Abstract
Background
Technosphere® [Bis-3,6(4-fumarylaminobutyl)-2,5-diketopiperazine (FDKP)] microparticles, the integral component of the Technosphere inhalation system, deliver drugs to the deep lung and have been used to administer insulin and glucagon-like peptide-1 via inhalation in clinical studies. Three studies were conducted to characterize FDKP pharmacokinetics, including assessments in subjects with diabetic nephropathy (DNP), in subjects with chronic liver disease (CLD), and in healthy subjects.
Methods
An open-label, nonrandomized, two-period, fixed-sequence crossover absorption, distribution, metabolism, and excretion (ADME) study was conducted in six healthy nonsmoking men who received single intravenous and oral doses of [14C]FDKP solution, with serial sampling of blood, urine, feces, and expired air. Additionally, two single-dose, open-label, parallel-design studies with 20 mg of inhaled FDKP were conducted in (1) 12 diabetic subjects with normal renal function and 24 DNP subjects and (2) 12 healthy subjects and 21 CLD subjects.
Results
In the ADME study, >95% of the intravenous dose and <3% of the oral dose were recovered in urine, with no evidence of metabolism. No significant pharmacokinetic differences were observed between healthy subjects and CLD subjects [geometric mean (% coefficient of variation) area under the curve from time 0 to 480 minutes (AUC0–480): 26,710 (34.8) and 31,477 (28.8) ng/ml·min, respectively]. Maximum observed drug concentration (Cmax) and AUC0–480 were higher in DNP subjects than in subjects with normal renal function [Cmax: 159.9 (59.4) ng/ml versus 147.0 (44.3) ng/ml; AUC0–480: 36,869 (47.2) ng/ml·min versus 30,474 (31.8) ng/ml·min]. None of the differences observed were considered clinically significant.
Conclusions
Fumaryl diketopiperazine is predominantly cleared unchanged by the kidney with essentially no oral bioavailability. Technosphere is a safe delivery vehicle for medications administered via inhalation.
Keywords: chronic liver disease, diabetic nephropathy, fumaryl diketopiperazine, inhalation, Technosphere®
Introduction
Technosphere® technology is a novel, versatile drug delivery platform that enables pulmonary administration of therapeutic agents.1 The resulting drug product is composed of the active agent adsorbed onto the Technosphere microparticle [Bis-3,6(4-fumarylaminobutyl)-2,5-diketopiperazine (FDKP) and polysorbate 80], which utilizes a proprietary, breath-actuated inhaler for pulmonary delivery of the particles.
Fumaryl diketopiperazine is a pharmaceutical excipient that, in solution and under slightly acidic conditions, forms an array of microcrystalline plates. The plates then self-assemble into microparticles with a large surface area onto which various peptides, proteins, or other agents can be adsorbed (Figure 1). When dried, the resulting dry powder is filled into unit-dose cartridges, which are then used in conjunction with an inhaler.
Figure 1.

Scanning electron micrograph of a Technosphere® particle.
Technosphere particles have an average diameter of 2.5 μm, with ≥90% of the particles in the respirable range for pulmonary delivery (0.5–5.8 μm), as confirmed by aerodynamic testing.1 These characteristics enable oral inhalation of therapeutics for a wide range of disease states,1 providing an alternative delivery route for agents currently dosed only by injection, and by making it possible to bypass hepatic first-pass metabolism by absorption directly into the arterial circulation.2,3 This technology can accommodate therapeutics over a wide molecular size range1 and has been used to administer insulin and glucagon-like peptide-1 by the pulmonary route in clinical studies.3,4 A number of other agents, including oxyntomodulin and peptide YY, have been administered in nonclinical studies.5
Therapeutics administered via the Technosphere technology demonstrate rapid absorption, believed to be associated with rapid dissolution of the microparticles rather than any FDKP-mediated absorption enhancement. In vitro studies using the Calu-3 lung cell line6 and in vivo rat studies (unpublished data) show that FDKP does not enhance drug absorption.
Neither in vitro nor preclinical in vivo investigations have found any evidence of a pharmacological effect of FDKP. Fumaryl diketopiperazine was evaluated in 63 in vitro receptor-binding assays (neurotransmitters, steroids, ion channels, secondary messengers, prostaglandin, growth factors/hormones, brain and gut peptides, and various enzymes) to evaluate potential effects. At concentrations up to 100 μM, FDKP did not inhibit binding of any substrate to these receptors. The biological activity of FDKP has been evaluated in comprehensive toxicology studies and in rat and dog pharmacology studies, with no observed effects on cardiac, central nervous system, pulmonary, or renal functions (unpublished data).
To characterize FDKP pharmacokinetics, an absorption, distribution, metabolism, and excretion (ADME) study was conducted in healthy human subjects utilizing 14C-radiolabeled FDKP administered by both intravenous and oral routes (study 1). Subsequently, two phase 1 studies were conducted to assess FDKP pharmacokinetics in: (1) subjects with diabetic nephropathy (DNP) and diabetic subjects with normal kidney function (study 2) and (2) subjects with chronic liver disease (CLD) compared with subjects with normal hepatic function (study 3). The results of these clinical studies are reported here.
