Abstract
Aims/Hypothesis
The Andres clamp technique, which requires accurate and timely determination of glucose, utilizes the Beckman or Yellow Springs Instruments (YSI) glucose analyzers. Both instruments require maintenance, a dedicated operator, preparation of a plasma sample, and a duplicate measurement that takes ≥2 minutes. The Nova StatStrip glucose meter was evaluated for accuracy, reliability, and near-real-time availability of glucose.
Methods
Blood samples from 24 patients who underwent 6-hour clamp studies and 12 patients who had a standardized meal tolerance test (SMT) were measured. Specimens were analyzed simultaneously and immediately upon collection by Beckman, YSI, and Nova.
Results
Of 1004 data pairs for the Nova device versus Beckman, the Nova data points ranged from 32 to 444, while Beckman ranged from 42 to 412. The coefficient for the slope of Beckman versus Nova was 1.009 (r = 0.978). Using error grid analysis, the number and percentage of values for Nova were 976 (97.2%) in the A zone and 28 (2.8%) in the B zone. Of 399 data pairs for the Nova device versus YSI, the Nova data points ranged from 46 to 255, whereas YSI ranged from 47 to 231. The coefficient for the slope of YSI versus Nova was 1.023 (r = 0.989). All Nova readings fell in the A zone. Time required for final reading, in duplicate, was 15 seconds for Nova and 120–180 seconds for Beckman and YSI.
Conclusions
The simplicity of Nova and its reliability, accuracy, and speed make it an acceptable replacement device for Beckman and YSI in the conduct of clamps, especially when perturbations require rapid glucose determination.
Keywords: Beckman, glucose clamp, glucose meter, plasma glucose, YSI
Introduction
The glucose clamp technique, first described by Andres and colleagues1 in 1966, is currently considered the state of the art technique for measurement of glucose homeostasis.2 The hyperinsulinemic–euglycemic clamp method allows assessment of peripheral tissue sensitivity to insulin. To evaluate beta-cell sensitivity to glucose, a hyperglycemic variant of the clamp can be used. As individuals with various disorders of metabolism—for example, the obese with type 2 diabetes mellitus—present to the medical system in ever-growing numbers,3,4 the clamp has become the “gold standard” methodology to assess and discriminate beta-cell responsiveness from insulin sensitivity.
In both the hyperinsulinemic–euglycemic and hyperglycemic stages of the clamp, the rate of glucose infusion is varied, based on the current blood glucose level, in order to maintain the subject at a targeted blood glucose level. Accordingly, the clamp method depends on accurate and timely determination of plasma glucose (PG) levels. Most investigators have used the Beckman or Yellow Springs Instruments (YSI) glucose analyzers, both of which utilize a glucose oxidase technology. They are considered the gold standard in near-real-time glucose measurement but have several important drawbacks. These devices require a dedicated technician to prepare a plasma sample from each whole blood specimen; the process from blood draw to measurement using Beckman or YSI can take 2 minutes or more. Given that the glucose infusion rate is typically changed every 5 minutes, this delay is significant. Moreover, Beckman was invented in the 1960s, and Beckman Coulter has announced that it will no longer support the device as of January 2009.5 The YSI instrument (YSI Life Sciences, Yellow Springs, OH), although as accurate as the Beckman device, takes even longer, and its self-calibration interferes with PG measurement. This limitation requires that a second YSI instrument be available during the clamp due to the long delay in calibration. Any inability to determine PG level and adjust the glucose infusion rate will result in significant changes from the goal during the clamp, which is especially apparent in volunteers with very good sensitivity to insulin or glucose. These devices also require significant amounts of supplies and regular maintenance.
The use of glucose meters in performing the clamp studies have been studied by several groups. The results were reported as suboptimal in two of these studies.6,7 A paper by Cohen and associates,8 however, found a handheld glucose meter to be an accurate device in the measurement of blood glucose during the clamp study.
