Abstract
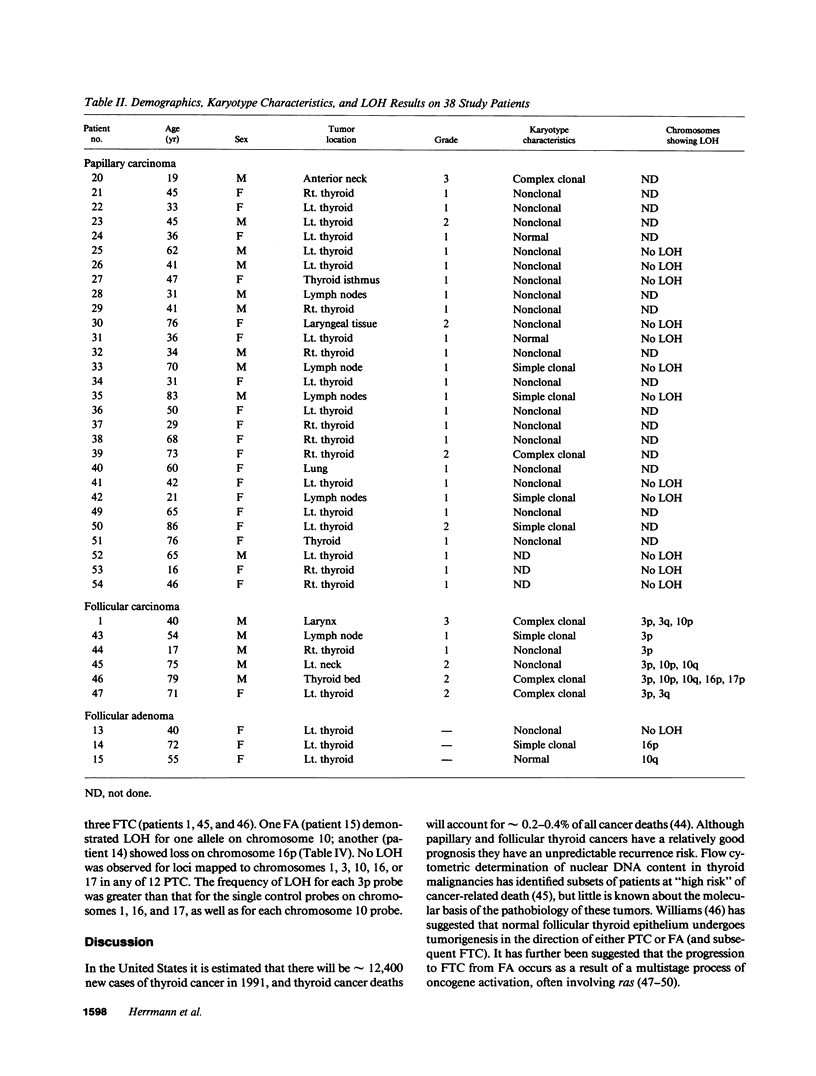
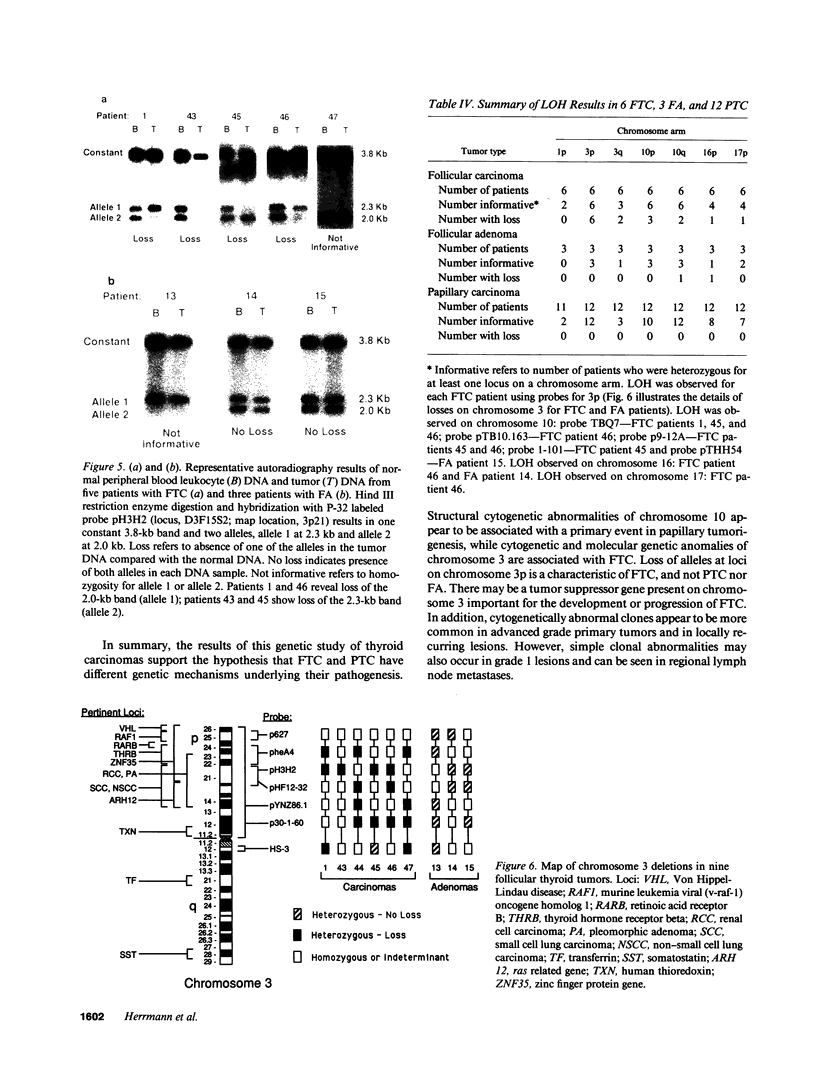
Cytogenetic studies have shown frequent clonal abnormalities in papillary carcinoma (PTC) and follicular carcinoma (FTC). Loss of heterozygosity (LOH) may suggest the presence of tumor suppressor genes and has not been reported in these neoplasms. These studies were undertaken to determine if consistent chromosomal abnormalities are associated with thyroid cancer, to determine likely regions for molecular genetic investigations, and to determine if there is allelic loss in thyroid tumors. Cytogenetic analysis of 26 PTC and 5 FTC showed clonal abnormalities in 9 and included -Y, +5, or inv(10)(q11.2q21.2) in PTC, and -Y or near haploidy in FTC. Using DNA probes specific for chromosomes 1, 3, 10, 16, and 17, we carried out restriction fragment length polymorphism analysis on 6 FTC, 3 follicular adenomas (FA), and 12 PTC. LOH of all informative loci on chromosome 3p was observed in all 6 FTC, but not in FA or PTC. No LOH was observed for loci mapped to chromosome 10 in PTC. Our results suggest: cytogenetic abnormalities of chromosome 10q are associated with PTC; cytogenetic and molecular abnormalities of chromosome 3 are associated with FTC; and a tumor suppressor gene may be present on the short arm of chromosome 3 important for the development or progression of FTC.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonini P., Venuat A. M., Linares G., Caillou B., Berger R., Parmentier C. A translocation (7;10)(q35;q21) in a differentiated papillary carcinoma of the thyroid. Cancer Genet Cytogenet. 1989 Aug;41(1):139–144. doi: 10.1016/0165-4608(89)90118-0. [DOI] [PubMed] [Google Scholar]
- Bartnitzke S., Herrmann M. E., Lobeck H., Zuschneid W., Neuhaus P., Bullerdiek J. Cytogenetic findings on eight follicular thyroid adenomas including one with a t(10;19). Cancer Genet Cytogenet. 1989 May;39(1):65–68. doi: 10.1016/0165-4608(89)90230-6. [DOI] [PubMed] [Google Scholar]
- Becher R., Gibas Z., Karakousis C., Sandberg A. A. Nonrandom chromosome changes in malignant melanoma. Cancer Res. 1983 Oct;43(10):5010–5016. [PubMed] [Google Scholar]
- Bondeson L., Bengtsson A., Bondeson A. G., Dahlenfors R., Grimelius L., Wedell B., Mark J. Chromosome studies in thyroid neoplasia. Cancer. 1989 Aug 1;64(3):680–685. doi: 10.1002/1097-0142(19890801)64:3<680::aid-cncr2820640319>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Boring C. C., Squires T. S., Tong T. Cancer statistics, 1991. CA Cancer J Clin. 1991 Jan-Feb;41(1):19–36. doi: 10.3322/canjclin.41.1.19. [DOI] [PubMed] [Google Scholar]
