Abstract
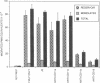
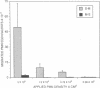
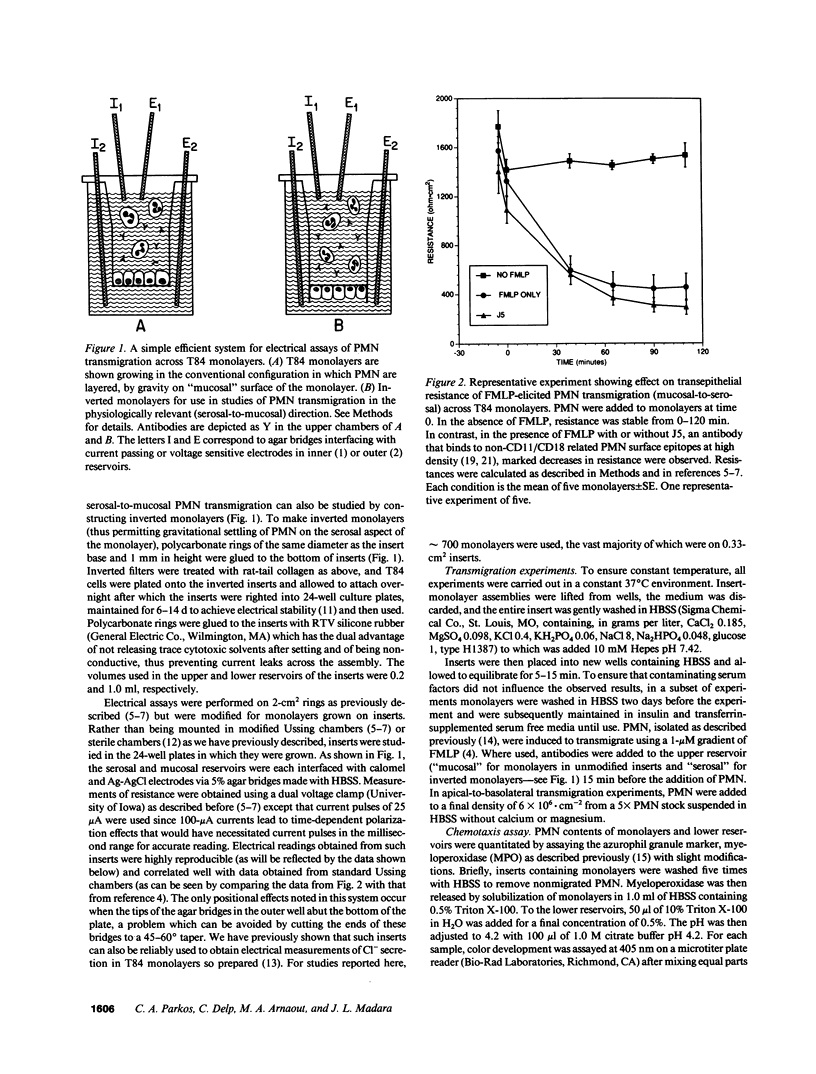
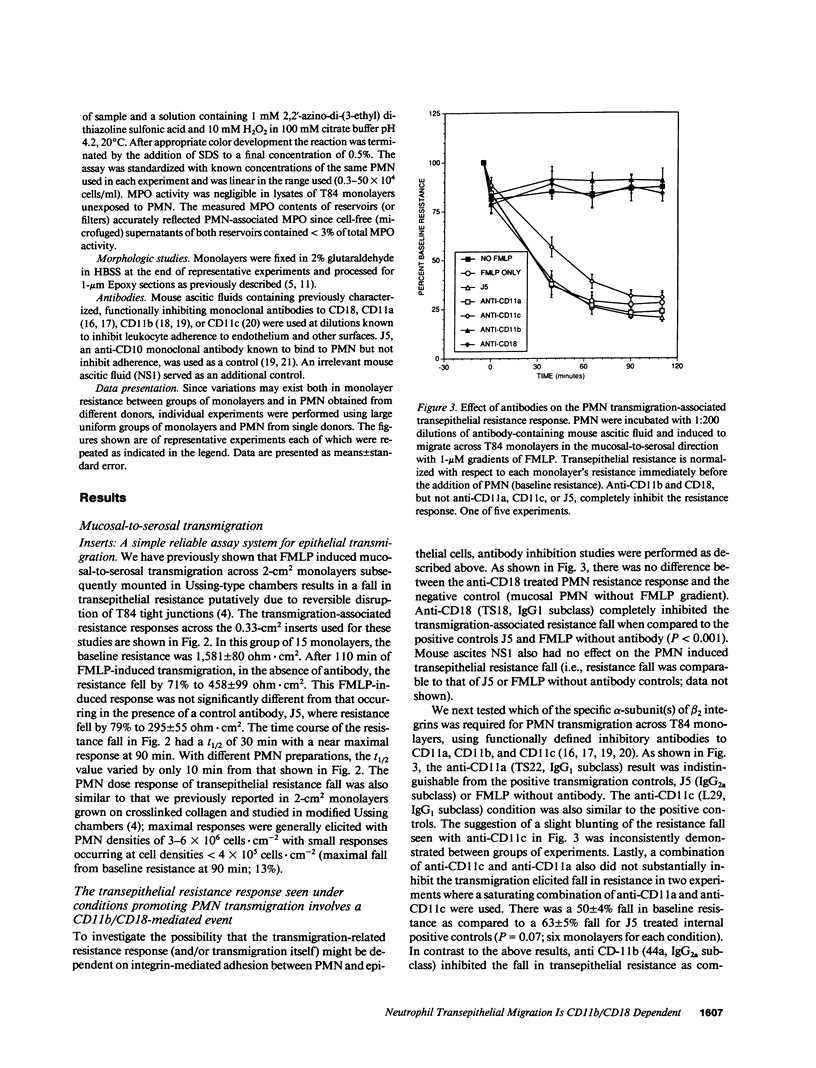
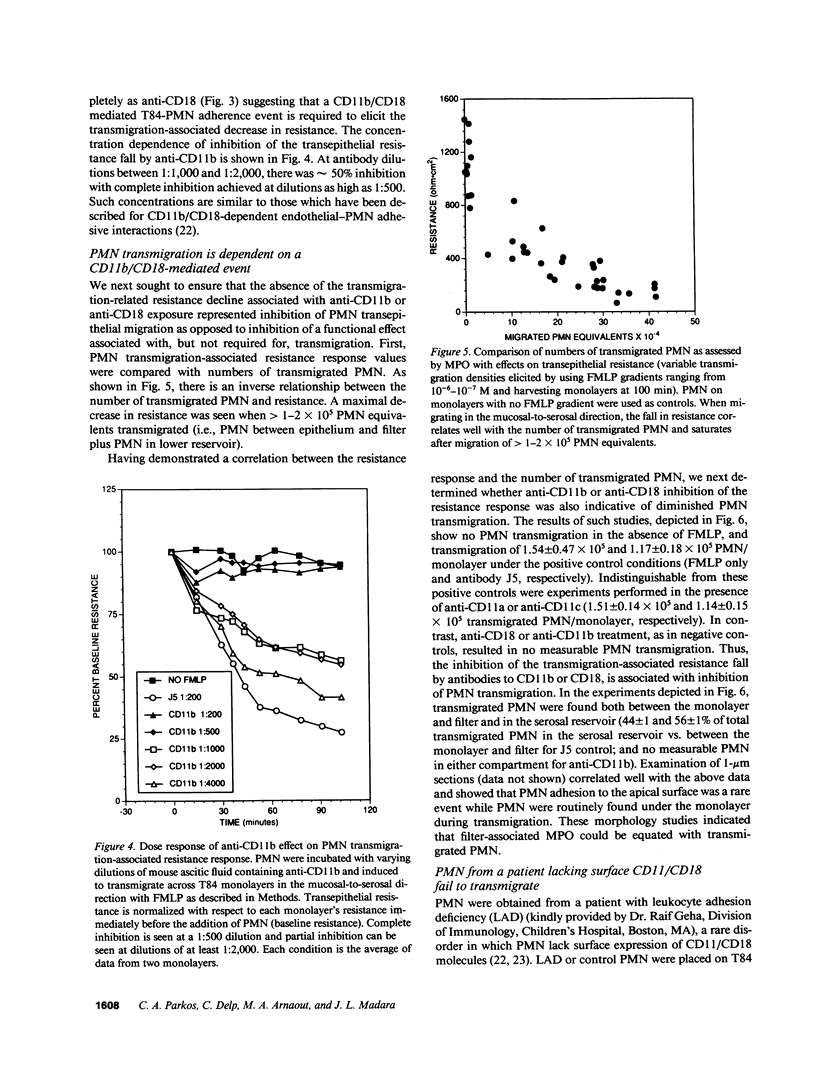
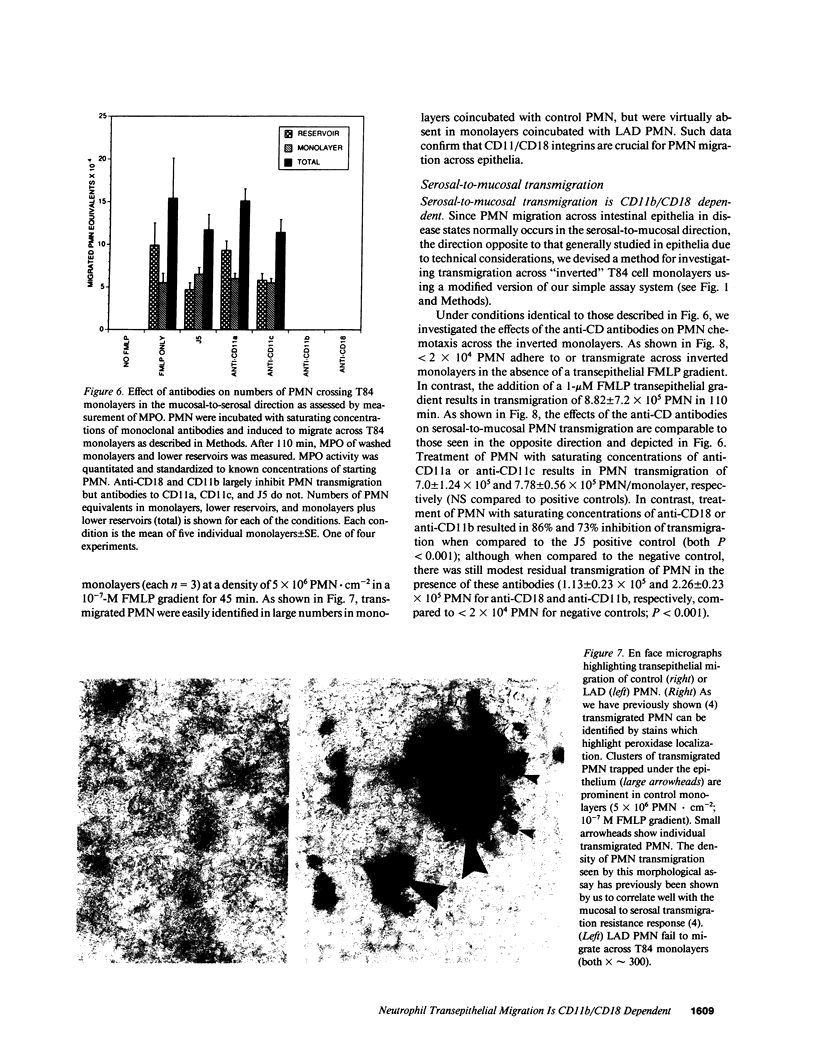
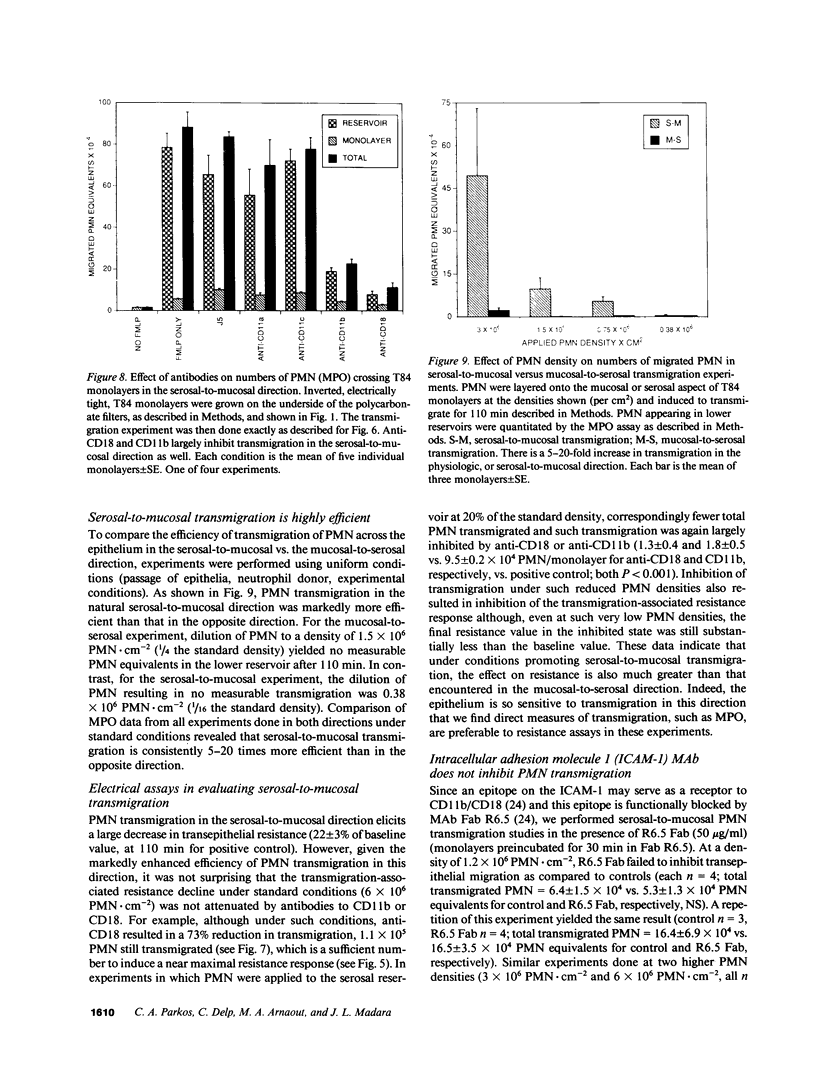
Neutrophils (PMN) migrate across intestinal epithelia in many disease states. Although such migration serves as a histological index of disease activity, little is known concerning the molecular events underlying PMN-intestinal epithelial interactions. We have studied chemotactic peptide-driven movement of PMN across cultured monolayers of the human intestinal epithelial cell line T84. Using a transmigration microassay, we show that both the decreased transepithelial resistance (76 +/- 3%) and transmigration (4 +/- 0.6 x 10(5) PMN.cm-2, when PMN applied at 6 x 10(6).cm-2) are largely prevented by MAbs which recognize either subunit of the PMN surface heterodimeric adhesion glycoprotein, CD11b/CD18. In contrast, such PMN-epithelial interactions are unaffected by MAbs recognizing either of the remaining two alpha subunits CD11a or CD11c. PMN from a leukocyte adherence deficiency patient also failed to migrate across epithelial monolayers thus confirming a requirement for CD11/18 integrins. By modifying our microassay, we were able to assess PMN transmigration across T84 monolayers in the physiological direction (which, for technical reasons, has not been studied in epithelia): transmigration was again largely attenuated by MAb to CD18 or CD11b (86 +/- 2% and 73 +/- 3% inhibition, respectively) but was unaffected by MAb to CD11a, CD11c. For standard conditions of PMN density, PMN transmigration in the physiological direction was 5-20 times more efficient than in the routinely studied