The need for an accurate and fast method for the determination of PG levels during a clamp study has led us to test the Nova StatStrip Glucose Monitor (Nova Biomedical, Waltham, MA). The device is a handheld, battery-operated meter that uses a modified glucose-oxidase-based amperometric technology with hematocrit correction. It also has the capability to upload PG data to a computer for further analysis. The aim of this current study was to assess whether or not the Nova device is accurate enough to serve as a replacement for Beckman or YSI during clamp studies.
Methods
Sample Testing
A total of 24 subjects who had undergone hyper-insulinemic–euglycemic and hyperglycemic clamps were included in the study. All were undergoing metabolic testing either before or after bariatric surgery. A new variant of the glucose clamp technique was used in which a hyperinsulinemic–euglycemic clamp was combined with a hyperglycemic clamp. The 6-hour test consists of three steps. The first is a hyperinsulinemic–euglycemic clamp. After establishing the basal PG level, a 10-minute prime infusion of insulin is followed by a continuous infusion at 480 pmol/m−2/min−1 for 2 hours. This part is followed by an hour of recovery, during which plasma insulin levels will return back to basal state. During the second step, the PG levels are raised by 98 mg/dl above basal levels and held there for 2 hours. Finally, during the last hour of hyperglycemia, glucagon-like peptide-1 (7–36) amide is infused in a primed-continuous manner (5 ng/kg−1/min−1). The fall in PG level is then followed for the next 30 minutes. During the 6-hour clamp used in this study, arterialized venous blood samples were taken every 5 minutes to measure PG and adjust the infusion rates using a negative feedback principal. Blood samples from 14 patients were then simultaneously measured by both the Beckman glucose meter analyzer and the Nova StatStrip glucose meter and from 10 patients for YSI and Nova.
To assess and compare the blood glucose values obtained during studies in which the prevailing blood glucose level is allowed to change continuously, i.e., not clamped and therefore represent a broader range of glucose values, simultaneous measurements were also performed during a standardized meal tolerance test (SMT) in the same subjects undergoing clamp studies. A total of 474 ml of Ensure plus (Abbott Laboratories, Columbus, OH) were consumed within 15 minutes. For the SMT, two samples were obtained before consumption of the liquid meal, then samples were obtained at 5, 10, 15, 20, 30, 40, 60, 90, 120, 150, and 180 minutes. These venous blood samples were then immediately and simultaneously tested with both the Beckman analyzer and the StatStrip glucose meter. Half a milliliter of whole blood was centrifuged to obtain plasma for the Beckman analyzer. For the Nova StatStrip device, a test strip was loaded and was touched by a droplet of blood from the tip of the sample syringe to the end of the strip.
Samples were always analyzed with the Beckman and YSI devices in duplicate. When duplicates were performed on either device, they were tested sequentially and immediately, one after the other. Both Beckman and YSI instruments were regularly calibrated and maintained.
Statistical Analyses
Data from the clamp and SMT studies were entered into a computer database after each clamp. The Beckman and YSI devices were chosen as reference methods. To assess the accuracy of the Nova readings, every Beckman or YSI value obtained during a clamp at a given time was paired with a corresponding Nova value for PG level. The values used for the Beckman or YSI were the mean of the duplicate values for each device. More than 97% of the Beckman and YSI values were within 3 mg/dl of each other (for the Nova instrument, the first value was always used). Using the Beckman (or YSI) values as the reference and therefore independent variable and the Nova values as dependent variables, two ordinary least squares linear regressions were run using SigmaPlot 11.0 (Systat Software Inc., San Jose, CA).
Clinical acceptability of Nova glucose values were also analyzed using point error grid analysis (EGA). The EGA divides a graph showing the linear regression of Nova values on Beckman/YSI (reference) values into five zones, A, B, C, D, and E.9,10 Zones A and B contain results considered to be accurate or acceptable. Zone C values are considered not accurate, and their use may result in unnecessary actions that lead to poorer outcomes. Values in zone D are considered dangerous failures that may result in life-threatening actions, and values in zone E are considered inaccurate and may result in treatment contradictory to that actually required.
