Abstract
Autologous platelet-rich plasma (PRP) may enhance wound healing through the formation of a platelet plug that provides both hemostasis and the secretion of biologically active proteins, including growth factors such as platelet-derived growth factor, transforming growth factor (TGF)-β, TGF-β2, and epidermal growth factor. The release of these growth factors into the wound may create an environment more conducive to tissue repair and could accelerate postoperative wound healing. To our knowledge, there are no reports of combining the use of PRP with curative diabetic foot surgery. This article provides a summary of the literature regarding PRP and wound healing and presents a case of a 49-year-old man with diabetes and a three-month history of a deep, nonhealing plantar hallux wound in which PRP was combined with a first metatarsophalangeal joint arthroplasty. Through the use of the PRP and bioengineered tissue to supplement curative diabetic foot surgery, the patient healed uneventfully at seven weeks.
Keywords: diabetic, foot surgery, platelet rich plasma, wound
Introduction
People with diabetes often develop many factors such as neuropathy, tissue hypoxia, and hyperglycemia-related immunopathy that contribute to their increased suscepti-bility to infection and decreased ability to heal. This is evidenced by research studies that report defects in matrix-related cells and imbalances in key protesases, cytokines, and growth factors.1–3
An increased understanding of the physiological roles of platelets in wound healing has furthered the role of platelets as a novel therapeutic tool in wounds.4 Studies have demonstrated a significant increase in the limb salvage rate/amputation prevention among high-risk diabetes patients,5–8 as well as reduced total treatment expenses9,10 when combined with a comprehensive local wound care protocol.
Regarding lower extremity wounds, the plantar hallux is one of the most common locations of foot wounds in people with diabetes.11,12 Armstrong and colleagues13 reported that first metatarsophalangeal joint arthroplasty is a safe and effective curative procedure in the treatment of noninfected, nonischemic wounds of the plantar hallux, leading to faster and more durable healing when compared with a matched cohort. We are unaware of reports in the literature combining autologous platelet-rich plasma (PRP) with curative diabetic foot surgery.
Significance of Platelet-Rich Plasma
In the natural healing process of the body, surgical intervention and trauma initiate the healing cascade through platelet activation (α-degranulation) and the formation of a platelet plug and blood clot, providing both hemostasis and the secretion of biologically active proteins. The naturally occurring hematoma is composed of 95% red blood cells, 4% platelets, and 1% white blood cells. Through the use of PRP, hematomas with a composition of 95% platelets, 4% red blood cells, and 1% white blood cells can be created.14,15 Platelet-rich plasma also contains the full complement of clotting factors and secretory proteins. Among the 30 biologically active proteins [e.g., platelet-derived growth factor (PDGF), transforming growth factor (TGF)-β1, TGF-β2, platelet factor IV, interleukin-1, platelet-derived angiogenesis factor, vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), insulin-like growth factor (IGF), osteocalcin, osteonectin, fibrinogen, vitronectin, fibronectin, and thrombospondin-1], the bulk of research focus has centered around PDGF, TGF-β, VEGF, EGF, and IGF.14–21
Autologous PRP is a limited volume of plasma enriched in platelets that is derived from the patient's whole blood. The whole blood is then separated by centrifuge into layers of platelet-poor plasma, red blood cells, and platelets with white blood cells. The platelet concentrate is then activated through thrombin generation by an anticoagulant, either ascorbic acid or 10% calcium citrate, resulting in the formation of a fibrin scaffold. This activation of the platelets causes the release of concentrated growth factors and proteins locally to create an environment conducive to tissue repair and accelerated postoperative wound healing.21–26 Platelet-derived growth factor, TGF-β, VEGF, and EGF have all been shown to be increased three to seven times in autologous PRP.27 The low concentration of IGF in PRP is to be expected, as it is secreted by the liver into the blood plasma, which is removed in the separation process, and platelets themselves only generate negligible amounts of IGF.28
