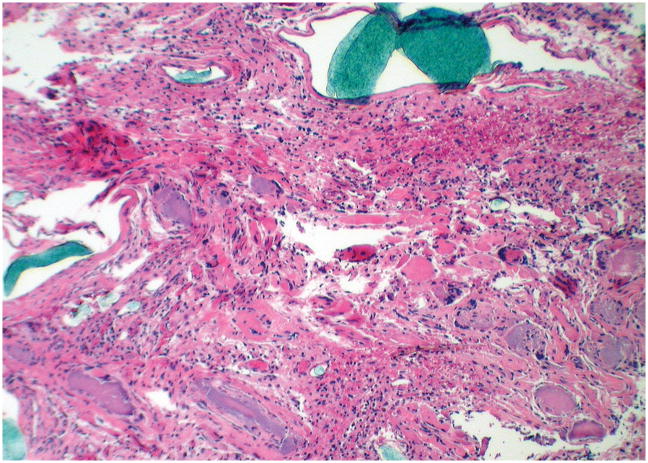
Figure 3.
Transverse section of a transplanted femur, hematoxylin-eosin stained, 400× magnification (Rat number: 1, control). Severe infiltration, vasculitis, bone resorption and hemorrhage. The blue intravascular polymer is visible on the left and top edges of the image.
One of six vascularized allotransplant skin monitor flaps developed hypovascularity and eventual necrosis. On sacrifice, the nutrient pedicle and the epigastric a/v bundle were found to be thrombosed. There was no measurable bone blood flow in the femur or tibia. The other five skin paddles remained viable and the corresponding pedicles and a/v bundles were found to be patent. Bone blood flow in the femur and tibia was measurable in all five transplants. Intravascular polymer casts showed neoangiogenesis from patent a/v bundles into the transplanted bones. The transplant nutrient circulation and the ambient lower leg circulation could also be visualized (Figures 4 and 5). Histologically, the transplants with patent pedicles had mild periosteal infiltrates but viable osteocytes and minimal cartilage breakdown (Figure 6). Complications included loss of femoral fixation in rat 6, and infection of the femoral interosseous wire in rat 5. The short survival time did allow osseous union in any animal (Figure 7). Stabile fixation in the vascularized transplant group resulted in full passive range of motion at three weeks with full weight bearing and outwardly normal use of the hind limb. No bone junctional loosening was observed in last three experimental animals. In all animals, ambulation and joint range of motion were consistently correlated.
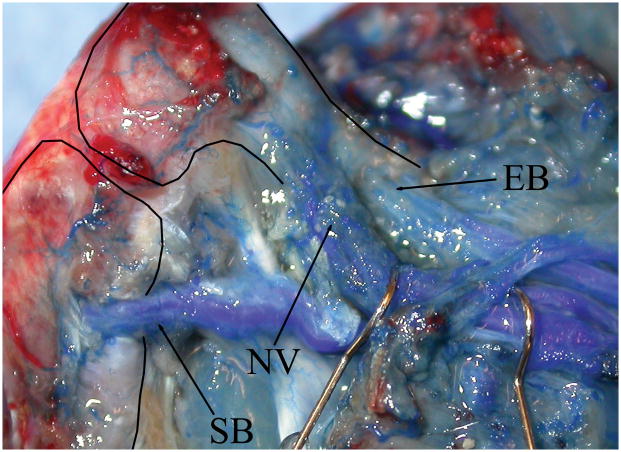
Figure 4.