To assess any bias in Nova measurements across the possible range of blood glucose values, the mean difference between Nova and Beckman/YSI values was plotted against the Beckman/YSI reference values using the Bland–Altman analysis.11,12 This method also permits assessment of limits of agreement between the two devices, as determined by plotting lines showing two standard deviations above and below the mean difference between the two devices. Furthermore, another Bland–Altman plot was created to compare the precision of Nova duplicate values to Beckman duplicates.
Finally, data were analyzed using locally smoothed median absolute difference curve in order to reveal glucose meter accuracy patterns.13
Results
From 14 patients, all of whom underwent a 6-hour glucose clamp study, 1004 matching data points were obtained comparing the Beckman analyzer to the Nova glucose meter. From another 10 patients who also had a 6 hour glucose clamp, 399 matching data points were obtained comparing the YSI analyzer to the Nova glucose meter. Figure 1 shows a representative comparison of PG during the clamp study as measured by Beckman and NOVA instruments. The Beckman–Nova regression yielded a slope coefficient of 1.009 and a constant coefficient of 2.602. Pearson's r was 0.978. The YSI–Nova regression yielded a slope coefficient of 1.023 and a constant coefficient of 5.854. Pearson's r was 0.989.
Figure 1.
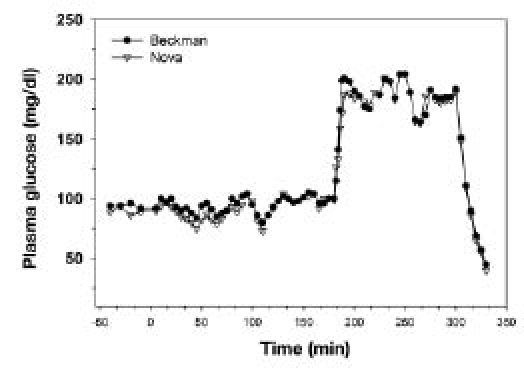
Comparison of PG values between Beckman and Nova devices during a hyperinsulinemic–euglycemic clamp (0–180 min), during a hyperglycemic clamp (180–200 min), and during recovery from hyperglycemia (300–330 min).
The same scatter plots were graphed using EGA in Figure 2. Using the EGA with Beckman values as reference, 976 (97.6%) of the data fell in the A zone and the remaining 28 (2.4%) fell in the B zone. No values fell in zones C, D, or E. Similarly, using YSI values as reference, 399 (100%) of values fell in the A zone.
Figure 2.
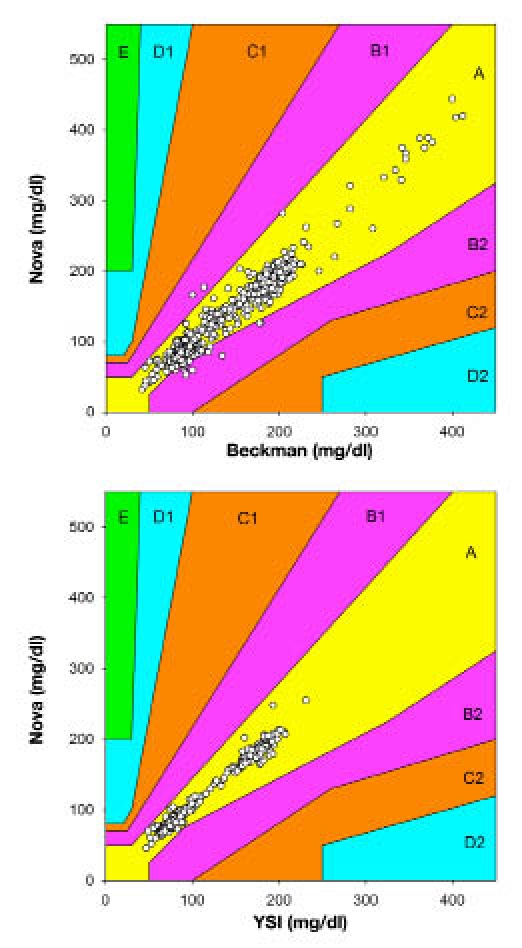
Scatter plot with EGA for pairs of Beckman and Nova (top) and YSI and Nova (bottom) blood glucose values using Beckman and YSI as the respective reference method.