- Bragg T., Nakamura Y., Fujimoto E., O'Connell P., Leppert M., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence (pTB10.163) on chromosome 10 [D10S22]. Nucleic Acids Res. 1988 May 11;16(9):4185–4185. doi: 10.1093/nar/16.9.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg T., Nakamura Y., Jones C., White R. Isolation and mapping of a polymorphic DNA sequence (cTBQ7) on chromosome 10 [D10S28]. Nucleic Acids Res. 1988 Dec 9;16(23):11395–11395. doi: 10.1093/nar/16.23.11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. D., Bergstralh E. J., van Heerden J. A., McConahey W. M. Follicular thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1991 Jan;66(1):11–22. doi: 10.1016/s0025-6196(12)61170-7. [DOI] [PubMed] [Google Scholar]
- Donghi R., Sozzi G., Pierotti M. A., Biunno I., Miozzo M., Fusco A., Grieco M., Santoro M., Vecchio G., Spurr N. K. The oncogene associated with human papillary thyroid carcinoma (PTC) is assigned to chromosome 10 q11-q12 in the same region as multiple endocrine neoplasia type 2A (MEN2A). Oncogene. 1989 Apr;4(4):521–523. [PubMed] [Google Scholar]
- Farrer L. A., Castiglione C. M., Kidd J. R., Myers S., Carson N., Simpson N. E., Kidd K. K. A linkage group of five DNA markers on human chromosome 10. Genomics. 1988 Jul;3(1):72–77. doi: 10.1016/0888-7543(88)90162-0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fusco A., Grieco M., Santoro M., Berlingieri M. T., Pilotti S., Pierotti M. A., Della Porta G., Vecchio G. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nature. 1987 Jul 9;328(6126):170–172. doi: 10.1038/328170a0. [DOI] [PubMed] [Google Scholar]
- Grieco M., Santoro M., Berlingieri M. T., Melillo R. M., Donghi R., Bongarzone I., Pierotti M. A., Della Porta G., Fusco A., Vecchio G. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990 Feb 23;60(4):557–563. doi: 10.1016/0092-8674(90)90659-3. [DOI] [PubMed] [Google Scholar]
- Hedinger C., Williams E. D., Sobin L. H. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer. 1989 Mar 1;63(5):908–911. doi: 10.1002/1097-0142(19890301)63:5<908::aid-cncr2820630520>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Heim S., Mandahl N., Jin Y., Strömblad S., Lindström E., Salford L. G., Mitelman F. Trisomy 7 and sex chromosome loss in human brain tissue. Cytogenet Cell Genet. 1989;52(3-4):136–138. doi: 10.1159/000132863. [DOI] [PubMed] [Google Scholar]
- Holm T., Nakamura Y., Ballard L., O'Connell P., Leppert M., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence pTHH54 on chromosome 10 [D10S13]. Nucleic Acids Res. 1988 Jan 11;16(1):372–372. doi: 10.1093/nar/16.1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R. B., Hay I. D., Herath J. F., Schultz C. G., Spurbeck J. L., Grant C. S., Goellner J. R., Dewald G. W. Frequent occurrence of cytogenetic abnormalities in sporadic nonmedullary thyroid carcinoma. Cancer. 1990 Sep 15;66(6):1213–1220. doi: 10.1002/1097-0142(19900915)66:6<1213::aid-cncr2820660622>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Khosla S., Patel V. M., Hay I. D., Schaid D. J., Grant C. S., van Heerden J. A., Thibodeau S. N. Loss of heterozygosity suggests multiple genetic alterations in pheochromocytomas and medullary thyroid carcinomas. J Clin Invest. 