Methods
Study Conduct and Study Subject Consent
Each study protocol and the accompanying patient information were approved by local ethics committees and institutional review boards, and each subject gave written informed consent before participation. Studies were conducted in compliance with the Declaration of Helsinki and under the current International Conference on Harmonization Guidelines for Good Clinical Practice. For studies 2 and 3, subjects were trained by site personnel on use of the MedTone® inhaler (MannKind Corporation, Danbury, CT).
Study Subject and Disease Characteristics
In all studies, subjects were nonsmokers willing to use adequate birth control. Exclusion criteria in all three studies included, but were not limited to, previous exposure to an inhaled insulin product, participation in another clinical study, or presence of clinically significant disease. In study 1, use of any medications or herbal remedies within 14 days before the first dosing (except for paracetamol) was also precluded. Complete physical examinations were performed at the screening and follow-up visits. Any clinically significant change was documented. Twelve-lead electrocardiograms were performed at protocol-specified time points with subjects in a supine position. Smoking and pulmonary status (studies 2 and 3) were reviewed at the screening, treatment, and follow-up visits.
Study 1: Human ADME Study
This phase 1, single-dose, open-label, nonrandomized, two-period, fixed-sequence crossover study was conducted in six healthy volunteers. Participants were men ≥18 and ≤60 years of age deemed to be healthy based on screening physical examination, medical history, normal clinical chemistry and urinalysis, fasting blood glucose ≤110 mg/dl, and body mass index (BMI) ≤30 kg/m2 (nonobese).
The study consisted of four clinic visits, with study treatment administration of 14C-labeled FDKP (see Figure 2 for FDKP structure) on visits 2 and 3. Subjects underwent serial sampling of blood, urine, feces, and expired air at both treatment visits. During visit 2, all eligibility criteria were confirmed and subjects received a single infusion of 10 ml FDKP (3.7 MBq of [14C]FDKP) intravenously over 5 minutes at 2 mg/min for a total dose of 10 mg FDKP. Urine and feces from all subjects were assessed daily by liquid scintillation counting (“quick counts”). If quick counts did not meet discharge criteria (<50 dpm/ml urine, <75 dpm/100 mg feces in 24-hour collection) on day 4, 24-hour urine and feces specimens were collected until criteria were met. After a minimum 2-week washout period, at visit 3, each subject received a single 100-ml oral dose of FDKP solution (0.2 mg/ml, containing 2.5 MBq of [14C]FDKP). The final 20-mg dose is an approximate equivalent of the FDKP amount in the 60-unit Technosphere Insulin dose, a clinically relevant dose studied frequently in the Technosphere Insulin development program.7–9
Figure 2.
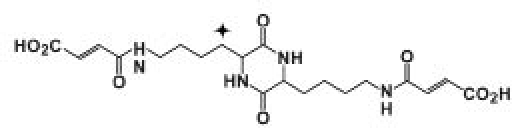
Structure of FDKP. The symbol indicates the position of the 14C label.
All subjects remained in the clinic until day 4, and quick counts were conducted as per visit 2. Visit 4 was a follow-up that took place 7–14 days after visit 3.
Study 2: Diabetic Subjects with Mild or Moderate Diabetic Nephropathy or Normal Renal Function
This single-dose, open-label, parallel, controlled study of FDKP was conducted in diabetic subjects with normal renal function and mild or moderate DNP. The study consisted of three clinic visits: screening, treatment, and follow-up. Each subject received one 20-mg (cartridge fill weight) dose of Technosphere inhalation powder at the treatment visit. Blood and urine samples were collected serially over 24 hours postdose.
Inclusion criteria consisted of men and women ≥18 and ≤80 years of age with type 1 or type 2 diabetes confirmed by medical records and a BMI ≥37 kg/m2. Staging of subjects with DNP was based on recommendations of the National Kidney Foundation Disease Outcomes Quality Initiative clinical practice guidelines.10 Mild DNP was defined as a glomerular filtration rate (GFR) of 60–89 ml/min/1.73 m2 and microalbuminuria (≥17 mg/g in men, ≥25 mg/g in women). Moderate DNP was defined as a GFR of 30–59 ml/min/1.73 m2 and microalbuminuria (≥17 mg/g in men, ≥25 mg/g in women). Normal renal function required a GFR ≥90 ml/min/1.73 m2 without microalbuminuria (<17 mg/g in men, <25 mg/g in women).
Study 3: Subjects with Mild or Moderate Chronic Liver Disease or Normal Liver Function
This single-dose, open-label, parallel, controlled study was conducted in nondiabetic subjects with normal liver function and mild or moderate CLD. This study consisted of screening, treatment, and follow-up visits and 20 mg of Technosphere inhalation powder (cartridge fill weight) administered at the treatment visit. Blood and urine samples were collected serially over 24 hours postdose.
Inclusion criteria consisted of men and women ≥18 and ≤80 years of age with a BMI ≤35 kg/m2. Chronic liver disease staging was based on the Child–Pugh score with recommendations of the American Association for the Study of Liver Diseases practice guidelines.11 Mild CLD was defined as Child–Pugh class A (5–6 points), and moderate CLD was defined as Child–Pugh class B (7–9 points). Disease history included histopathological confirmation or clinical imaging and laboratory findings. Subjects without CLD had an absence of clinical evidence of liver disease, negative hepatitis B and hepatitis C serology, and normal liver function tests.