Platelet-derived growth factor is the only growth factor approved by the Food and Drug Administration for clinical use, and it has been shown through phase III of a multicenter clinical human trial to generate a 10% increase in the rate of complete healing in diabetic wounds.29–31 Topical application of platelet-derived wound- healing factors has been shown to stimulate repair of chronically nonhealing human wounds, thereby resulting in accelerated granulation tissue formation and epithelialization.32 Platelet-derived growth factor is a key growth factor in wound healing that is secreted by macrophages, endothelial cells, fibroblasts, and megakaryocytes. Its actions include activation of immune cells and fibro- blasts, extracellular matrix deposition, collagen synthesis, matrix metalloproteinase and tissue inhibitor of metallo-proteinase synthesis, and angiogenesis.33–35 Platelet-derived growth factor, a powerful mitogen for fibroblasts and smooth muscle cells, is involved in all three phases of healing, including angiogenesis, formation of fibrous tissue, and reepithelialization. Release of PDGF into the wound bed also has a chemotactic effect on monocytes, neutrophils, fibtoblasts, mesenchymal stem cells, and osteoblasts.36 Platelet-derived growth factor is a stable protein and has been shown to have resistance to changes in heat and pH. This property enables PDGF to be resistant to destructive proteases present in the hostile, metalloproteinase-rich environment.37
TGF-β is a potent inhibitor of immune reactivity that attracts macrophages that then stimulate the secretion of additional cytokines.38 TGF-β also enhances fibroblast and smooth muscle cell chemotaxis, induces the deposition of bone matrix, and stimulates the synthesis of type I collagen.27 TGF-β also promotes angiogenesis and extracellular matrix production.39,40
TGF-β2 has shown improvement in healing rates in diabetic foot ulcers in phase II of a clinical human trial, but TGF-β1 is the predominant isoform in wound healing and has never been tested in human clinical trials. Studies conducted on young rabbits have shown it to increase wound healing by new granulation tissue formation and reepithelialization in ischemic and non-ischemic wounds.41
Many different cells produce VEGF or release factors that regulate the expression of VEGF. Fibroblast growth factor-4, PDGF, tumor necrosis factor-α, IGF, and some interleukins potentiate the effects of VEGF while nitric oxide enhances VEGF's effects on blood vessels and hydrogen peroxide activates it.42,43 VEGF indirectly stimulates endothelial growth and promotes angiogenesis while enhancing capillary permeability and leakage of tissue plasma extracellularly.38,44 Unlike PDGF, VEGF almost exclusively binds to endothelial cells45,46 and does not act on macrophages, fibroblasts, or smooth muscle. Human clinical trials studying the effects of VEGF on wound healing have not yet been conducted, but animal studies on rabbits have shown a doubling of granulation tissue formation in ischemic wounds.47
Epidermal growth factor is a cytokine that has been linked with angiogenesis and collagen deposition at wound sites, and it has been shown to stimulate wound repair in fibroblasts and epithelial cells.38,44 It is a mitogen for fibroblasts, endothelial cells, and keratinocytes, and it also stimulates reepithelialization, augments angio-genesis, and influences the synthesis and turnover of extracellular matrix.40
Case Presentation
The following is a presentation of a 49-year-old man with type 2 diabetes that presented with a three-month history of a nonhealing nonischemic neuropathic plantar hallux wound of his right foot. Prior to surgery, the patient's physical exam revealed a full-thickness non-infected ulceration with a punched-out appearance at the plantar hallux of the right foot measuring 1.5 cm around with depth of 2–3 mm that did not probe to bone (Figure 1). There was a granular base with a small central area of fibrotic tissue. There was no undermining of the wound edges, no purulence, and no serosanguinous drainage noted. Dorsiflexion motion at the first metatarsophalangeal joint (MTPJ) was <10°, both loaded and unloaded. A dorsal contracture of the second digit at the MTPJ and corresponding flexion contracture at the proximal interphalangeal joint (PIPJ) was also observed in the right foot.
Figure 1.
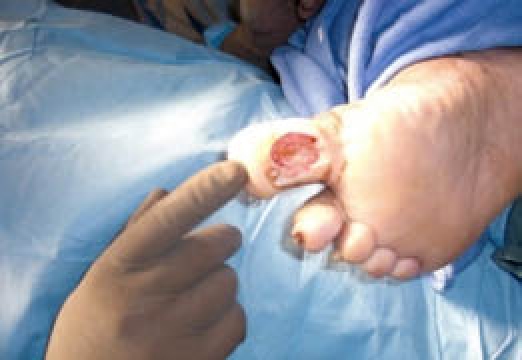
Noninfected ulceration at the plantar hallux of the right foot.
