Abstract
Sardinia and Finland are the “hottest” areas for type 1 diabetes mellitus (T1DM) worldwide. Its genetic and epidemiological background make Sardinia an ideal region for investigating environmental, immunological, and genetic factors related to the etiopathogenesis of T1DM. Consequently, in 1990, the Insulin-Dependent Diabetes Mellitus Sardinia Project was launched in order to map the geographical distribution of T1DM in the island and to investigate preclinical phases of T1DM in a large cohort of people genetically at risk.
The final goal would be to design models of prediction and to formulate safe preventive measures, especially addressed to the general population living in areas at high risk.
Keywords: diabetes registries, Sardinian epidemiology, type 1 diabetes mellitus
Introduction
Type 1 diabetes mellitus (T1DM) results from an autoimmune destruction of insulin-producing β cells.1 Although T1DM accounts for 5–10% of the total burden of diabetes mellitus, it is the predominant endocrine disease in childhood and adolescence.
Since 1980, there has been an increase in the incidence of T1DM,2 with an estimated overall annual increase of 2–5 % worldwide3 and 3.2% in Europe,4 and with a higher rate of increase among children <5 years of age.5 The increase is steeper in the populations with a lower incidence, and in countries with the highest rates, there has not been any leveling off.2,3,6
From an epidemiological point of view, Sardinia, the second largest Italian Mediterranean island, has the second highest incidence of T1DM in the world, after Finland.4,7
Moreover, the history of the Sardinian population, characterized by a prolonged isolation with a small number of founders and by a long history of settlements together with the pressure of selecting factors (such as malaria), has made the island a genetic isolate with a unique and stable distribution of alleles.8 These genetic peculiarities together with the high incidence make Sardinia an ideal model to study T1DM,5,9,10 allowing for much deeper insights into its natural history and potential solutions to prevent and to control T1DM and its complications.
Good data on the prevalence and the incidence of T1DM with reliable geographical resolution could provide health care planners and providers a good instrument for rational allocation of limited resources.
Epidemiological data are usually drawn out from registers, which are searchable lists of all patients with a particular chronic condition, that can often interface with an electronic medical record.11,12
Type 1 diabetes mellitus is ideally suited to be studied using a register currently based on capture–recapture method, and T1DM is particularly suitable for being captured because it is not too severe, it is not too frequent, it has a classic set of symptoms, and a simple test rapidly diagnoses the disease, so there are few misdiagnosed cases. Registers need a definition of cases: precise diagnosis of T1DM and the description of the population at risk (age range, geographical data, time period evaluated). Very simple data are needed for a register: name, date of birth, date of diagnosis, place of residence, and ascertainment status. Several sources of ascertainment are available to monitor diabetes. The choice of data sources is important in determining the accuracy of the estimated prevalence.10
On the basis of the assumption that Sardinia is an ideal epidemiological observatory for T1DM, we have focused our efforts, and in 1990, the Insulin-Dependent Diabetes Mellitus Sardinia Project was started13 with two main objectives: (1) to map the geographical distribution of the incidence of T1DM in the island and (2) to investigate pre-T1DM in a large children cohort. Achieving these two main objectives should allow for eventual identification of areas with a high or low prevalence of T1DM and pre-T1DM across Sardinia (“hot and cold spots project”). This should lead to design models of prediction to formulate safe preventive measures especially addressed to the general population living in areas at high risk. To these aims, several studies have been designed and others have branched out from the initial project. The studies can be divided into four main groups, with subgroups on the basis of the area of investigation:
-
Studies for monitoring the T1DM incidence: the Sardinian T1DM Registry
- Conscripts study
- Migrants study
-
Studies on pre-T1DM
-
Prediction studies:
- Newborns Sardinia Insulin-Dependent Diabetes Mellitus Study (NSIS)
- Sardinia Schoolchildren Insulin-Dependent Diabetes Mellitus Study (SSIS)
-
Prevention studies:
- Trial to Reduce Insulin-Dependent Diabetes in Ethnically At-Risk (TRIGR)
- Environmental Factor Study
- The Ecological/Environmental/Veterinarian Variables T1DM Study
-
In this review, a survey on these studies is reported.