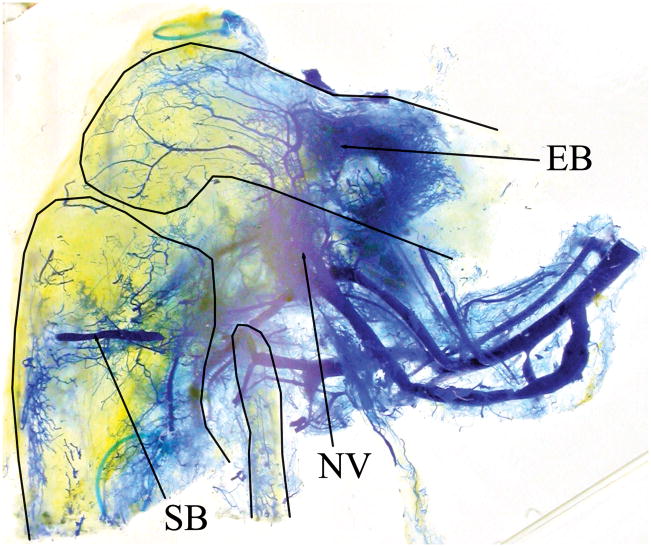
Knee transplant vascularity at sacrifice (Rat number: 6, right side, from medial). Thin solid lines indicate the outlines of the femur and tibia. Vessels are visualized by blue endovascular polymer. Adductor, medial biceps and quadriceps muscles are detached. The saphenous (SB) and epigastric (EB) recipient-derived a/v bundles are shown entering the transplanted tibia and femur, respectively. The transplant nutrient vessels (NV) are marked by hooks behind the pedicle. (Refer to Figure 2 for orientation.)
Figure 5.
Optically cleared knee allotransplant (right side, from medial). Thin solid lines show the outlines of the femur, tibia and fibula. Blue endovascular polymer visualizes capillary networks from three locations: saphenous (SB) and epigastric (EB) a/v bundles and the transplant nutrient vessels (NV). (Refer to Figure 2 for orientation.)
Figure 6.
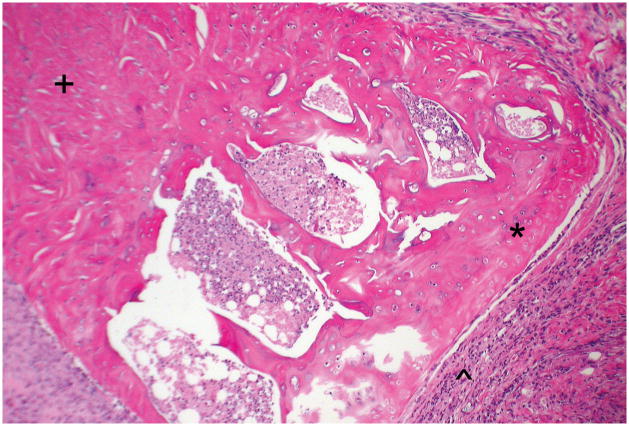
Longitudinal, hematoxylin-eosin stained section of the transplant tibial plateau at 400× magnification (Rat number: 4). The cartilage is viable (*), with mild synovial infiltration (^). For orientation, the metaphysis is marked (+). There are no empty lacunae in the bone.
Figure 7.
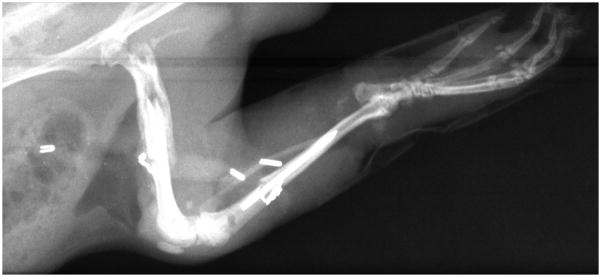
X-ray of a whole knee allotransplant three weeks post-transplantation. There is no evidence yet of bony union, neither of pseudoarthrosis or stress fractures. Full weight bearing was seen at this point.