Bioanalytical Methods
Serial blood samples were collected into tubes on ice. In study 1, plasma was analyzed for radioactivity by liquid scintillation counting (LSC). Urine and feces were collected and pooled for specified timed intervals. Urine samples were analyzed for radioactivity (LSC), whereas the radioactivity in feces was measured via sample combustion (Packard 307 sample oxidizer). Expired air was assessed for 14CO2 with hyamine hydroxide as a trapping solution and phenolphthalein in ethanol as an indicator. Subjects exhaled via a tube placed into a vial containing the solution until the solution became colorless (≈2 minutes), indicating neutralization by CO2. Vials were stored at 4 °C until assay on site for 14C radioactivity by LSC. For studies 2 and 3, samples for the FDKP assay were centrifuged at 4 °C within 1 hour of collection and frozen until FDKP quantification by liquid chromatography with tandem mass spectrometry.
Metabolites in Urine and Feces
Urine and plasma samples with a level of radioactivity >4000 DPM/ml were analyzed by high-performance liquid chromatography (HPLC) with radiochemical detection (Table 1), a method sensitive enough to separate the cis and trans FDKP isomers. The injection volume was ≤75 μl to prevent overloading of the column.
Table 1.
High-Performance Liquid Chromatography: Conditions for Profiling Serum and Urine Samplesa
| Column | Luna phenyl-hexyl 3-μm packing, 150 × 3.0 mm ID | ||
| Guard cartridge | Phenyl, 3 μm, 4 × 2 mm ID | ||
| Mobile phases | A 1000:1 (v/v) water:TFA B 900:100:1 (v/v) MeOH:THF:TFA | ||
| Mobile phase gradient | Time (min) | %A | %B |
| 0 | 95 | 5 | |
| 35 | 65 | 35 | |
| 37 | 5 | 95 | |
| 40 | 5 | 95 | |
| 41 | 95 | 5 | |
| Flow rate | 0.300 ml/min | ||
ID, inside diameter; TFA, trifluoroacetic acid; MeOH, methanol; THF, tetrahydrofuran.
Pharmacokinetic Parameters
Pharmacokinetic parameters included maximum plasma drug concentration (Cmax), time to Cmax (tmax), and area under the curve from time 0 to t (AUC0–t, calculated by the linear-trapezoidal method). Terminal elimination half-life (t1/2) was calculated as ln(2)/λz, where λz is the terminal elimination rate constant estimated from log-linear regression analysis of the terminal elimination phase of the concentration–time profile, and area under the curve from time 0 to infinity (AUC0–∞) was calculated as AUC0–tlast + Clast/λz, where Clast is the last observed drug concentration and tlast is the time corresponding to Clast. In study 1, additional parameters included mass balance, calculated as the sum of the amount of 14C radioactivity excreted in urine, feces, and expired air divided by the amount of radioactivity dosed. Pharmacokinetic analyses were performed using WinNonlin Professional, version 5.0.1 or higher (Pharsight, Inc., Mountain View, CA), and summary statistics and statistical analyses were performed using SAS®, version 8.2 or higher (SAS Institute, Inc., Cary, NC).
Results
Study 1: Human ADME
Four of the six enrolled subjects completed the study. Two subjects were withdrawn after the intravenous dose but before oral dosing (prior to starting visit 3) due to elevated levels of urinary cotinine (>100 ng/ml), in violation of inclusion criteria (i.e., nonsmoking status). Hence, the pharmacokinetic analysis included six healthy subjects following intravenous dosing and four subjects following oral dosing (Table 2).
Table 2.
Mean (SDa) Demographics and Baseline Characteristics of Subjects in Study 1
| Intravenous dose population (n = 6) | Oral dose population (n = 4) | |
|---|---|---|
| Age, years (SD) | 29.8 (14.9) | 34.0 (17.2) |
| Weight, kg (SD) | 77.7 (10.5) | 77.2 (13.6) |
| Height, cm (SD) | 180.3 (12.7) | 177.3 (15.2) |
| BMI, kg/m2 (SD) | 23.9 (1.2) | 24.5 (1.0) |
| Race, n (%) | ||
| Caucasian | 5 (83.3) | 3 (75.0) |
| Asian | 1 (16.7) | 1 (25.0) |
Standard deviation.
Following intravenous administration, >90% of total radioactivity elimination occurred within 8 hours of dosing (Table 3, Figure 3A). An average of 97.2% of total radioactivity was recovered in the urine and 1.65% was recovered in the feces. Analyses of urine and dosing materials by HPLC demonstrated that all radioactivity peaks detected in urine were present in the dosing material in the same proportions. There was no evidence of metabolism following intravenous dosing. The radio-chromatogram from a representative urine sample (Figure 4) shows the cis and trans FDKP isomers, with no other radioactivity peaks evident.
Table 3.