Studies for Monitoring T1DM Incidence: Epidemiology and Prevention of Diabetes; Aetiology of Childhood Diabetes on an Epidemiological Basis; Type 1 Genetic Epidemiologic Resource
The Epidemiology and Prevention of Diabetes (EURODIAB) started in 1989, supported by the European Union, with the objective to define the incidence, the complications, and the social impact of T1DM across Europe. The study has included teams from different places that belong to the European Union and from some countries that do not belong to the European Union (i.e., Israel) and comprises 40 different groups working together collecting data from a total of almost 30 million children initially aged 0–14 years, later extended up to 29 years.14
Different phases of the initial project have followed each other, and from 1996 until the writing of this article, the last one, EURODIAB TIGER (Type 1 Genetic Epidemiologic Resource), is still ongoing, with the aim to define precisely the incidence of T1DM in the European population.
Current data confirm the North-to-South European gradient, with the highest incidence rate in Scandinavian countries and, interestingly, the exception of Sardinia, where the disease has a risk higher than expected, approaching that of Finland (Figure 1).
Figure 1.
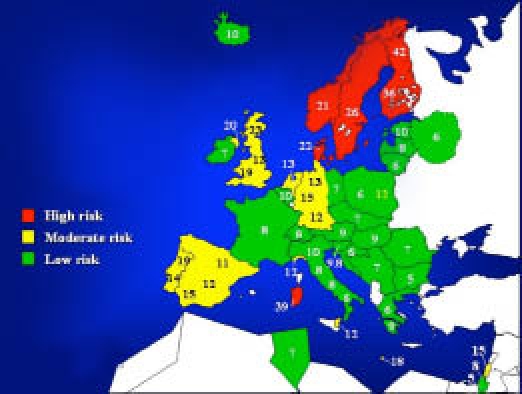
The T1DM incidence among children aged 0–14 years in Europe and in some Mediterranean countries based on EURODIAB data.
Since 1989, in Sardinia, a population-based and prospective register of T1DM occurring in children aged <15 years and living in the island was developed according to the EURODIAB criteria.15
EURODIAB data analysis of an 11-year period (from 1989 to 1999) had shown these results for Sardinia:
The average yearly standardized incidence rate of T1DM is 38.8/100,000 (95% confidence interval [CI] 36.7– 41.1), five to seven times higher than continental Italy. Interestingly, the incidence rate is the second (after Finland) highest worldwide.4
The male-to-female ratio is 1.4 (95% CI 1.3–1.8). The highest male-to-female ratio was registered in the age range 10–14 years (1.8).
The median age at the onset was 9 years for both sexes.
For both sexes, incidence was quite high at the age of 3 years (41.0/100,000).
A statistically significant (p = .002) rising trend of incidence was observed from the beginning to the end of the study, with an estimated average yearly increase of 2.8% (95% CI 1.0–4.7), similar to the mean increase across Europe (Figure 2).
Figure 2.
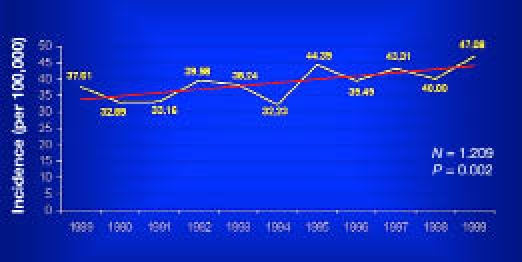
Incidence rate trend in Sardinia among children aged 0–14 years from 1989–1999 (p = .002).
The geographical distribution of T1DM risk within the four provinces of the island was mapped, showing that the highest incidence (“hot spot”) was in the middle-western part of the island (Oristano Province, 45/100,000) followed by Cagliari (38/100,000) and Nuoro (35/100,000).
The province with the lowest incidence (“cold spot”) was in the northwestern part (Sassari Province, 30/100,000) of the island (Figures 3 and 4). However, it is remarkable that the incidence, even in the cold spot area, was three times greater than the mean incidence in mainland Italy5,16–19 (Figure 5).
Figure 3.
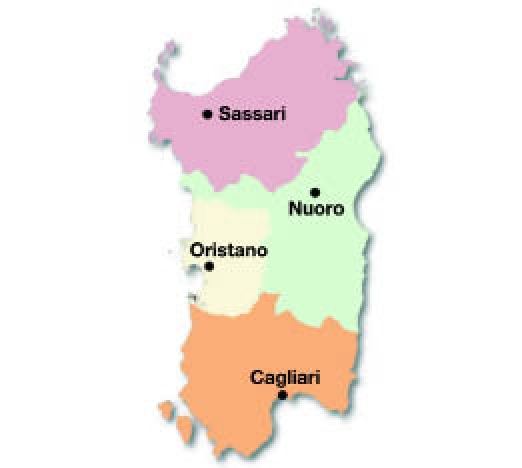
Four Sardinian provinces.