1991 May;87(5):1691–1699. doi: 10.1172/JCI115186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok K., Osinga J., Carritt B., Davis M. B., van der Hout A. H., van der Veen A. Y., Landsvater R. M., de Leij L. F., Berendsen H. H., Postmus P. E. Deletion of a DNA sequence at the chromosomal region 3p21 in all major types of lung cancer. Nature. 1987 Dec 10;330(6148):578–581. doi: 10.1038/330578a0. [DOI] [PubMed] [Google Scholar]
- Kovacs G., Erlandsson R., Boldog F., Ingvarsson S., Müller-Brechlin R., Klein G., Sümegi J. Consistent chromosome 3p deletion and loss of heterozygosity in renal cell carcinoma. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1571–1575. doi: 10.1073/pnas.85.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine N. R., Mayall E. S., Wyllie F. S., Farr C. J., Hughes D., Padua R. A., Thurston V., Williams E. D., Wynford-Thomas D. Activated ras oncogenes in human thyroid cancers. Cancer Res. 1988 Aug 15;48(16):4459–4463. [PubMed] [Google Scholar]
- Lemoine N. R., Mayall E. S., Wyllie F. S., Williams E. D., Goyns M., Stringer B., Wynford-Thomas D. High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene. 1989 Feb;4(2):159–164. [PubMed] [Google Scholar]
- Liou G. I., Li Y., Wang C., Fong S. L., Bhattacharya S., Bridges C. D. Bgl II RFLP recognized by a human IRBP cDNA localized to chromosome 10. Nucleic Acids Res. 1987 Apr 10;15(7):3196–3196. doi: 10.1093/nar/15.7.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt M., Litt R., Ballard L., Leppert M. An anonymous single-copy clone, p30-1-60, identifies a frequent RFLP on chromosome 3p [HGM9 no. D3S86]. Nucleic Acids Res. 1989 Apr 11;17(7):2883–2883. doi: 10.1093/nar/17.7.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt M., Mueller O. T., Shows T. B., Litt R. A single copy subclone, p1-101, from cosmid 3-3B, defines three RFLPs on 10pter-q23 [HGM9 no. D10S4]. Nucleic Acids Res. 1987 Mar 25;15(6):2783–2783. doi: 10.1093/nar/15.6.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark J., Ekedahl C., Dahlenfors R., Westermark B. Cytogenetical observations in five human anaplastic thyroid carcinomas. Hereditas. 1987;107(2):163–174. doi: 10.1111/j.1601-5223.1987.tb00281.x. [DOI] [PubMed] [Google Scholar]
- McDermid H. E., Goodfellow P. J., Duncan A. M., Brasch K. R., Simpson N. E., Souza C. D., Holden J. J., White B. N. A polymorphic locus, D10S5, at 10q21.1. Nucleic Acids Res. 1987 Jul 10;15(13):5498–5498. doi: 10.1093/nar/15.13.5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman F., Kaneko Y., Trent J. M. Report of the committee on chromosome changes in neoplasia. Cytogenet Cell Genet. 1990;55(1-4):358–386. doi: 10.1159/000133022. [DOI] [PubMed] [Google Scholar]