Summary of 14C Radioactivity Pharmacokinetic Parameters in Urine, Feces, and Expired Air: Study 1
| Parameter | 10-mg IV dose (n = 6) | 20-mg oral dose (n = 4a) | ||||
|---|---|---|---|---|---|---|
| Urine | Feces | Expired air | Urine | Feces | Expired air | |
| Ae,b μg | 9720 | 165.0 | 0 | 490.0 | 19,030 | 5 |
| Fe, % | 97.2 | 1.65 | 0 | 2.45 | 95.1 | 0.03 |
| Mass balance, % (SD) | 98.9 (2.1) | 97.6 (1.5) | ||||
Two subjects did not receive oral dose of study drug because they were withdrawn from the study.
Ae, total amount of 14C radioactivity excreted in urine or feces, or estimated total amount of 14C radioactivity excreted in expired air; Fe, percentage of dose of 14C radioactivity excreted in urine, feces, or expired air; IV, intravenous; SD, standard deviation.
Figure 3.
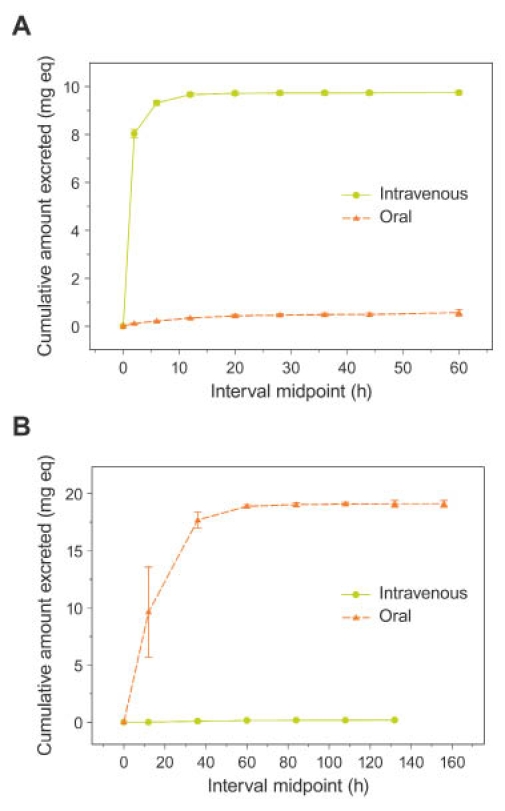
Mean (±SE) cumulative excretion of total radioactivity in (A) urine and (B) feces following a 10-mg intravenous and a 20-mg oral FDKP dose.
Figure 4.
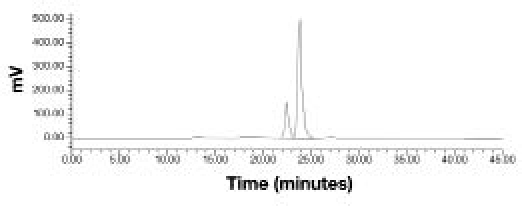
Representative radiochromatogram of a 0- to 4-hour urine sample following intravenous administration of 14C-labeled FDKP showing the cis (first peak) and trans (second peak) isomers of FDKP.
Following oral dosing, >90% of radiolabel was eliminated within 48 hours postdose, with an average of 2.45% of total radioactivity recovered in the urine and approximately 95.1% recovered in the feces (Table 3, Figure 3B). A negligible fraction of the dose was recovered in expired air (0.03% of the dose). Pharmacokinetic parameters and 14C radioactivity of FDKP in plasma are summarized in Table 4 and Figures 5 and 6.
Table 4.
Summary of Pharmacokinetic Parameters and 14C Radioactivity of FDKP in Plasma: Study 1
| Parametera | Intravenous dose (10 mg) n = 6 | Oral dose (20 mg) n = 4 |
|---|---|---|
| AUC0−∞, ng/ml·min | 75,240 (5640) | NC |
| AUC0−tlast, ng/ml·min | 74,791 (5640) | 5,587 (3028) |
| Cmax, ng/ml | 722.7 (130.5) | 8.5 (1.6) |
| tmax, h | NC | 6 (1, 16) |
| t1/2, min | 150 (24) | NC |
| Foral | NA | 0.0364 (0.0174) |
Parameters are presented as mean (standard deviation) except for tmax, which is presented as median (min, max).
AUC0–tlast, area under the plasma concentration–time curve from time 0 to t, where t is time of last quantifiable concentration; Foral, bioavailability following oral dose; NA, not applicable; NC, not calculated.
Figure 5.
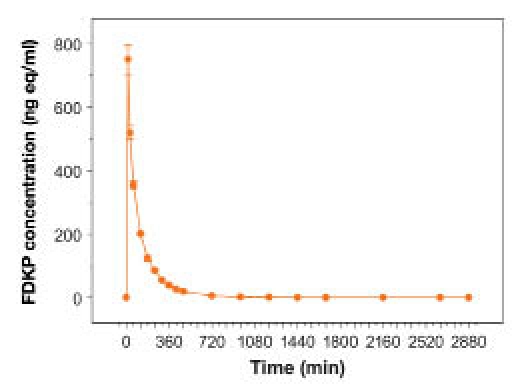
Mean (±SE) total radioactivity in plasma following a 10-mg FDKP intravenous dose.