Figure 4.
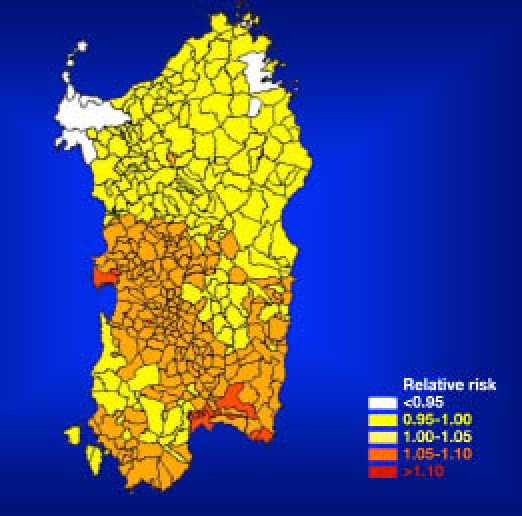
Estimated risk of T1DM among children aged 0–14 years in the four Sardinian provinces from 1989–1999.
Figure 5.
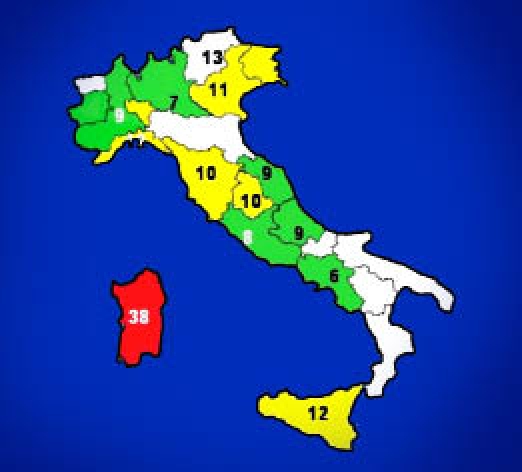
The T1DM incidence in Italy according to RIDI (Italian Registry of T1DM).
Conscript Study
Using the Italian National Service Register (Conscript Register) related only to Sardinia, where T1DM is a cause of rejection, a series of birth cohorts of male army conscripts aged 18–19 years were screened to obtain new and more detailed information about temporal trends and geographical distribution of T1DM in Sardinia.20
In the first report, an analysis was made of the birth cohort from 1936 to 1973,18 identifying a total of 678 Sardinian (born and permanently residing) diabetic subjects. The point prevalence (× 1000) at the age of 20 years in the birth cohorts ranged from values close to zero for the first 10 cohorts (1936–1945) up to a maximum of 3.08 (95% CI 22.8–4.08) for the 1966 cohort and continued to be high thereafter, although an apparent decrease was observed from the early 1970s birth cohorts. Type 1 diabetes mellitus was distributed throughout the four provinces of Sardinia without any significant heterogeneity; however, according to the geographical distribution of diabetes from EURODIAB data, the highest prevalence of disease was observed in the Cagliari and Oristano provinces, followed by Nuoro and Sassari.
These data suggest a gradually increasing trend of male T1DM in Sardinia, with a 29-fold increase between the late 1930s and the late 1960s birth cohorts. This confirms the high incidence of T1DM in the 0–14 year age groups reported among Sardinians during EURODIAB-ACE (Aetiology of Childhood Diabetes on an Epidemiological Basis).17
To update the prevalence, a second retrospective analysis was conducted on a total of 83,807 Sardinian males aged 18 years (birth cohort 1974 to 1979), virtually representative of the whole Sardinian population of that age and sex. A total of 307 Sardinian T1DM subjects were identified, and T1DM prevalence was 3.66/1000 (95% CI 3.28–4.09). These data were concordant with the first survey and the EURODIAB data confirming the south-to-north gradient of T1DM across the island.
No significant temporal trend of prevalence was seen in the six years examined. By pooling data of both birth cohorts studied, the increasing trend of prevalence and the progressive increase of the T1DM risk among males in Sardinia were confirmed20 (Figure 6).