The patient was treated surgically with a combination of arthroplasties, a first MTPJ arthroplasty (Keller) and second PIPJ arthroplasty, and autologous PRP as a curative procedure for a plantar hallux wound. The patient's past medical history included diabetes, hypertension, and hypercholesterolemia. Previous wound care included local wound care with serial sharp debridements, topical aquacell, and traditional offloading.
The patient was treated surgically with sharp debridement of the wound and an arthroplasty of the hallux and an arthroplasty of the second proximal interphalangeal joint (Figure 2). Additionally, the wound was filled with an injection of 3 cc of Angel (Sorin Inc., Mirandola, Italy) autologous PRP to fill the wound defect (Figures 3–5).
Figure 2.
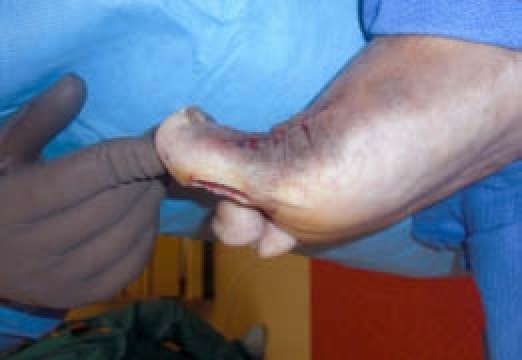
Increased dorsiflexion achieved through the arthroplasty of the hallux.
Figure 3.
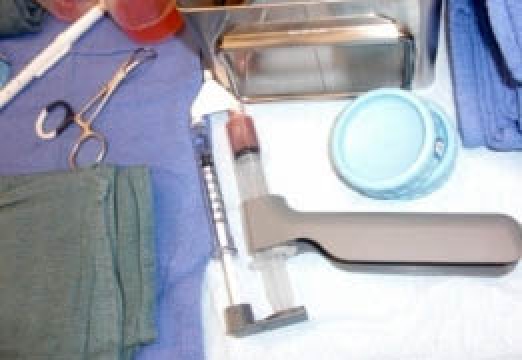
Dual syringe used for PRP.
Figure 5.
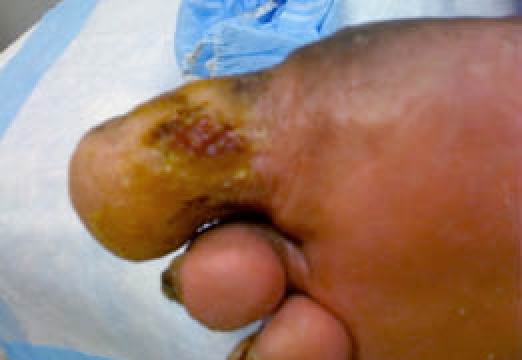
Superficial wound at four weeks postoperation. At this time, a Dermagraft bioengineered graft was applied to the remaining superficial wound, and by seven weeks postoperation, the wound had completely epithelized.
Figure 4.
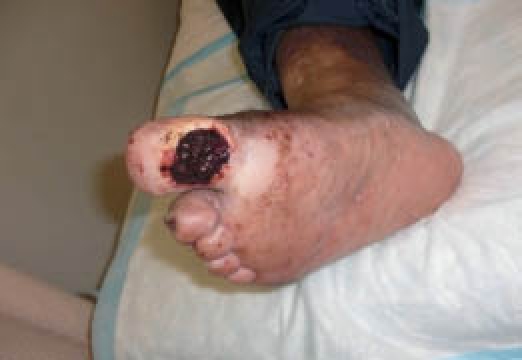
Wound defect filled with 3 cc of PRP.
Conclusion
Platelet concentrates may be potentially useful in wound- healing applications because they function as both a tissue sealant and a delivery system that contains a variety of mitogenic and chemotactic growth factors. Studies have shown that platelets can be sequestered and concentrated eight-fold from whole blood without premature activation.48 There is an increasing cause for optimism in the use of PRP in treatment of diabetic and other chronic wounds as numerous clinical trials have reported favorable results in wounds treated with PRP and platelet-rich concentrate.49–56
This article described the novel use of autologous PRP to augment healing of a stalled plantar foot ulceration when combined with curative diabetic foot surgery. Proper offloading of the wound afforded by the first MTPJ resectional arthroplasty was paramount to healing in our patient. The authors look forward to further works to better determine whether augmentation with PRP during curative diabetic foot surgery provides a more consistent, cost-effective treatment approach in a difficult population.