Figure 6.
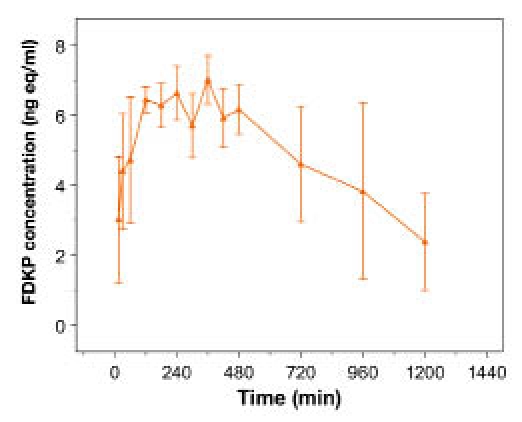
Mean (±SE) total radioactivity in plasma following a 20-mg FDKP oral dose (linear scale).
Study 2: Diabetic Subjects with Diabetic Nephropathy and Normal Renal Function
All 36 enrolled subjects completed the study (Table 5). No significant differences were noted in subject characteristics among the three renal function groups, with the exception of race; the majority of subjects in the mild DNP group were of Hispanic origin. Given the small number of subjects, the determination of racial differences on FDKP pharmacokinetics was not possible; however, there is no expectation that FDKP clearance is affected by race, as there were no obvious differences in FDKP parameters in the four racial groups within each renal function category.
Table 5.
Mean (SDa) Demographics and Baseline Characteristics of Subjects in Studies 2 and 3
| Diabetic subjects without DNP (n = 12) | Mild DNP (n = 15) | Moderate DNP (n = 9) | Normal (n = 12) | Mild CLD (n = 15) | Moderate CLD (n = 6) | |
|---|---|---|---|---|---|---|
| Age, years (SD) | 58 (7) | 63 (9) | 52 (13) | 51 (7) | 58 (8) | 58 (3) |
| BMI, kg/m2 (SD) | 31 (4) | 29 (4) | 33 (3) | 28 (4) | 30 (4) | 28 (2) |
| Sex | ||||||
| Male (%) | 7 (58) | 11 (73) | 6 (67) | 12 | 14 | 6 |
| Female (%) | 5 (42) | 4 (27) | 3 (33) | 0 | 1 | 0 |
| Weight, kg (SD) | 88 (16) | 80 (13) | 97 (11) | 92 (18) | 95 (15) | 88 (11) |
| Height, cm (SD) | 167 (11) | 165 (7) | 170 (8) | 181 (8) | 178 (10) | 176 (7) |
| Race, n | ||||||
| Caucasian | 4 | 2 | 3 | 9 | 8 | 3 |
| Black | 1 | 0 | 1 | 2 | 5 | 2 |
| Hispanic | 7 | 13 | 4 | 1 | 2 | 1 |
| Other | 0 | 0 | 1 | 0 | 0 | 0 |
Standard deviation.
Fumaryl diketopiperazine exposure [FDKP area under the curve from time 0 to 480 minutes (AUC0–480)] was approximately 18 and 25% higher in subjects with mild and moderate DNP, respectively, compared with subjects with normal renal function (Table 6, Figure 7). Fumaryl diketopiperazine t1/2 was similar in subjects with normal renal function and those with mild DNP and was ≈80 minutes longer in subjects with moderate DNP. This difference in t1/2 resulted in a more noticeable difference in exposure over a longer sampling period in subjects with moderate DNP, with an approximate 52% greater exposure when AUC0–∞ was compared with subjects with normal renal function. The longer tmax observed in subjects with moderate DNP (30 minutes versus 12 minutes in subjects with normal renal function and 15 minutes in those with mild DNP) is most likely also attributable to the observed longer t1/2.
Table 6.
Geometric Mean (% Coefficient of Variation) FDKP Pharmacokinetic Parameters in Diabetic Nephropathy: Study 2
| Parameter | Diabetic without DNP (n = 12) | Diabetic nephropathy | ||
|---|---|---|---|---|
| Mild (n = 15) | Moderate (n = 9) | Mild and moderate (n = 24) | ||
| AUC0–480, ng/ml·min | 30,474 (32) | 36,090 (43) | 38,206 (54) | 36,869 (47) |
| AUC0−∞, ng/ml·min | 37,137 (29) | 44,168 (43) | 56,393 (58) | 48,406 (53) |
| Cmax, ng/ml | 147.0 (44) | 184.1 (55) | 126.4 (66) | 159.9 (59) |
| tmax, mina | 12 (3–45) | 15 (3–76) | 30 (9–153) | 16 (3–153) |
| t1/2, min | 190.6 (52) | 196.2 (46) | 269.8 (65) | 223.8 (64) |
| Ae0–1440, mgb | 3.7 (1.2) | 3.7 (1.7) | 2.8 (1.7) | 3.3 (1.7) |
tmax is presented as median (range).
Ae is presented as arithmetic mean (standard deviation).
Figure 7.