Figure 6.
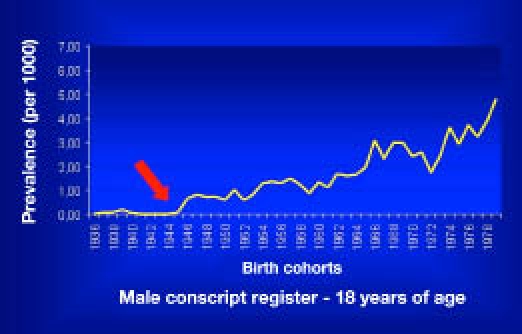
Secular trend of T1DM in Sardinia.
Migrant Studies
The etiology of T1DM is still largely unknown, but the most widely accepted hypothesis is that the disorder has a multifactorial origin, involving a complex interaction among genetic predisposition,21 immunological determinants, and environmental agents.22
One way to understand the potential role of environmental factors compared with genetic predisposition toward T1DM is to study the migrant populations.
For this purpose, different reports have been carried out on the Sardinians and their offspring migrated to mainland Italy.
Muntoni and coworkers23 evaluated the incidence of T1DM in children born in Lazio (low-incidence region) but whose parents came from Sardinia (high-incidence region). The results of the study demonstrated that the incidence of T1DM in Sardinian-heritage children born and residing in Lazio was 33.8/100,000 (95% CI 0–99.0) for those with both Sardinian parents and one-half (15.7/100,000, 95% CI 8.7–26.6) for those with only one Sardinian parent. Incidence rates overlapped with ones recorded in Sardinia (34.4/100,000, CI 31.3–37.9) and was four times as high as that among the indigenous population.24
Calori and colleagues25 studied Sardinian-heritage children in Lombardy (low-incidence region, as Lazio). Sardinian-heritage children with both Sardinian parents had a relative risk of developing T1DM of 3.4 (CI 1.3–9) and 2.0 (CI 1.2–3.3) if only one parent was Sardinian, compared with children whose parents were not Sardinian.
Tenconi and associates26 carried out a prevalence study of T1DM in the Sardinian population that migrated from the island to Pavia. A total of 10 patients affected by T1DM were identified (prevalence = 4.4/1000, CI 95% 2.1–8.1).
Preliminary data showed that the prevalence of T1DM in the Sardinian emigrants is still higher compared to the general population of the same geographical area and that, interestingly, the age of the disease onset seems to be delayed in the individuals who developed diabetes after emigration compared with the ones who live on the island.
In the second part of the study (called “wet”), a total of 554 Sardinian immigrants and their 226 offspring (21.7% with both Sardinian parents and 78.3% with only one Sardinian parent) were evaluated for the presence of risk haplotypes and/or autoantibody positivity. High-risk patients (genetic and/or autoantibody positivity) were then followed up on for seven years. Results showed a higher prevalence of glutamic acid decarboxylase autoantibodies (GADA) positivity within Sardinian immigrants. GADA positivity may be considered as the detectable marker of pre-diabetes and, in high-risk individuals, may allow diagnosis of a subclinical phase of T1DM.27,28
We can conclude that studies on Sardinian immigrants (genetically prone to the disease) appear to be in contrast with the environmental hypothesis and emphasize the pivotal role of genetic susceptibility in developing T1DM.25,29,30
Studies on Pre-Type 1 Diabetes Mellitus
The autoimmune attack of β cells often initiates several years before the clinical onset of T1DM. This “preclinical phase” (pre-T1DM) has been described as related to the presence of several islet cell autoantibodies. Four are considered as predictive markers of T1DM: islet-cell autoantibodies (ICA),31 GADA,32 autoantibodies to insulin,33 and autoantibodies to tyrosine phosphatase-like protein (IA-2).34,35
It is noteworthy that, even in individuals at risk, the predictive value of diabetes-related autoantibodies is dependent on titer and on the multiplicity of circulating antibodies.36 Moreover, the occurrence of T1DM is sporadic in about 90% of cases, and studies carried out on unselected individuals suggest that multiple islet antibodies determination also has a significant predictive value in the general population.36,37
Newborns Sardinia Insulin-Dependent Diabetes Mellitus Study
Susceptibility to develop T1DM is largely inherited, residing predominantly in specific human leukocyte antigen genotypes.38,39
The next step requires exposure to one or more environmental triggers that alter the immune function, thereby initiating β-cell destruction. Various environmental triggers, e.g., certain viruses,40,41 dietary components,42 and environmental toxins,43 have been proposed as triggering the autoimmune process.