- Mooibroek H., Osinga J., Postmus P. E., Carritt B., Buys C. H. Loss of heterozygosity for a chromosome 3 sequence presumably at 3p21 in small cell lung cancer. Cancer Genet Cytogenet. 1987 Aug;27(2):361–365. doi: 10.1016/0165-4608(87)90020-3. [DOI] [PubMed] [Google Scholar]
- Müllenbach R., Lagoda P. J., Welter C. An efficient salt-chloroform extraction of DNA from blood and tissues. Trends Genet. 1989 Dec;5(12):391–391. [PubMed] [Google Scholar]
- Nakamura Y., Ballard L., Leppert M., O'Connell P., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence (pYNZ22) on chromosome 17p [D17S30]. Nucleic Acids Res. 1988 Jun 24;16(12):5707–5707. doi: 10.1093/nar/16.12.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Bragg T., Myers R., O'Connell P., Leppert M., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence (pTB10.171) on chromosome 10 [D10S19]. Nucleic Acids Res. 1988 May 11;16(9):4187–4187. doi: 10.1093/nar/16.9.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Culver M., Gillilan S., O'Connell P., Leppert M., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence pYNZ86.1 on chromosome 3 (D3S30). Nucleic Acids Res. 1987 Dec 10;15(23):10079–10079. doi: 10.1093/nar/15.23.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor S. L., Carritt B. Report of the committee on the genetic constitution of chromosome 3. Cytogenet Cell Genet. 1990;55(1-4):92–96. doi: 10.1159/000132999. [DOI] [PubMed] [Google Scholar]
- Naylor S. L., Sakaguchi A. Y., Barker D., White R., Shows T. B. DNA polymorphic loci mapped to human chromosomes 3, 5, 9, 11, 17, 18, and 22. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2447–2451. doi: 10.1073/pnas.81.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah E., Balogh E., Bojan F., Juhasz F., Stenszky V., Farid N. R. Cytogenetic analyses of three papillary carcinomas and a follicular adenoma of the thyroid. Cancer Genet Cytogenet. 1990 Jan;44(1):119–129. doi: 10.1016/0165-4608(90)90204-n. [DOI] [PubMed] [Google Scholar]
- Pedersen M. I., Bennett J. W., Wang N. Nonrandom chromosome structural aberrations and oncogene loci in human malignant melanoma. Cancer Genet Cytogenet. 1986 Feb 1;20(1-2):11–27. doi: 10.1016/0165-4608(86)90103-2. [DOI] [PubMed] [Google Scholar]
- Pierre R. V., Hoagland H. C. 45,X cell lines in adult men: loss of Y chromosome, a normal aging phenomenon? Mayo Clin Proc. 1971 Jan;46(1):52–55. [PubMed] [Google Scholar]
- Popescu N. C., Chahinian A. P., DiPaolo J. A. Nonrandom chromosome alterations in human malignant mesothelioma. Cancer Res. 1988 Jan 1;48(1):142–147. [PubMed] [Google Scholar]