DISCUSSION
Living joint allotransplants at present remain experimental. Routine use is problematic due to the significant health risks attendant to the need for long-term immune modulation. Drug therapy complications include opportunistic infections, malignancy and organ toxicity23–26, while induction of donor-specific tolerance is difficult to accomplish and carries the risk of inducing graft versus host disease27–29. Such risks are difficult to defend ethically for non-life-critical reconstructive problems. For these reasons, bone and joint allotransplantation has seldom been performed clinically. A series of six whole knee and femoral diaphyseal allotransplants has been reported by Hofmann30–35. Postoperative immunosuppression included long-term maintenance with cyclosporine and azathioprine, or FK-506 and mycophenolate mofetil31,36. Long-term results have been poor, with survival of one transplant, one loss from a surgical site infection, one by non-compliance and three by late rejection37. Doi described a case of vascularized fibula allografting from the mother of a 2-year old boy with congenital pseudoarthrosis of the right tibia and fibula and persistent non-union despite two previous osteotomies38. The patient was treated with cyclosporine until union at both ends was achieved at 7 months. Despite occlusion of the arterial anastomosis at 3 months, the allograft survived and maintained structural support 7 years postoperatively.
More research is needed before vascularized bone, joint or other composite allotransplantation becomes routine in clinical practice. The development of joint allotransplant research models has a long and varied history (Table 2). Heterotopic39,40 and orthotopic41–44 whole knee joint and orthotopic hemi-knee joint45 allotransplantation in animal models have shown long-term survival under immunosuppression with FK506 and/or cyclosporin A. However, in these models the anatomic integrity of the recipient musculoskeletal structures was largely compromised, limiting the value of any functional assessments reported. Also, viability could not be maintained or reasonably expected after removal of short-term immunosuppression46,47. Large animal osteochondral models that study function and survival after the withdrawal of immunosuppression are lacking.
Table 2.
Historical development of joint CTA models.
| Authors | New Knee Allograft Model or Modification | Outcome |
|---|---|---|
| (Slome and Reeves 1966)41 | Canine vascularized orthotopic knee allograft | Successful 20-hour non-survival experiment with patent vessels and maintained synovial isotope clearance |
| (Yaremchuk, Sedacca et al. 1983)43 | Rabbit vascularized orthotopic whole knee allograft with anastomosis on popliteal vessels and muscle reattachment | Longest survival 3 months, vascular rejection limited graft survival; authors conclude joint allografting not yet a clinical option |
| (Yaremchuk, Nettelblad et al. 1985)39 | Rat heterotopic whole knee allograft (subcutaneous placement) | Major histocompatibility mismatch leads to complete graft rejection by 4–6 weeks |
| (Muramatsu 1992)47 | Rat vascularized orthotopic whole knee joint transplantation | Complete bony union achieved; continuous low-dose Cyclosporin A needed to maintain joint function |
| (Lee, Pan et al. 1995)46 | Rat vascularized orthotopic hemi- knee joint allograft | Pulsed or early cessation of Cyclosporin A leads to graft rejection, continuous treatment allows indefinite survival and function |
An ideal osteochondral allotransplant model has the following properties: a) vascularized, b) orthotopic, c) full functional recovery possible (bony union and spared host neuromuscular structures), d) genetically well-defined, e) allow serial assessments of transplant survival, and f) potential survival after removal of immunosuppression.
In previous and ongoing studies from our laboratory we have demonstrated that implanted vessels of host origin (‘surgical angiogenesis’) can maintain blood flow to and viability of, heterotopic femoral allotransplants after removal of short-term postoperative immunosuppression48,49. The currently described vascularized orthotopic joint allotransplantation model preserves the innervation, circulation and musculature in the host hind limb and allows an approximation of a clinically relevant problem combined with the advantages of functional and mechanical assessment and potential survival following the removal of immunosuppression.
Diefenbeck and Hofmann used the skin component of their vascularized human knee joints in order to monitor rejection. In one patient, a rejection episode was accurately signaled by the skin paddle and histology obtained from both the skin and the articular cartilage revealed the same amount of lymphocyte infiltration, proving this method to accurately predict rejection episodes13. In our model, the skin paddle was used to monitor transplant perfusion. Early pedicle thrombosis was presumed to cause a change in functional outcome before experiment termination. We do not plan to use the skin paddle in long term studies because skin is historically seen as a strong barrier to composite tissue allotransplantation (CTA) 50. Use of skin in a CTA model aiming for survival after tapering of immunosuppression may negate the hope of eradicating immunosuppression altogether.