It is completely unknown precisely when those external triggers start to act, and one cannot exclude the possibility that they may even act as early as in utero. The NSIS was developed with the intention of further clarifying the natural history of the disease as early as from birth and identifying the island areas where individuals are at higher potential risk of developing T1DM.
Cordal sera of all newborns of the island were collected between 1994 and 1996. A preliminary evaluation of the appearance of ICA was tested in cord blood samples to verify the role of their transplacental crossing into fetal blood as a possible risk factor for T1DM. Subsequently, children at risk recruited were investigated from the clinical, genetic, and immunological testing (ICA, GADA, and IA-2A) standpoint and followed up with over time for new cases of T1DM.
Initial results on the prevalence of ICA detected in cordal sera were as follows:
The overall prevalence of ICA is 3.9% for titers ≥5 JDF44 units, 3.0% for titers 5–20 JDF units, and 0.9% JDF units for titers >20.
The geographical pattern of ICA prevalence followed a north-to-south gradient different from that of the incidence of overt T1DM.15,28
Follow-up data indicate that (1) GADA tends to appear before ICA and IA-2A at year 1, (2) there is a significant increase in the development of ICA at year 2 when compared with year 1, and (3) children developing T1DM before 3 years of age appeared to be ICA negative at birth (cord blood) but positive at the onset of the disease. Moreover, from the epidemiological point of view, it appears that there is a possible increase in the incidence of T1DM in children under 3 years of age.15,45
To the best of our knowledge, the NSIS is the first study that identified the initial and subsequent appearance of the known islet-related immunological markers in a population living in an area of high incidence of T1DM, such as Sardinia.
Sardinia Schoolchildren Insulin-Dependent Diabetes Mellitus Study
This prospective study was conducted between 1986 and 1994 on 8448 of the same schoolchildren studied for the incidence of goiter in Sardinia.45 Purposes of the SSIS have been (1) to clarify the natural history of the disease in “sporadic” versus “familial” cases and (2) to define the predictive value of autoantibody positivity (single or combined) in relation to the risk of future T1DM.
All schoolchildren were evaluated for the presence of three diabetes-related autoantibodies (i.e., ICA, GADA, and IA-2A) and have been followed up with for a period of 10 years. Schoolchildren with T1DM at the time of recruitment were excluded from the study.
The main conclusions from this study are as follows:
Any single antibody tested showed a sensitivity greater than 64.9% and a positive predictive value lower than 16%. Positivity to any single auto-antibody gave a specificity to develop T1DM greater than 97%.
The risk of developing T1DM within 10 years from enrollment was 55.3 times higher in positive to any single autoantibody tested and increased 14.5 times for any double positivity.
Only 3 out of 43 (7%) schoolchildren who developed T1DM within these 10 years were negative to all autoantibodies.
Combination of ICA and IA-2A positivity reached a positive predictive value of 51.3%.
These data support the importance of screening multiple autoantibodies among young Sardinians to identify prog-ressor individuals (Dr. Songini personal communication).
Trial to Reduce Insulin-Dependent Diabetes in Ethnically At-Risk
The TRIGR project is an ongoing international multi-centric study in which 2800 newborns in the world have been enrolled (around half of Italian cases are from Sardinia).
The project aims to assess the hypothetical diabetogenic role of cow's milk consumed during the first months of life in genetically at-risk newborns (first-degree relatives with diabetes).
In this double-blind randomized study, one group of newborns is being fed with conventional cow's milk whereas the other group is being fed with casein hydrolyzed milk.
This study is based on the hypothesis that the second group should develop diabetes-related autoantibodies and thus overt disease with lower incidence compared to the control group.
The study is still ongoing, and if the hypothesis is confirmed, a primary prevention of T1DM may be implemented.
Environmental Factor Study and the Ecological/Environmental/Veterinarian Variables Type 1 Diabetes Mellitus Study
Several environmental factors and their relation to T1DM have been investigated in Sardinia:
Seasonal patterns. As already reported in other countries, the peak of incidence of T1DM occurs in the autumn–winter seasons,47 and the prevalence of T1DM is higher in older males in high-risk areas.18
Temperatures and rates of precipitation. The Sassari province, which has the lowest incidence of T1DM on the island, also has the lowest temperature, with the highest rainfall.10,19
-
Correlations.