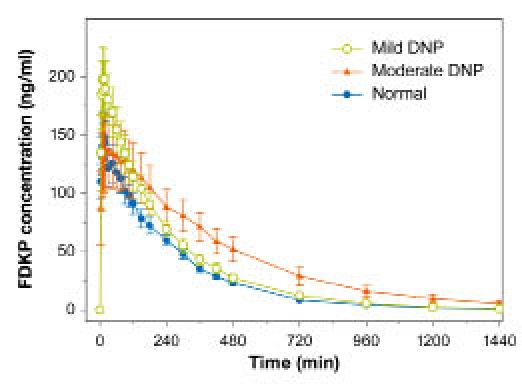
Mean (±SE) FDKP concentration–time profiles in subjects with diabetes and diabetic nephropathy and normal kidney function (study 2).
Over 24 hours, urinary elimination was almost complete, with approximately 20% of the FDKP dose excreted in the urine of all subjects, with slower accumulation in subjects with moderate DNP compared with the other subjects (Figure 8).
Figure 8.
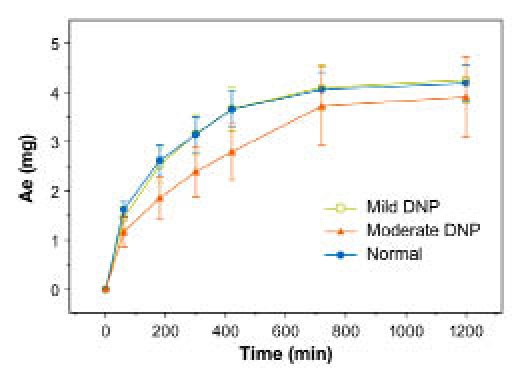
Mean (±SE) cumulative amount of FDKP excreted in urine in subjects with diabetes and diabetic nephropathy and normal kidney function (study 2).
Study 3: Subjects with Chronic Liver Disease and Normal Liver Function
Study enrollment was stopped at 33 subjects before the target enrollment of 36 subjects was achieved (Table 5) due to the prolonged enrollment period necessary to identify CLD subjects. No significant differences in subject characteristics were noted among the three hepatic function groups.
Fumaryl diketopiperazine exposure (AUC0–480) was higher in subjects with CLD than in those with normal liver function; however, this difference was slight (Table 7, Figure 9). No differences in FDKP Cmax and tmax and t1/2 were observed. Over 24 hours, urinary elimination was almost complete with, on average, <25% of the FDKP dose excreted in urine for all subjects (Figure 10). Differences in mean cumulative excretion amounts were skewed for the mild CLD group by one subject with high excretion values; this apparent difference was negated when that subject was excluded.
Table 7.
Geometric Mean (% Coefficient of Variation) FDKP Pharmacokinetic Parameters in Chronic Liver Disease: Study 3
| Parameter | Normal (n = 12) | Chronic liver disease | ||
|---|---|---|---|---|
| Mild (n = 15) | Moderate (n = 6) | Mild and moderate (n = 21) | ||
| AUC0–480, ng/ml·min | 26,710 (35) | 31,001 (26) | 32,700 (36) | 31,477 (29) |
| AUC0−∞, ng/ml·min | 32,320 (33) | 36,505 (26) | 40,085 (41) | 37,494 (32) |
| Cmax, ng/ml | 143.4 (49) | 161.5 (35) | 156.8 (42) | 160.2 (36) |
| tmax, mina | 7.5 (3–20) | 6.0 (3–60) | 6.0 (3–45) | 6.0 (3–60) |
| t1/2, min | 190 (27) | 173 (30) | 198 (45) | 180 (36) |
| Ae0–1440, mgb | 3.2 (1.1) | 4.5 (2.7) | 3.4 (1.2) | 4.1 (2.4) |
tmax is presented as median.
Ae is presented as arithmetic mean (standard deviation).
Figure 9.
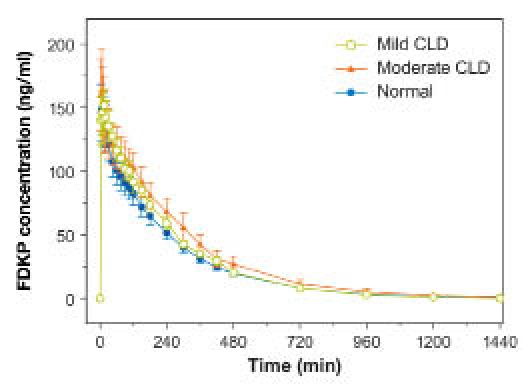
Mean (±SE) FDKP concentration–time profiles in subjects with normal and impaired hepatic function (study 3).
Figure 10.
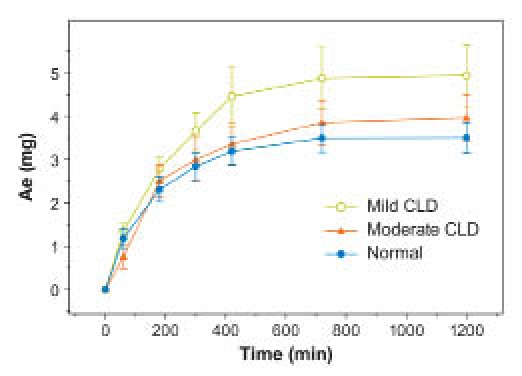
Mean (±SE) cumulative amount of FDKP excreted in urine (Ae) in subjects with impaired and normal hepatic function (study 3).