Bland–Altman plots of the mean difference between Beckman and Nova, or YSI and Nova, values against reference Beckman/YSI values are shown in Figure 3. Using Beckman values as reference, the mean difference between data pairs was 1.3 mg/dl, with a standard deviation of 11.6 mg/dl. As expected, of the 1004 data pairs, 48 (4.7%) disagreed by more than two standard deviations. Meanwhile, using YSI values as reference, the mean difference between data pairs was 8.5 mg/dl, with a standard deviation of 7.2 mg/dl. Of the 399 data pairs, 12 (3.0%) disagreed by more than two standard deviations.
Figure 3.
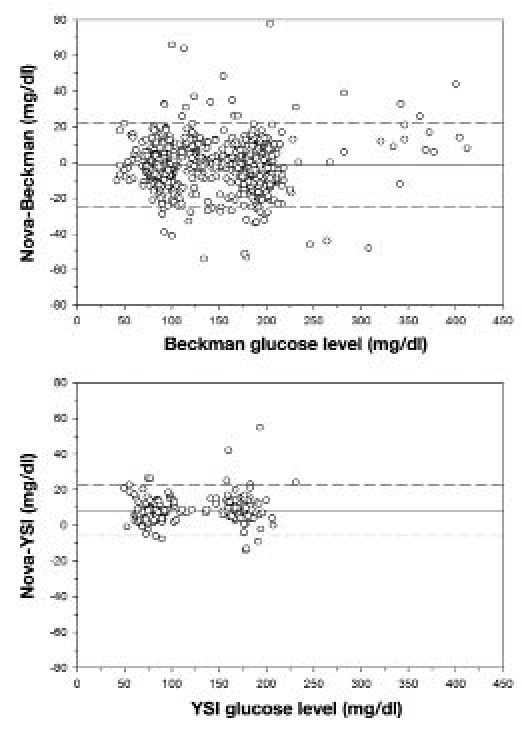
Bland–Altman plot comparing the difference between Nova and Beckman (top) and Nova and YSI (bottom) readings of the same blood sample. Solid line represents mean difference, and dashed lines show two standard deviations above and below the mean (n = 1004 for Nova–Beckman comparison; n = 399 for Nova–YSI comparison).
Bland–Altman plots were computed to compare differences between Beckman duplicates with differences between Nova duplicates (Figure 4). The left panel of Figure 4 shows the differences between the Beckman duplicates, with the solid line showing the mean difference of 0.915 mg/dl and the dashed lines indicating two standard deviations above and below (sigma = 2.79 mg/dl). The right panel of Figure 4 shows differences between the Nova duplicates, which had a mean difference of 0.767 mg/dl but a standard deviation of 5.563 mg/dl, nearly twice that of the Beckman duplicates. Of the 210 Nova duplicates, 168 (80%) were within two standard deviations of the Beckman analyzer values (i.e., within 2 × 2.79 = 5.58 mg/dl).
Figure 4.
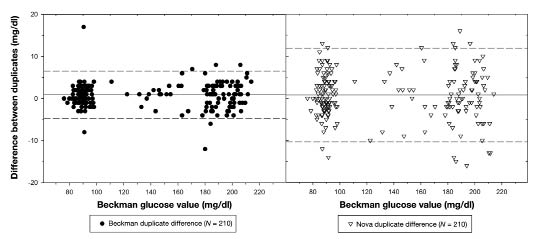
Bland–Altman plot comparing Beckman duplicate differences with Nova duplicate differences. Solid line represents mean difference, and dashed lines represent two standard deviations above and below each device's respective mean difference.