The duration of the study interval was not long enough to evaluate rejection or tolerance. A longer follow-up is needed to adequately study the outcome of this surgical model in immunological terms, as well as to evaluate time to complete bone healing. The interval of three weeks was chosen based on vascularized femoral allotransplants, for which immunosuppression was given in a short two-week course48,49. Revascularization was found to be significantly similar after two weeks or four weeks of immunosuppression. We anticipated requiring a slightly longer course (3 weeks) to revascularize the femur and the tibia in the knee allotransplant. Also, the use of continuous FK-506 makes any study of rejection impossible. For the purpose of this study, however, we have demonstrated the function and feasibility of our model.
This study confirms the surgical feasibility, functional return, vessel sprouting from implanted host-derived vessels and survival of vascularized rat whole knee allotransplants under FK-506 immunosuppression. Tissue survival was negatively affected by diminished vascularity in non-vascularized controls and in case of early pedicle thrombosis, although in the latter this had little obvious effect on function after three weeks. A/v bundle patency was lower (3/8) in the transplants with thrombosed pedicles than in those with patent pedicles (10/10). A/v bundle patency has been greater in previous heterotopic vascularized transplants than non-vascularized transplants48. This suggests an interaction between the a/v bundle and its environment, for which further research is warranted. Bone blood flow was measurable and bone viability was maintained in the vascularized transplants with patent pedicles on sacrifice. New capillary networks grew into the allotransplants from implanted host-derived a/v bundles. When these are allowed to grow sufficiently, they may be sufficient to provide perfusion to the transplant and long-term survival after the removal of immunosuppression, consistent with our findings in the heterotopic femoral model. Articular structures are subject to destruction due to necrosis of the subchondral bone. This is countered by implanting the a/v bundles in the metaphyseal areas. Bone allotransplants have been shown to be colonized by recipient osteocytes. The resulting bone remodeling by autogenous cells supplied from a neoangiogenic autogenous blood supply ultimately provides a mechanism of survival after removal of immunosuppression51.
We encountered some postoperative problems consisting of widening of bone junctions, which may be overcome with improved osteosynthesis. The goal of the present study was to determine the feasibility of a larger allotransplant orthotopic model in the rat hind limb during the time that the short-term course of immunosuppression is administered. Based on the favorable results here presented, in future studies we will use a longer follow-up interval and compare biomechanical and radiographic results after short-term and long-term courses of immunosuppression. This will be done in line with our rabbit orthotopic model52, which compares favorably to the literature53,54 in showing bone healing after two weeks. We did not immediately expect bony union in the rat model after less than 10 weeks, despite the evidence from our rabbit model. Future studies in a larger animal model provides the advantages of improved osteosynthesis and the availability of larger joint surfaces for accurate biomechanical testing.
CONCLUSIONS
Previous papers have shown that surgical neoangiogenesis from implanted vascularized recipient tissue allows long-term bone allotransplant survival without long-term immunosuppression. We have developed a novel rat knee CTA model to study if the whole joint allotransplants similarly treated might also survive and function. Longer survival periods will be needed to fully evaluate the feasibility of this method for composite whole joint allotransplantation.
Acknowledgments
Grant sponsor: National Institutes of Health
Grant number: R01-AR-49718
The authors thank Fujisawa Pharmaceutical Co., Ltd., Osaka, Japan, for the generous donation of FK-506.