- Reeders S. T., Breuning M. H., Davies K. E., Nicholls R. D., Jarman A. P., Higgs D. R., Pearson P. L., Weatherall D. J. A highly polymorphic DNA marker linked to adult polycystic kidney disease on chromosome 16. Nature. 1985 Oct 10;317(6037):542–544. doi: 10.1038/317542a0. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Rouleau G. A., Ozelius L. J., Lane A. H., Farmer G. E., Lamiell J. M., Haines J., Yuen J. W., Collins D., Majoor-Krakauer D. Von Hippel-Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature. 1988 Mar 17;332(6161):268–269. doi: 10.1038/332268a0. [DOI] [PubMed] [Google Scholar]
- Smeets W., Pauwels R., Laarakkers L., Debruyne F., Geraedts J. Chromosomal analysis of bladder cancer. III. Nonrandom alterations. Cancer Genet Cytogenet. 1987 Nov;29(1):29–41. doi: 10.1016/0165-4608(87)90028-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spurbeck J. L., Carlson R. O., Allen J. E., Dewald G. W. Culturing and robotic harvesting of bone marrow, lymph nodes, peripheral blood, fibroblasts, and solid tumors with in situ techniques. Cancer Genet Cytogenet. 1988 May;32(1):59–66. doi: 10.1016/0165-4608(88)90312-3. [DOI] [PubMed] [Google Scholar]
- Teyssier J. R., Liautaud-Roger F., Ferre D., Patey M., Dufer J. Chromosomal changes in thyroid tumors. Relation with DNA content, karyotypic features, and clinical data. Cancer Genet Cytogenet. 1990 Dec;50(2):249–263. doi: 10.1016/0165-4608(90)90184-c. [DOI] [PubMed] [Google Scholar]
- Teyssier J. R. Nonrandom chromosomal changes in human solid tumors: application of an improved culture method. J Natl Cancer Inst. 1987 Dec;79(6):1189–1198. [PubMed] [Google Scholar]
- Teyssier J. R. The chromosomal analysis of human solid tumors. A triple challenge. Cancer Genet Cytogenet. 1989 Jan;37(1):103–125. doi: 10.1016/0165-4608(89)90080-0. [DOI] [PubMed] [Google Scholar]
- Vanni R., Scarpa R. M., Nieddu M., Usai E. Cytogenetic investigation on 30 bladder carcinomas. Cancer Genet Cytogenet. 1988 Jan;30(1):35–42. doi: 10.1016/0165-4608(88)90090-8. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Williams E. D. The aetiology of thyroid tumours. Clin Endocrinol Metab. 1979 Mar;8(1):193–207. doi: 10.1016/s0300-595x(79)80017-1. [DOI] [PubMed] [Google Scholar]
- Wright P. A., Lemoine N. R., Mayall E. S., Wyllie F. S., Hughes D., Williams E. D., Wynford-Thomas D. Papillary and follicular thyroid carcinomas show a different pattern of ras oncogene mutation. Br J Cancer. 1989 Oct;60(4):576–577. doi: 10.1038/bjc.1989.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie F. S., Lemoine N. R., Williams E. D., Wynford-Thomas D. Structure and expression of nuclear oncogenes in multi-stage thyroid tumorigenesis. Br J Cancer. 1989 Oct;60(4):561–565. doi: 10.1038/bjc.1989.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg E., Oosterhuis J. W., de Jong B., Buist J., Vos A., Dam A., Vermeij B. Cytogenetics of thyroid follicular adenomas. Cancer Genet Cytogenet. 1990 Feb;44(2):217–222. doi: 10.1016/0165-4608(90)90050-k. [DOI] [PubMed] [Google Scholar]