opposite direction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altieri D. C., Agbanyo F. R., Plescia J., Ginsberg M. H., Edgington T. S., Plow E. F. A unique recognition site mediates the interaction of fibrinogen with the leukocyte integrin Mac-1 (CD11b/CD18). J Biol Chem. 1990 Jul 25;265(21):12119–12122. [PubMed] [Google Scholar]
- Altieri D. C., Edgington T. S. The saturable high affinity association of factor X to ADP-stimulated monocytes defines a novel function of the Mac-1 receptor. J Biol Chem. 1988 May 25;263(15):7007–7015. [PubMed] [Google Scholar]
- Anderson D. C., Schmalstieg F. C., Arnaout M. A., Kohl S., Tosi M. F., Dana N., Buffone G. J., Hughes B. J., Brinkley B. R., Dickey W. D. Abnormalities of polymorphonuclear leukocyte function associated with a heritable deficiency of high molecular weight surface glycoproteins (GP138): common relationship to diminished cell adherence. J Clin Invest. 1984 Aug;74(2):536–551. doi: 10.1172/JCI111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A., Lanier L. L., Faller D. V. Relative contribution of the leukocyte molecules Mo1, LFA-1, and p150,95 (LeuM5) in adhesion of granulocytes and monocytes to vascular endothelium is tissue- and stimulus-specific. J Cell Physiol. 1988 Nov;137(2):305–309. doi: 10.1002/jcp.1041370214. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A. Leukocyte adhesion molecules deficiency: its structural basis, pathophysiology and implications for modulating the inflammatory response. Immunol Rev. 1990 Apr;114:145–180. doi: 10.1111/j.1600-065x.1990.tb00564.x. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Pitt J., Cohen H. J., Melamed J., Rosen F. S., Colten H. R. Deficiency of a granulocyte-membrane glycoprotein (gp150) in a boy with recurrent bacterial infections. N Engl J Med. 1982 Mar 25;306(12):693–699. doi: 10.1056/NEJM198203253061201. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Todd R. F., 3rd, Dana N., Melamed J., Schlossman S. F., Colten H. R. Inhibition of phagocytosis of complement C3- or immunoglobulin G-coated particles and of C3bi binding by monoclonal antibodies to a monocyte-granulocyte membrane glycoprotein (Mol). J Clin Invest. 1983 Jul;72(1):171–179. doi: 10.1172/JCI110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M., Robbins E. S., Dolan W. J., Rotunno C. A., Sabatini D. D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978 Jun;77(3):853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatila T. A., Geha R. S., Arnaout M. A. Constitutive and stimulus-induced phosphorylation of CD11/CD18 leukocyte adhesion molecules. J Cell Biol. 1989 Dec;109(6 Pt 2):3435–3444. doi: 10.1083/jcb.109.6.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer E. B., Milks L. C., Brontoli M. J., Ojakian G. K., Wright S. D., Showell H. J. Effect of human serum and some of its components on neutrophil adherence and migration across an epithelium. J Cell Biol. 1986 May;102(5):1868–1877. doi: 10.1083/jcb.102.5.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana N., Todd R. F., 3rd, Pitt J., Springer T. A., Arnaout M. A. Deficiency of a surface membrane glycoprotein (Mo1) in man. J Clin Invest. 1984 Jan;73(1):153–159. doi: 10.1172/JCI111186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Madara J. L. Established intestinal cell lines as model systems for electrolyte transport studies. Methods Enzymol. 1990;192:354–389. doi: 10.1016/0076-6879(90)92082-o. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., McRoberts J. A., Mandel K. G., Tisdale L. D., Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984 Feb;246(2 Pt 1):G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., de Fougerolles A. R., Stacker S. A., Garcia-Aguilar J., Hibbs M. L., Springer T. A. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J Cell Biol. 1990 Dec;111(6 Pt 2):3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Entman M. L., Youker K., Shappell S. B., Siegel C., Rothlein R., Dreyer W. J., Schmalstieg F. C., Smith C. W. Neutrophil adherence to isolated adult canine myocytes. Evidence for a CD18-dependent mechanism. J Clin Invest. 1990 May;85(5):1497–1506. doi: 10.1172/JCI114596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C. W., Taylor J. E., Walker J. D., Simmons N. L. Transepithelial chemotaxis of rat peritoneal exudate cells. Br J Exp Pathol. 1983 Dec;64(6):644–654. [PMC free article] [PubMed] [Google Scholar]
- Hawker P. C., McKay J. S., Turnberg L. A. Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterology. 1980 Sep;79(3):508–511. [PubMed] [Google Scholar]
- Hecht G., Pothoulakis C., LaMont J. T., Madara J. L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988 Nov;82(5):1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. Secretion of granule constituents and increased oxidation of glucose. J Clin Invest. 1975 Oct;56(4):1053–1061. doi: 10.1172/JCI108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. B., Nostrant T. T., Appelman H. D. The histopathologic spectrum of acute self-limited colitis (acute infectious-type colitis). Am J Surg Pathol. 1982 Sep;6(6):523–529. doi: 10.1097/00000478-198209000-00004. [DOI] [PubMed] [Google Scholar]
- Luscinskas F. W., Brock A. F., Arnaout M. A., Gimbrone M. A., Jr Endothelial-leukocyte adhesion molecule-1-dependent and leukocyte (CD11/CD18)-dependent mechanisms contribute to polymorphonuclear leukocyte adhesion to cytokine-activated human vascular endothelium. J Immunol. 1989 Apr 1;142(7):2257–2263. [PubMed] [Google Scholar]