Abbreviations
- EGF
epidermal growth factor
- IGF
insulin-like growth factor
- MTPJ
metatarsophalangeal joint
- PDGF
platelet-derived growth factor
- PIPJ
proximal interphalangeal joint
- PRP
platelet-rich plasma
- TGF
transforming growth factor
- VEGF
vascular endothelial growth factor
References
- 1.Nwomeh BC, Yager DR, Cohen IK. Physiology of the chronic wound. Clin Plast Surg. 1998;25(3) 341.56. [PubMed] [Google Scholar]
- 2.Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen. 1996;4(4):411–420. doi: 10.1046/j.1524-475X.1996.40404.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen C, Schultz GS, Bloch M, Edwards PD, Tebes S, Mast BA. Molecular and mechanistic validation of delayed healing rat wounds as a model for human chronic wounds. Wound Repair Regen. 1999;7(6):486–494. doi: 10.1046/j.1524-475x.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- 4.Slavkin HC, Bartold PM. Challenges and potential in tissue engineering. Periodontol 2000. 2006;41:9–15. doi: 10.1111/j.1600-0757.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- 5.Doucette MM, Fylling C, Knighton DR. Amputation prevention in a high-risk population through a comprehensive wound-healing protocol. Arch Phys Med Rehabil. 1989;70(10):780–785. [PubMed] [Google Scholar]
- 6.Knighton DR, Fylling CP, Doucette MM. Wound healing and amputation in a high-risk diabetic population. Wounds. 1989;2:107–114. [Google Scholar]
- 7.Knighton DR, Fylling CP, Fiegel VD, Cerra F. Amputation prevention in an independently reviewed at-risk diabetic population using a comprehensive wound care protocol. Am J Surg. 1990;160(5):466–471. doi: 10.1016/s0002-9610(05)81005-0. [DOI] [PubMed] [Google Scholar]
- 8.Glover JL, Weingarten MS, Buchbinder DS, Poucher RL, Deitrick GA, 3rd, Fylling CP. A 4-year outcome-based retrospective study of wound healing and limb salvage in patients with chronic wounds. Adv Wound Care. 1997;10(1):33–38. [PubMed] [Google Scholar]
- 9.Fylling CP, McKeown PC. Cost and healing efficacy of treating diabetic foot ulcers in a comprehensive wound management program with growth factor technology. Diabetes. 1990;39:18S. [Google Scholar]
- 10.Bentkover JD, Champion AH. Economic evaluation of alternative methods of treatment for diabetic foot ulcer patients: cost-effectiveness of platelet releasate and wound care clinics. Wounds. 1993;5:207–215. [Google Scholar]
- 11.Armstrong DG, Lavery LA, Bushman TR. Peak foot pressures influence healing time of diabetic ulcers treated with total contact casts. J Rehabil Res Dev. 1998;35(1):1–5. [PubMed] [Google Scholar]
- 12.Lavery LA, Armstrong DG, Vela SA, Quebedeaux TL, Fleischli JG. Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch Intern Med. 1998;158(2):157–162. doi: 10.1001/archinte.158.2.157. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong DG, Lavery LA, Vazquez JR, Short B, Kimbriel HR, Nixon BP, Boulton AJ. Clinical efficacy of the first meta-tarsophalangeal joint arthroplasty as a curative procedure for hallux interphalangeal joint wounds in patients with diabetes. Diabetes Care. 2003;26(12):3284–3287. doi: 10.2337/diacare.26.12.3284. [DOI] [PubMed] [Google Scholar]
- 14.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 16.Martinowitz U, Spotnitz WD. Fibrin tissue adhesives. Thromb Haemost. 1997;78(1):661–666. [PubMed] [Google Scholar]
- 17.Lin PH, Hirko MK, von Fraunhofer JA, Greisler HP. Chu CC, von Fraunhofer JA, Greisler HP. Wound closure biomaterials and devices. Boca Raton: CRC Press; 1997. Wound healing and inflammatory response to biomaterials; pp. 7–24. [Google Scholar]
- 18.Slater M, Patava J, Kingham K, Mason RS. Involvement of platelets in stimulating osteogenic activity. J Orthop Res. 1995;13(5):655–663. doi: 10.1002/jor.1100130504. [DOI] [PubMed] [Google Scholar]