Safety: All Studies
Administration of FDKP was generally well tolerated in all studies, and treatment-related adverse events were mild with no associated discontinuations. No severe adverse events or deaths occurred during these studies.
In study 1, all treatment-related adverse events were mild in intensity except for one instance of iridocyclitis of moderate intensity, not related to the study drug. Cough, the most common treatment-emergent adverse event, was reported by 19 (52.8%) subjects in study 2 and by 17 (51.5%) subjects in study 3. In both studies, all coughs were nonproductive, mild in intensity, and occurred within 10 minutes of inhalation and as a single or intermittent occurrence.
Discussion
The Technosphere inhalation system can be used to deliver various therapeutic agents via the pulmonary route. Fumaryl diketopiperazine, the primary component of Technosphere particles, is a novel, pharmacologically inert compound that is absorbed rapidly into the systemic circulation.1 Because administration of Technosphere particles results in systemic FDKP exposure, the pharmacokinetic properties of this compound were characterized.
Inhaled drugs may enter the systemic circulation via two routes: (1) a fraction of the dose enters through the lung (directly into the central compartment) and (2) a fraction of the dose is swallowed. Therefore, FDKP metabolism was assessed following both intravenous and oral routes of administration to fully characterize its clearance after inhalation. Following intravenous administration, almost complete renal clearance of unchanged FDKP was observed. In the same study, FDKP demonstrated essentially no oral bioavailability; hence, the route of elimination of drug following pulmonary dosing is expected to be predominantly renal, as any swallowed drug is not expected to be systemically bioavailable at clinically relevant doses.
In studies 2 and 3, the cumulative amount of FDKP excreted in the urine after inhalation was measured following dosing. Approximately 20–23% of the dose was excreted at 24 hours in all groups studied. Because the ADME study showed almost complete urinary FDKP elimination by 8 hours following intravenous dosing and because FDKP is absorbed rapidly from the lung, this quantity was assumed to reflect FDKP bioavailability following inhalation. Absolute bioavailability was also calculated from mean FDKP AUC0–∞ in studies 2 and 3 and FDKP AUC0–∞ following intravenous administration in study 1 (24.7 and 21.5%, respectively), confirming the value determined from urinary excretion data. Fumaryl diketopiperazine bioavailability less than unity can be attributed to the fact that some of the drug product remains in the inhaler and some is swallowed during inhalation.
Differences observed in FDKP rate and extent of exposure between subjects with DNP and diabetic subjects without renal disease are consistent with the predominant renal clearance of the compound. However, differences observed were not considered clinically significant, as FDKP exposure is expected to remain within the safety margins with repeated dosing, based on the NOAEL (no observed adverse effect level) established in nonclinical studies (unpublished data) and an accumulation ratio (R = AUC0–∞/AUC0–480, based on geometric means of AUCs) of 1.22 (normal renal function) and 1.31 (mild and moderate DNP) with three-times-per-day dosing. When data from subjects with moderate DNP were examined, an approximate 50% increase in FDKP exposure (based on AUC0–∞) was observed compared with diabetic subjects without renal impairment. This finding was without expected clinical implications due to the lack of FDKP pharmacological activity following extensive in vitro, preclinical, and toxicological studies.
Interestingly, although inhaled FDKP is cleared predominantly by the kidneys, study 3 showed an increase in FDKP exposure in subjects with mild and moderate CLD compared with healthy subjects. It is well established that decreased renal function occurs in subjects with hepatic disease12,13 and that reduced renal excretion of a number of drugs cleared unchanged in urine (i.e., furosemide, bumetanide, cimetidine, and ranitidine) in subjects with hepatic impairment has been reported.13 Therefore, it is likely that the slightly elevated FDKP exposure in subjects with CLD is linked to impaired renal clearance associated with reduced hepatic function. However, this small, relative increase in FDKP exposure was not considered clinically significant.
In studies 2 and 3, pharmacokinetic data following inhalation were available from subjects without CLD or DNP. Most parameters were comparable between these two groups, with somewhat higher exposure (Cmax) and later tmax in study 2. This difference is most likely attributed to the difference in populations and the potential effect of the diabetic state on renal clearance of FDKP in study 2.
In studies 2 and 3, cough was the common treatment-emergent adverse event. The cough was mild and was observed as a single or intermittent occurrence. This finding was not surprising, as cough is often associated with the inhalation of dry powder.14
Conclusions
In an ADME study conducted in healthy male volunteers, >95% of the intravenous FDKP dose and <3% of the oral FDKP dose were recovered unchanged in urine. Oral bioavailability was negligible, and no evidence of metabolism was observed. FDKP was well tolerated in all populations studied. The increase in overall exposure due to mild or moderately impaired renal function was not clinically significant. Fumaryl diketopiperazine (Technosphere) is a safe delivery vehicle for medications administered via inhalation.