References
- 1.DeGroot H, 3rd, Mankin H. Total knee arthroplasty in patients who have massive osteoarticular allografts. Clin Orthop Relat Res. 2000;(373):62–72. doi: 10.1097/00003086-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Fox EJ, Hau MA, Gebhardt MC, Hornicek FJ, Tomford WW, Mankin HJ. Long-term followup of proximal femoral allografts. Clin Orthop Relat Res. 2002;(397):106–113. doi: 10.1097/00003086-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Heck DA, Chao EY, Sim FH, Pritchard DJ, Shives TC. Titanium fibermetal segmental replacement prostheses. A radiographic analysis and review of current status. Clin Orthop Relat Res. 1986;(204):266–285. [PubMed] [Google Scholar]
- 4.Abudu A, Carter SR, Grimer RJ. The outcome and functional results of diaphyseal endoprostheses after tumour excision. J Bone Joint Surg Br. 1996;78(4):652–657. [PubMed] [Google Scholar]
- 5.Damron TA, Sim FH, Shives TC, An KN, Rock MG, Pritchard DJ. Intercalary spacers in the treatment of segmentally destructive diaphyseal humeral lesions in disseminated malignancies. Clin Orthop Relat Res. 1996;(324):233–243. doi: 10.1097/00003086-199603000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Schurmann M, Gradl G, Andress HJ, Kauschke T, Hertlein H, Lob G. Metastatic lesions of the humerus treated with the isoelastic diaphysis prosthesis. Clin Orthop Relat Res. 2000;(380):204–214. doi: 10.1097/00003086-200011000-00028. [DOI] [PubMed] [Google Scholar]
- 7.Weiland AJ, Kleinert HE, Kutz JE, Daniel RK. Free vascularized bone grafts in surgery of the upper extremity. J Hand Surg [Am] 1979;4(2):129–144. doi: 10.1016/s0363-5023(79)80129-x. [DOI] [PubMed] [Google Scholar]
- 8.Pho RW. Free vascularised fibular transplant for replacement of the lower radius. J Bone Joint Surg Br. 1979;61-B(3):362–365. doi: 10.1302/0301-620X.61B3.479261. [DOI] [PubMed] [Google Scholar]
- 9.del Pinal F, Innocenti M. Evolving concepts in the management of the bone gap in the upper limb. Long and small defects. J Plast Reconstr Aesthet Surg. 2007;60(7):776–792. doi: 10.1016/j.bjps.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Peterson CA, 2nd, Koch LD, Wood MB. Tibia-hindfoot osteomusculocutaneous rotationplasty with calcaneopelvic arthrodesis for extensive loss of bone from the proximal part of the femur. A report of two cases. J Bone Joint Surg [Am] 1997;79(10):1504–1509. doi: 10.2106/00004623-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Van Nes C. Rotation-plasty for congenital defects of the femur. Making use of the ankle of the shortened limb to control the knee joint of a prosthesis. J Bone Joint Surg. 1950;32-B(1):12–16. [Google Scholar]
- 12.Deirmengian CA, Lonner JH. What’s new in adult reconstructive knee surgery. J Bone Joint Surg [Am] 2009;91(12):3008–3018. doi: 10.2106/JBJS.I.01062. [DOI] [PubMed] [Google Scholar]
- 13.Diefenbeck M, Hofmann GO. Vascularized Knee Transplantation. In: Hewitt CW, Lee WPA, Gordon CR, editors. Transplantation of composite tissue allografts. New York: Springer; 2008. pp. 293–306. [Google Scholar]
- 14.Malm K, Dahlback B, Arnljots B. Low-molecular-weight heparin (dalteparin) effectively prevents thrombosis in a rat model of deep arterial injury. Plast Reconstr Surg. 2003;111(5):1659–1666. doi: 10.1097/01.PRS.0000053549.45063.A1. [DOI] [PubMed] [Google Scholar]