- Madara J. L., Dharmsathaphorn K. Occluding junction structure-function relationships in a cultured epithelial monolayer. J Cell Biol. 1985 Dec;101(6):2124–2133. doi: 10.1083/jcb.101.6.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L., Stafford J., Dharmsathaphorn K., Carlson S. Structural analysis of a human intestinal epithelial cell line. Gastroenterology. 1987 May;92(5 Pt 1):1133–1145. doi: 10.1016/s0016-5085(87)91069-9. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Burakoff S. J., Faller D. V. Adhesion of T lymphocytes to human endothelial cells is regulated by the LFA-1 membrane molecule. J Cell Physiol. 1986 Feb;126(2):285–290. doi: 10.1002/jcp.1041260219. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Crimmins M. A., Burakoff S. J., Faller D. V. Alpha and beta subunits of the LFA-1 membrane molecule are involved in human monocyte-endothelial cell adhesion. J Cell Physiol. 1987 Mar;130(3):410–415. doi: 10.1002/jcp.1041300314. [DOI] [PubMed] [Google Scholar]
- Migliorisi G., Folkes E., Cramer E. B. Differences in the ability of neutrophils and monocytes to traverse epithelial occluding junctions. J Leukoc Biol. 1988 Dec;44(6):485–492. doi: 10.1002/jlb.44.6.485. [DOI] [PubMed] [Google Scholar]
- Nash S., Parkos C., Nusrat A., Delp C., Madara J. L. In vitro model of intestinal crypt abscess. A novel neutrophil-derived secretagogue activity. J Clin Invest. 1991 Apr;87(4):1474–1477. doi: 10.1172/JCI115156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash S., Stafford J., Madara J. L. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1987 Oct;80(4):1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash S., Stafford J., Madara J. L. The selective and superoxide-independent disruption of intestinal epithelial tight junctions during leukocyte transmigration. Lab Invest. 1988 Oct;59(4):531–537. [PubMed] [Google Scholar]