Locally smoothed median absolute difference curves were created using a bandwidth of 15 mg/dl and X range from 57 to 218 mg/dl (Figure 5). A nearly flat curve was observed in the euglycemic range of 60–100 mg/dl. The curve was also flat in the range of 120–220 mg/dl at the higher level of 10 mg/dl.
Figure 5.
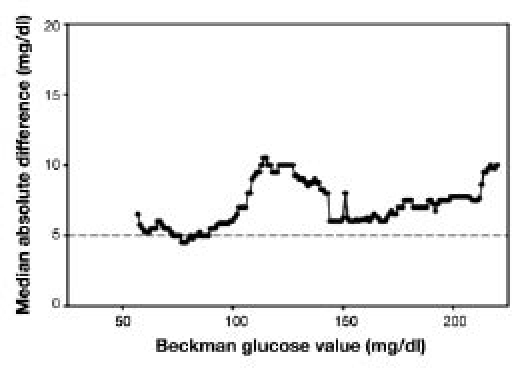
Locally smoothed median absolute difference curve. The dashed horizontal line represents the recommended error tolerance (5 mg/dl).
Discussion
The glucose clamp methodology is the state-of-the-art technique to assess peripheral tissue sensitivity to insulin and beta-cell sensitivity to glucose in a controlled setting. Thus, the changes in these variables following various interventions can be assessed longitudinally because the stimulus remains unchanged. Many investigators across the world have used different variations of this technique to answer various questions about fuel homeostasis. Regardless of the study design, the clamp technique is unique in that it can assess glucose homeostasis in a precise and reproducible manner.
The collective effort required on the part of staff (nurses, technicians, and investigators), however, makes this technique a labor-intensive and expensive method for assessment of fuel homeostasis. One of the principal limitations is the requirement for timely, accurate, and precise measurement of PG levels to determine the 20% glucose infusion rates in a negative-feedback-controlled system.
The glucose clamp methodology creates unique demands for PG measurement—specifically, that the measurement device used be fast, accurate, and able to withstand repeated testing over the course of several hours. Accordingly, since Beckman Coulter has announced it will no longer support its glucose analyzer, the Nova StatStrip device was tested to see if it could fulfill these requirements as an effective replacement.
The linear regression of all 1004 Beckman–Nova pairs of data returned a slope coefficient of near unity and a Pearson's r of 0.978. The strong correlation of the Nova with the Beckman is essential to running a successful clamp study. Error grid analysis also confirmed the high accuracy of the Nova device, with almost 98% of values falling in the zone considered to be accurate and acceptable. Moreover, no values fell in any of the zones considered to indicate an unacceptable reading. Similar results were seen using the YSI analyzer as a reference method, though the limited number of data pairs analyzed with the YSI device makes the Beckman comparison somewhat more meaningful.
The Bland–Altman plot of the difference between Nova and Beckman readings for a given blood sample as compared to the reference Beckman readings did not show any upward or downward trend across a wide range of blood glucose values. No trend was observed in the same plot of the difference between Nova and YSI values. The Nova glucose meter, therefore, does not appear to bias readings in any direction across the range of PG levels measured. The vast majority of values were within two standard deviations of the mean difference of 1.3 mg/dl. However, with a standard deviation of the differences of nearly 12 mg/dl, this meant that some readings were rather widely discrepant. Values this far apart can, at times, make it difficult to run a successful clamp when targeting a tight range of PG values. Note that the data tend to cluster in two parts, representing, respectively, the euglycemic and hyperglycemic stages of the clamp study.