Acknowledgments
We acknowledge the following principal investigators: Robert J. Schwab, M.D., Qualia Clinical Research, Omaha, NE; Sherwyn L. Schwartz, M.D., Cetero Research, Inc., San Antonio, TX; Ernesto Fuentes, M.D., Elite Research Institute, Miami, FL; Krishna K. Pudi, M.D., Upstate Pharmaceutical Research, Greenville, SC; and Richard A. Preston, M.D., M.B.A., Division of Clinical Pharmacology, University of Miami, West Miami, FL.
Abbreviations
- ADME
absorption, distribution, metabolism, and excretion
- AUC0–480
area under the curve from time 0 to 480 minutes
- AUC0–∞
area under the curve from time 0 to infinity
- AUC0–t
area under the curve from time 0 to t
- BMI
body mass index
- Clast
last observed insulin concentration
- CLD
chronic liver disease
- Cmax
maximum observed drug concentration
- DNP
diabetic nephropathy
- FDKP
Bis-3,6(4-fumarylaminobutyl)-2, 5-diketopiperazine
- GFR
glomerular filtration rate
- HPLC
high-performance liquid chromatography
- LSC
liquid scintillation counting
- t1/2
terminal elimination half-life
- tlast
time corresponding to last observed drug concentration
- tmax
time to maximum observed drug concentration
References
- 1.Leone-Bay A, Grant M. Technosphere/insulin: mimicking endogenous insulin release. In: Rathbone MJ, Hadgraft M, Roberts M, Lane M, editors. Modified release drug delivery technology. 2nd ed. Vol 2. New York: Informa Healthcare USA, Inc.; 2008. pp. 673–679. [Google Scholar]
- 2.Rave K, Potocka E, Boss AH, Marino M, Costello D, Chen R. Pharmacokinetics and linear exposure of AFRESA compared with the subcutaneous injection of regular human insulin. Diabetes Obes Metab. 2009;11(7):715–720. doi: 10.1111/j.1463-1326.2009.01039.x. [DOI] [PubMed] [Google Scholar]
- 3.Marino MT, Costello D, Baughman R, Boss A, Cassidy J, Damico C, van Marle S, van Vliet A, Richardson PC. Pharmacokinetics and pharmacodynamics of inhaled GLP-1 (MKC253): proof-of-concept studies in healthy normal volunteers and in patients with type 2 diabetes. Clin Pharmacol Ther. 2010;88(2):243–250. doi: 10.1038/clpt.2010.85. [DOI] [PubMed] [Google Scholar]
- 4.Boss AH, Yu W, Ellerman K. Prandial insulin: is inhaled enough? Drug Dev Res. 2008;69:138–142. [Google Scholar]
- 5.Grant ML, Greene S, Stowell GW, Daniels S, Smithson A, Villanueva S, Leone-Bay A. Bloomington, IN: Presented at: 21st American Peptide Symposium; June 7–12; Mimicking endogenous peptide secretion by inhalation [poster] [Google Scholar]
- 6.Angelo R, Rousseau K, Grant M, Leone-Bay A, Richardson P. Technosphere insulin: defining the role of Technosphere particles at the cellular level. J Diabetes Sci Technol. 2009;3(3):545–554. doi: 10.1177/193229680900300320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips M, Amin N, Boss AH, Richardson P. Pulmonary functions (over 2 years) in diabetic subjects treated with Technosphere insulin or usual antidiabetic treatment [abstract] Diabetologia. 2009;52(Suppl 1):S361. Abstract 920. [Google Scholar]
- 8.Rosenstock J, Lorber DL, Gnudi L, Howard CP, Bilheimer DW, Chang PC, Petrucci RE, Boss AH, Richardson PC. Prandial inhaled insulin plus basal insulin glargine versus twice daily biaspart insulin for type 2 diabetes: a multicentre randomised trial. Lancet. 2010;375(9753):2244–2253. doi: 10.1016/S0140-6736(10)60632-0. [DOI] [PubMed] [Google Scholar]
- 9.Peyrot M, Rubin RR. Patient reported outcomes in adults with type 2 diabetes using mealtime insulin monomer human (rDNA origin) inhalation powder (Technosphere insulin inhalation powder) or metformin + secretagogue or both [abstract] Diabetologia. 2009;52(Suppl 1):S396. Abstract 1009. [Google Scholar]
- 10.National Kidney Foundation. Part 5. Evaluation of laboratory measurements for clinical assessment of kidney disease. Am J Kidney Dis. 2002;39(2 Suppl 1):S76–10. [PubMed] [Google Scholar]
- 11.Murray KF, Carithers RL., Jr AASLD. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology. 2005;41(6):1407–1432. doi: 10.1002/hep.20704. [DOI] [PubMed] [Google Scholar]
- 12.Morgan DJ, McLean AJ. Clinical pharmacokinetic and pharmacodynamic considerations in patients with liver disease. An update. Clin Pharmacokinet. 1995;29(5):370–391. doi: 10.2165/00003088-199529050-00005. [DOI] [PubMed] [Google Scholar]
- 13.Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64(12):1147–1161. doi: 10.1007/s00228-008-0553-z. [DOI] [PubMed] [Google Scholar]
- 14.Siekmeier R, Scheuch G. Inhaled insulin–does it become reality? J Physiol Pharmacol. 2008;59(Suppl 6):81–113. [PubMed] [Google Scholar]