- 15.Tung TH, Mohanakumar T, Mackinnon SE. A subcutaneous heterotopic limb transplantation model in the mouse for prolonged allograft survival. Microsurgery. 2001;21(7):298–305. doi: 10.1002/micr.1056. [DOI] [PubMed] [Google Scholar]
- 16.Larsen M, Pelzer M, Friedrich PF, Bishop AT. Measurement of bone blood flow using the hydrogen washout technique-part II: Validation by comparison to microsphere entrapment. J Orthop Res. 2008;26(6):746–752. doi: 10.1002/jor.20561. [DOI] [PubMed] [Google Scholar]
- 17.Pelzer M, Larsen M, Friedrich PF, Bishop AT. Measurement of bone blood flow using the hydrogen washout Technique-Part I: Quantitative evaluation of tissue perfusion in the laboratory rat. J Orthop Res. 2008;26(6):741–745. doi: 10.1002/jor.20562. [DOI] [PubMed] [Google Scholar]
- 18.Spalteholz K. Nebst Anhang: Über Knochenfärbung. Leipzig: S. Hirzel; 1914. Über das Durchsichtigmachen von menschlichen und tierischen Präparaten und seine theoretischen Bedingungen. [Google Scholar]
- 19.Sheetz KK, Bishop AT, Berger RA. The arterial blood supply of the distal radius and ulna and its potential use in vascularized pedicled bone grafts. J Hand Surg [Am] 1995;20(6):902–914. doi: 10.1016/S0363-5023(05)80136-4. [DOI] [PubMed] [Google Scholar]
- 20.Plissonnier D, Nochy D, Poncet P, Mandet C, Hinglais N, Bariety J, Michel JB. Sequential immunological targeting of chronic experimental arterial allograft. Transplantation. 1995;60(5):414–424. doi: 10.1097/00007890-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Buttemeyer R, Jones NF, Min Z, Rao U. Rejection of the component tissues of limb allografts in rats immunosuppressed with FK-506 and cyclosporine. Plast Reconstr Surg. 1996;97(1):139–148. doi: 10.1097/00006534-199601000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Muramatsu K, Doi K, Kawai S. Limb allotransplantation in rats: combined immunosuppression by FK-506 and 15-deoxyspergualin. J Hand Surg [Am] 1999;24(3):586–593. doi: 10.1053/jhsu.1999.0586. [DOI] [PubMed] [Google Scholar]
- 23.Rubin RH, Cosimi AB, Hirsch MS, Herrin JT, Russell PS, Tolkoff-Rubin NE. Effects of antithymocyte globulin on cytomegalovirus infection in renal transplant recipients. Transplantation. 1981;31(2):143–145. doi: 10.1097/00007890-198102000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Peterson PK, Ferguson R, Fryd DS, Balfour HHJ, Rynasiewicz J, Simmons RL. Infectious diseases in hospitalized renal transplant recipients: a prospective study of a complex and evolving problem. Medicine (Baltimore) 1982;61(6):360–372. doi: 10.1097/00005792-198211000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Krause JR, Ayuyang HQ, Ellis LD. Secondary non-hematopoietic cancers arising following treatment of hematopoietic disorders. Cancer. 1985;55(3):512–515. doi: 10.1002/1097-0142(19850201)55:3<512::aid-cncr2820550307>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Brenner MJ, Tung TH, Jensen JN, Mackinnon SE. The spectrum of complications of immunosuppression: is the time right for hand transplantation? J Bone Joint Surg [Am] 2002;84-A(10):1861–1870. [PubMed] [Google Scholar]
- 27.Korngold B, Sprent J. Lethal graft-versus-host disease after bone marrow transplantation across minor histocompatibility barriers in mice. Prevention by removing mature T cells from marrow. J Exp Med. 1978;148(6):1687–1698. doi: 10.1084/jem.148.6.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdick JF, Vogelsang GB, Smith WJ, Farmer ER, Bias WB, Kaufmann SH, Horn J, Colombani PM, Pitt HA, Perler BA. Severe graft-versus-host disease in a liver-transplant recipient. N Engl J Med. 1988;318(11):689–691. doi: 10.1056/NEJM198803173181107. [DOI] [PubMed] [Google Scholar]