The precision of duplicate values taken on the Nova glucose meter were compared to the Beckman analyzer and assessed (Figure 4). Though the mean differences between duplicates were similar for both devices, the standard deviation of difference for Nova duplicates was nearly twice that of Beckman duplicates. If the Nova is held to the Beckman standard by asking how many of the Nova duplicate differences fall within two standard deviations of the Beckman duplicate differences, only 168 of 210 (80%) of Nova duplicates are within those two standard deviations. This result reflects an occasional tendency of the Nova glucose meter to give readings that are not sufficiently accurate for the clamp study. For the Beckman instrument, manufacturer—s recommendation was followed that duplicates be within 3 mg/dl of each other. If they were not, a triplicate or, at times, even a quadruplicate was run. For the Nova device, the authors recommend that the duplicates must be within 5 mg/dl of each other and that a third or fourth measurement should be taken if necessary. From experience, even if a sample is run four times, an accurate value can be obtained in 30 seconds; duplicates are available within 15 seconds.
The locally smoothed median absolute difference curve clearly showed that, in both the euglycemic and the hyperglycemic glucose ranges, a relatively flat pattern was observed. However, in the hyperglycemic range, the differences are higher, and the glucose values must be carefully evaluated with respect to both the previous glucose level as well as the change in the 20% glucose infusion rate. If there is uncertainty, a third or even forth reading should be performed. Still, the rapid analysis of glucose determination has led the authors to believe that the performance of the instrument is acceptable and that the 5 mg/dl difference in duplicates can be achieved even during a hyperglycemic clamp.
The authors acknowledge that the accuracy of the Nova instrument is not as robust as the Beckman or YSI. However, the management of the glucose clamp, even with sophisticated software, requires familiarity with the “art” of the clamp. Therefore, it is suggested that unreasonable glucose values as determined by the Nova glucose meter can be identified easily with repeated glucose measurements. The previous PG level and the change in the 20% glucose infusion rate should always be considered as a guide for the reliability of the current value. It should be noted that discrepant values also occur with both the Beckman and the YSI, in which case a third reading is necessary.
The Nova glucose meter has several key advantages not necessarily reflected in the data of this study that make it particularly suitable for the glucose clamp study. First of all, its real time of 6 seconds makes it ideal when the investigator needs to constantly vary the glucose infusion rate to maintain a stable euglycemia or hyperglycemia. Even if it becomes necessary to run a sample in triplicate on the Nova glucose meter, 20 seconds (three readings of 6 seconds each) is a far shorter time period than the time it takes for one sample to be measured with the Beckman or YSI devices. Moreover, unlike the Beckman, which usually takes at least 120–180 seconds to confirm a value, or the YSI, which takes at least 60 seconds longer than the Beckman (mechanical sampling arm versus manual pipetting, respectively), the Nova can confirm a value within 15 seconds (two readings of 6 seconds each). The Nova glucose meter is simple to use and does not require a dedicated technician. Supplies and maintenance for the Nova analyzer are also far less intensive, and the Nova instrument is significantly less costly to purchase and operate.
This study has some limitations in evaluating the Nova glucose meter's precision and accuracy in the hospital setting. The study group consists of obese patients without any major medical illnesses, such as anemia or acidosis. Therefore, the meter's lack of interference with hematocrit and other variables claimed by the manufacturer could not be investigated by the design of our study. Furthermore, it was not possible to assess drug interference because the study population was not on any medications aside from oral vitamin supplements. The occasional use of Tylenol during the clamp studies was not observed to cause any changes in measured blood glucose values. Finally, though in critically ill patients, blood glucose measurements are usually performed with finger sticks, this study analyzed blood samples taken from intravenous lines. However, it should be noted that the instrument has been evaluated for hematocrit effect as well as analytical interference in a hospital setting.14,15
In summary, results indicate that the Nova device is able to replicate blood glucose values obtained with the Beckman or YSI analyzers to a high degree of fidelity. Moreover, the Nova glucose meter is significantly easier to operate and is well suited to the glucose clamp study. The authors conclude that the Nova StatStrip glucose meter is an acceptable replacement for the Beckman or YSI glucose analyzers for real-time glucose determinations in glucose clamp testing. Moreover, the speed in which results can be obtained could counterbalance less precise agreement of duplicate readings.