- 19.Kevy SV, Jacobson MS. Preparation of growth factor enriched autologous platelet gel. Presented at the 27th Annual Meeting of the Society for Biomaterials, St. Paul; MN. 2001. April 26. [Google Scholar]
- 20.Spencer EM, Tokunaga A, Hunt TK. Insulin-like growth factor binding protein-3 is present in the alpha-granules of platelets. Endocrinology. 1993;132(3):996–1001. doi: 10.1210/endo.132.3.7679986. [DOI] [PubMed] [Google Scholar]
- 21.Harrison P, Cramer EM. Platelet alpha-granules. Blood Rev. 1993;7(1):52–62. doi: 10.1016/0268-960x(93)90024-x. [DOI] [PubMed] [Google Scholar]
- 22.Gibble JW, Ness PM. Fibrin glue: the perfect operative sealant? Transfusion. 1990;30(8):741–747. doi: 10.1046/j.1537-2995.1990.30891020337.x. [DOI] [PubMed] [Google Scholar]
- 23.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 24.Ogino Y, Ayukawa Y, Tsukiyama Y, Koyano K. The effect of platelet-rich plasma on the cellular response of rat bone marrow cells in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(3):302–307. doi: 10.1016/j.tripleo.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 26.Doucette MM, Fylling C, Knighton DR. Amputation prevention in a high-risk population through a comprehensive wound-healing protocol. Arch Phys Med Rehabil. 1989;70(10):780–785. [PubMed] [Google Scholar]
- 27.Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114(6):1502–1508. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 28.Rubin R, Baserga R. Biology of disease: insulin-like growth factor receptor, its role in cell proliferation, apoptosis, and tumorigenicity. Lab Invest. 1995;73(3):311–331. [PubMed] [Google Scholar]
- 29.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21(5):822–827. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- 30.d'fHemecourt PA, Smiell JM, Karim MR. Sodium carboxy-methylcellulose aqueous-based gel versus becaplermin gel in patients with nonhealing, lower extremity diabetic ulcers. Wounds. 1998;10(3):69. [Google Scholar]
- 31.Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen. 1999;7(5):335–346. doi: 10.1046/j.1524-475x.1999.00335.x. [DOI] [PubMed] [Google Scholar]
- 32.Knighton DR, Ciresi KF, Fiegel VD, Austin LL, Butler EL. Classification and treatment of chronic nonhealing wounds. Successful treatment with autologous platelet-derived wound healing factors (PDWHF) Ann Surg. 1986;204(3):322–330. doi: 10.1097/00000658-198609000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deuel TF, Kawahara RS, Mustoe TA, Pierce AF. Growth factors and wound healing: platelet-derived growth factor as a model cytokine. Annu Rev Med. 1991;42:567–584. doi: 10.1146/annurev.me.42.020191.003031. [DOI] [PubMed] [Google Scholar]
- 34.Ross R. Platelet-derived growth factor. Lancet. 1989;1(8648):1179–1182. doi: 10.1016/s0140-6736(89)92760-8. [DOI] [PubMed] [Google Scholar]
- 35.Lobmann R, Schultz G, Lehnert H. Proteases and the diabetic foot syndrome: mechanisms and therapeutic implications. Diabetes Care. 2005;28(2):461–471. doi: 10.2337/diacare.28.2.461. [DOI] [PubMed] [Google Scholar]
- 36.Hosgood G. Wound healing. The role of platelet-derived growth factor and transforming growth factor beta. Vet Surg. 1993;22(6):490–495. doi: 10.1111/j.1532-950x.1993.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 37.Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet-derived growth factor in wound healing. J Cell Biochem. 1991;45(4):319–326. doi: 10.1002/jcb.240450403. [DOI] [PubMed] [Google Scholar]
- 38.Pfeilschifter J, Oechsner M, Naumann A, Gronwald RG, Minne HW, Ziegler R. Stimulation of bone matrix apposition in vitro by local growth factors: a comparison between insulin-like growth factor I, platelet-derived growth factor, and transforming growth factor beta. Endocrinology. 1990;127(1):69–75. doi: 10.1210/endo-127-1-69. [DOI] [PubMed] [Google Scholar]