- 29.Kernan NA, Bartsch G, Ash RC, Beatty PG, Champlin R, Filipovich A, Gajewski J, Hansen JA, Henslee-Downey J, McCullough J, et al. Analysis of 462 transplantations from unrelated donors facilitated by the National Marrow Donor Program. N Engl J Med. 1993;328(9):593–602. doi: 10.1056/NEJM199303043280901. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann GO, Kirschner MH, Buhren V, Land W. Allogenic vascularized transplantation of a human femoral diaphysis under cyclosporin A immunosuppression. Transpl Int. 1995;8(5):418–419. doi: 10.1007/BF00337179. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann G, Kirschner MH, Wagner FD, Brauns L, Gonschorek O, Buhren V. Allogeneic vascularized transplantation of human femoral diaphyses and total knee joints--first clinical experiences. Transplant Proc. 1998;30(6):2754–2761. doi: 10.1016/s0041-1345(98)00803-3. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann GO, Kirschner MH, Brauns L, Wagner FD, Land W, Buhren V. Vascularized knee joint transplantation in man: a report on the first cases. Transpl Int. 1998;11 (Suppl 1):S487–490. doi: 10.1007/s001470050525. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann GO, Kirschner MH. Clinical experience in allogeneic vascularized bone and joint allografting. Microsurgery. 2000;20(8):375–383. doi: 10.1002/1098-2752(2000)20:8<375::aid-micr6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Kirschner MH, Wagner FD, Nerlich A, Land W, Buhren V, Hofmann GO. Allogenic grafting of vascularized bone segments under immunosuppression. Clinical results in the transplantation of femoral diaphyses. Transpl Int. 1998;11(3):195–203. doi: 10.1007/s001470050127. [DOI] [PubMed] [Google Scholar]
- 35.Kirschner MH, Brauns L, Gonschorek O, Buhren V, Hofmann GO. Vascularised knee joint transplantation in man: the first two years experience. Eur J Surg. 2000;166(4):320–327. doi: 10.1080/110241500750009186. [DOI] [PubMed] [Google Scholar]
- 36.Diefenbeck M, Wagner F, Kirschner MH, Nerlich A, Muckley T, Hofmann GO. Management of acute rejection 2 years after allogeneic vascularized knee joint transplantation. Transpl Int. 2006;19(7):604–606. doi: 10.1111/j.1432-2277.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 37.Diefenbeck M, Wagner F, Kirschner MH, Nerlich A, Muckley T, Hofmann GO. Outcome of allogeneic vascularized knee transplants. Transpl Int. 2007;20(5):410–418. doi: 10.1111/j.1432-2277.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- 38.Doi K, Kawai S, Shigetomi M. Congenital tibial pseudoarthrosis treated with vascularised bone allograft. Lancet. 1996;347(9006):970–971. doi: 10.1016/s0140-6736(96)91458-0. [DOI] [PubMed] [Google Scholar]
- 39.Yaremchuk MJ, Nettelblad H, Randolph MA, Weiland AJ. Vascularized bone allograft transplantation in a genetically defined rat model. Plast Reconstr Surg. 1985;75(3):355–362. doi: 10.1097/00006534-198503000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Schwind JV. Homotransplantation by parabiosis. J Surg Res. 1962;2:332–336. doi: 10.1016/s0022-4804(62)80043-2. [DOI] [PubMed] [Google Scholar]
- 41.Slome D, Reeves B. Experimental homotransplantation of the knee-joint. Lancet. 1966;2(7456):205. doi: 10.1016/s0140-6736(66)92483-4. [DOI] [PubMed] [Google Scholar]