- Nathan C. F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987 Dec;80(6):1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Srimal S., Farber C., Sanchez E., Kabbash L., Asch A., Gailit J., Wright S. D. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989 Sep;109(3):1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos C. A., Cochrane C. G., Schmitt M., Jesaitis A. J. Regulation of the oxidative response of human granulocytes to chemoattractants. No evidence for stimulated traffic of redox enzymes between endo and plasma membranes. J Biol Chem. 1985 Jun 10;260(11):6541–6547. [PubMed] [Google Scholar]
- Parsons P. E., Sugahara K., Cott G. R., Mason R. J., Henson P. M. The effect of neutrophil migration and prolonged neutrophil contact on epithelial permeability. Am J Pathol. 1987 Nov;129(2):302–312. [PMC free article] [PubMed] [Google Scholar]
- Ritz J., Pesando J. M., Notis-McConarty J., Lazarus H., Schlossman S. F. A monoclonal antibody to human acute lymphoblastic leukaemia antigen. Nature. 1980 Feb 7;283(5747):583–585. doi: 10.1038/283583a0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Rothlein R., Hughes B. J., Mariscalco M. M., Rudloff H. E., Schmalstieg F. C., Anderson D. C. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988 Nov;82(5):1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Wright S. D., Levin S. M., Jong M. T., Chad Z., Kabbash L. G. CR3 (CD11b/CD18) expresses one binding site for Arg-Gly-Asp-containing peptides and a second site for bacterial lipopolysaccharide. J Exp Med. 1989 Jan 1;169(1):175–183. doi: 10.1084/jem.169.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]