Acknowledgments
The authors would like to thank Nova Biomedical Inc. for providing glucose testing equipment and supplies.
Abbreviations
- EGA
error grid analysis
- PG
plasma glucose
- SMT
standardized meal tolerance test
- YSI
Yellow Springs Instruments
References
- 1.Andres R, Swerdloff R, Pozefsky T, Coleman D. Manual feedback technique for control of glucose concentration. In: Skeggs LT Jr, editor. Automation in analytic chemistry. New York: Medaid, Inc.; 1966. pp. 486–501. [Google Scholar]
- 2.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894(i–xii):1–253. [PubMed] [Google Scholar]
- 4.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S.,2005–2050. Diabetes Care. 2006;29(9):2114–2116. doi: 10.2337/dc06-1136. [DOI] [PubMed] [Google Scholar]
- 5.Elahi D, Jeicher R. Personal communication. July 31, 2009.
- 6.Brunner GA, Ellmerer M, Sendlhofer G, Wutte A, Trajanoski Z, Schaupp L, Quehenberger F, Wach P, Krejs GJ, Pieber TR. Validation of home blood glucose meters with respect to clinical and analytical approaches. Diabetes Care. 1998;21(4):585–590. doi: 10.2337/diacare.21.4.585. [DOI] [PubMed] [Google Scholar]
- 7.Stork AD, Kemperman H, Erkelens DW, Veneman TF. Comparison of the accuracy of the HemoCue glucose analyzer with the Yellow Springs Instrument glucose oxidase analyzer, particularly in hypoglycemia. Eur J Endocrinol. 2005;153(2):275–281. doi: 10.1530/eje.1.01952. [DOI] [PubMed] [Google Scholar]
- 8.Cohen O, Shaklai S, Gabis E, Pani MA. FreeStyle Mini blood glucose results are accurate and suitable for use in glycemic clamp protocols. J Diabetes Sci Technol. 2008;2(5):890–895. doi: 10.1177/193229680800200521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8):1143–1148. doi: 10.2337/diacare.23.8.1143. [DOI] [PubMed] [Google Scholar]
- 10.Clarke WL. The original Clarke Error Grid Analysis (EGA) Diabetes Technol Ther. 2005;7(5):776–779. doi: 10.1089/dia.2005.7.776. [DOI] [PubMed] [Google Scholar]
- 11.Bland JM, Altman DJ. Regression analysis. Lancet. 1986;1(8486):908–909. doi: 10.1016/s0140-6736(86)91008-1. [DOI] [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 2--correlation between subjects. BMJ. 1995;310(6980):633. doi: 10.1136/bmj.310.6980.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kost GJ, Tran NK, Abad VJ, Louie RF. Evaluation of point-of-care glucose testing accuracy using locally-smoothed median absolute difference curves. Clin Chim Acta. 2008;389(1–2):31–39. doi: 10.1016/j.cca.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karon BS, Griesmann L, Scott R, Bryant SC, Dubois JA, Shirey TL, Presti S, Santrach PJ. Evaluation of the impact of hematocrit and other interference on the accuracy of hospital-based glucose meters. Diabetes Technol Ther. 2008;10(2):111–120. doi: 10.1089/dia.2007.0257. [DOI] [PubMed] [Google Scholar]
- 15.Holtzinger C, Szelag E, DuBois JA, Shirey TL, Presti S. Evaluation of a new POCT bedside glucose meter and strip with hematocrit and interference corrections. Point Care. 2008;7(1):1–6. [Google Scholar]