- 42.Doi K, DeSantis G, Singer DI, Hurley JV, O’Brien B, McKay SM, Hickey MJ, Murphy BF. The effect of immunosuppression on vascularised allografts. A preliminary report. J Bone Joint Surg Br. 1989;71(4):576–582. doi: 10.1302/0301-620X.71B4.2768300. [DOI] [PubMed] [Google Scholar]
- 43.Yaremchuk MJ, Sedacca T, Schiller AL, May JW., Jr Vascular knee allograft transplantation in a rabbit model. Plast Reconstr Surg. 1983;71(4):461–472. [PubMed] [Google Scholar]
- 44.Siliski JM, Simpkin S, Green CJ. Vascularized whole knee joint allografts in rabbits immunosuppressed with cyclosporin A. Arch Orthop Trauma Surg. 1984;103(1):26–35. doi: 10.1007/BF00451315. [DOI] [PubMed] [Google Scholar]
- 45.Lee WP, Randolph MA, Weiland AJ, Yaremchuk MJ. Prolonged survival of vascularized limb tissue allografts by donor irradiation. J Surg Res. 1995;59(5):578–588. doi: 10.1006/jsre.1995.1208. [DOI] [PubMed] [Google Scholar]
- 46.Lee WP, Pan YC, Kesmarky S, Randolph MA, Fiala TS, Amarante MT, Weiland AJ, Yaremchuk MJ. Experimental orthotopic transplantation of vascularized skeletal allografts: functional assessment and long-term survival. Plast Reconstr Surg. 1995;95(2):336–349. [PubMed] [Google Scholar]
- 47.Muramatsu K. Experimental studies on vascularized allogeneic joint transplantation in rats. Nippon Seikeigeka Gakkai Zasshi. 1992;66(4):315–325. [PubMed] [Google Scholar]
- 48.Ohno T, Pelzer M, Larsen M, Friedrich PF, Bishop AT. Host-derived angiogenesis maintains bone blood flow after withdrawal of immunosuppression. Microsurgery. 2007;27(8):657–663. doi: 10.1002/micr.20427. [DOI] [PubMed] [Google Scholar]
- 49.Pelzer M, Larsen M, Chung YG, Ohno T, Platt JL, Friedrich PF, Bishop AT. Short-term immunosuppression and surgical neoangiogenesis with host vessels maintains long-term viability of vascularized bone allografts. J Orthop Res. 2007;25(3):370–377. doi: 10.1002/jor.20313. [DOI] [PubMed] [Google Scholar]
- 50.Lee WP, Yaremchuk MJ, Pan YC, Randolph MA, Tan CM, Weiland AJ. Relative antigenicity of components of a vascularized limb allograft. Plast Reconstr Surg. 1991;87(3):401–411. doi: 10.1097/00006534-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Pelzer M, Larsen M, Friedrich PF, Aleff RA, Bishop AT. Repopulation of vascularized bone allotransplants with recipient-derived cells: detection by laser capture microdissection and real-time PCR. J Orthop Res. 2009;27(11):1514–1520. doi: 10.1002/jor.20915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giessler GA, Zobitz M, Friedrich PF, Bishop AT. Transplantation of a vascularized rabbit femoral diaphyseal segment: mechanical and histologic properties of a new living bone transplantation model. Microsurgery. 2008;28(4):291–299. doi: 10.1002/micr.20492. [DOI] [PubMed] [Google Scholar]
- 53.Friedrich JB, Moran SL, Bishop AT, Wood CM, Shin AY. Free vascularized fibular graft salvage of complications of long-bone allograft after tumor reconstruction. J Bone Joint Surg [Am] 2008;90(1):93–100. doi: 10.2106/JBJS.G.00551. [DOI] [PubMed] [Google Scholar]
- 54.Lee WP, Pan YC, Kesmarky S, Randolph MA, Fiala TS, Amarante MT, Weiland AJ, Yaremchuk MJ. Experimental orthotopic transplantation of vascularized skeletal allografts: functional assessment and long-term survival. Plast Reconstr Surg. 1995;95(2):336–349. discussion 350–333. [PubMed] [Google Scholar]