- 39.Hosgood G. Wound healing. The role of platelet-derived growth factor and transforming growth factor beta. Vet Surg. 1993;22(6):490–495. doi: 10.1111/j.1532-950x.1993.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 40.Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg. 2003;90(2):133–146. doi: 10.1002/bjs.4019. [DOI] [PubMed] [Google Scholar]
- 41.Wu L, Xia YP, Roth SI, Gruskin E, Mustoe TA. Transforming growth factor-b1 fails to stimulate wound healing and impairs its signal transduction in an aged ischemic ulcer model: importance of oxygen and age. Am J Pathol. 1999;154:301–309. doi: 10.1016/s0002-9440(10)65276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9–22. [PubMed] [Google Scholar]
- 43.Brauchle M, Funk JO, Kind P, Werner S. Ultraviolet B and H2O2 are potent inducers of vascular endothelial growth factor expression in cultured keratinocytes. J Biol Chem. 1996;271(36):21793–21797. doi: 10.1074/jbc.271.36.21793. [DOI] [PubMed] [Google Scholar]
- 44.Diegelmann RF, Evans MC. Wound healing: an overview of acute fibrotic and delayed healing. Front Biosci. 2004 doi: 10.2741/1184. 9-283-9. [DOI] [PubMed] [Google Scholar]
- 45.Vaisman N, Gospodarowicz D, Neufeld G. Characterization of the receptors for vascular endothelial growth factor. J Biol Chem. 1990;265(32):19461–19466. [PubMed] [Google Scholar]
- 46.Plouet J, Moukadiri H. Characterization of the receptor to vasculotropin on bovine adrenal cortex-derived capillary endothelial cells. J Biol Chem. 1990;265(36):22071–22074. [PubMed] [Google Scholar]
- 47.Corral CJ, Siddiqui A, Wu L, Farrell CL, Lyons D, Mustoe TA. Vascular endothelial growth factor is more important than basic fibroblastic growth factor during ischemic wound healing. Arch Surg. 1999;134(2):200–205. doi: 10.1001/archsurg.134.2.200. [DOI] [PubMed] [Google Scholar]
- 48.Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114(6):1502–1508. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 49.Knighton DR, Phillips GD, Fiegel VD. Wound healing angiogenesis: indirect stimulation by basic fibroblast growth factor. J Trauma. 1990;30(12 Suppl):S134–144. [PubMed] [Google Scholar]
- 50.Ganio C, Tenewitz FE, Wilson R, Moyles BG. The treatment of chronic nonhealing wounds using autologous platelet-derived growth factors. J Foot Ankle Surg. 1993;32(3):263–268. [PubMed] [Google Scholar]
- 51.Mazzucco L, Medici D, Serra M, Panizza R, Rivara G, Orecchia S, Libener R, Cattana E, Levis A, Betta PG, Borzini P. The use of autologous platelet gel to treat difficult-to-heal wounds: a pilot study. Transfusion. 2004;44(7):1013–1018. doi: 10.1111/j.1537-2995.2004.03366.x. [DOI] [PubMed] [Google Scholar]
- 52.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 53.Henderson JL, Cupp CL, Ross EV, Shick PC, Keefe MA, Wester DC, Hannon T, McConnell D. The effects of autologous platelet gel on wound healing. Ear Nose Throat J. 2003;82(8):598–602. [PubMed] [Google Scholar]
- 54.Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, Moroni M, Carabelli A. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci. 2004;30(2):145–151. doi: 10.1016/j.transci.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Everts PA, Devilee RJ, Brown Mahoney C, Eeftinck-Schattenkerk M, Box HA, Knape JT, van Zundert A. Platelet gel and fibrin sealant reduce allogeneic blood transfusions in total knee arthroplasty. Acta Anaesthesiol Scand. 2006;50(5):593–599. doi: 10.1111/j.1399-6576.2006.001005.x. [DOI] [PubMed] [Google Scholar]
- 56.Hom DB, Linzie BM, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9(3):174–183. doi: 10.1001/archfaci.9.3.174. [DOI] [PubMed] [Google Scholar]