- On the island, there is no overlap between areas with high prevalence of T1DM and areas of both past and present epidemic disorders, such as thalassemia, malaria, and glucose-6-phosphate dehydrogenase deficiency.10
- A positive correlation between the occurrence of T1DM in cow's milk rather than breastfed children has not been confirmed, although the study was carried out in the province of Sassari only.48
- No correlation between T1DM incidence and nitrate intake with drinking water.49
- No correlation has been found between T1DM incidence and intake of casein A1 and B, which are supposed to be diabetogenic. Conflicting data are reported regarding the association between cow's milk intake and incidence of T1DM on the island, being the Italian region with both the highest diabetes incidence and the highest cow's milk intake. However, Sardinia lies far from the regression line between cow's milk intake and T1DM incidence drawn from European data.50,51
The impact of environmental factors will be extended by comparing “hot” and “cold” areas for incidence of the disease. A possible relationship between T1DM and disease affecting domestic animals (e.g., dogs, cats, cattle, equine, and swine) will also be investigated.
Conclusions
Historical and geographical peculiarities confer a rare ethnic homogeneity to Sardinia that has been maintained for several centuries. Together with its high incidence of T1DM, this creates a unique epidemiological observatory, where the dynamics of the events leading to T1DM could be investigated deeply.
By taking advantage of our framework, following epidemiological trends of T1DM, our ultimate objective is to map the island for “hot and cold spots” of T1DM and pre-T1DM in order to identify areas where the resident population could have an increased risk for developing T1DM. Identifying “hot areas” and studying environmental, genetic, and immunological characteristics of residing population should allow scientists to gain decisive insights into the etiopathogenesis of the disease.
Abbreviations
- CI
confidence interval
- EURODIAB
Europe Diabetes Epidemiology and Prevention of Diabetes
- GADA
glutamic acid decarboxylase autoantibodies
- IA-2
autoantibodies to tyrosine phosphatase-like protein
- ICA
islet-cell autoantibodies
- NSIS
Newborns Sardinia Insulin-Dependent Diabetes Mellitus Study
- SSIS
Sardinia Schoolchildren Insulin-Dependent Diabetes Mellitus Study
- T1DM
type 1 diabetes mellitus
- TRIGR
Trial to Reduce Insulin-Dependent Diabetes in Genetically At-Risk
References
- 1.Borg WP, Sherwin RS. Classification of diabetes mellitus. Adv Intern Med. 2000;45:279–295. [PubMed] [Google Scholar]
- 2.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51(12):3353–3361. doi: 10.2337/diabetes.51.12.3353. [DOI] [PubMed] [Google Scholar]
- 3.Onkamo P, Väänänen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of type I diabetes—the analysis of the data on published incidence trends. Diabetologia. 1999;42(12):1395–1403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- 4.Green A, Patterson CC, EURODIAB TIGER Study Group-Europe and Diabetes Trends in the incidence of childhood-onset diabetes in Europe 1989–1998. Diabetologia. 2001;44(Suppl 3):B3–8. doi: 10.1007/pl00002950. [DOI] [PubMed] [Google Scholar]
- 5.Casu A, Pascutto C, Bernardinelli L, Songini M. Type 1 diabetes among sardinian children is increasing: the Sardinian diabetes register for children aged 0–14 years (1989–1999) Diabetes Care. 2004;27(7):1623–1629. doi: 10.2337/diacare.27.7.1623. [DOI] [PubMed] [Google Scholar]
- 6.Gillespie KM, Bain SC, Barnett AH, Bingley PJ, Christie MR, Gill GV, Gale EA. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 2004;364(9446):1699–1700. doi: 10.1016/S0140-6736(04)17357-1. [DOI] [PubMed] [Google Scholar]
- 7.DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23(8):857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 8.Cappello N, Rendine S, Griffo R, Mameli GE, Succa V, Vona G, Piazza A. Genetic analysis of Sardinia: I Data on 12 polymorphisms in 21 linguistic domains. Ann Hum Genet. 1996;60(Pt 2):125–141. doi: 10.1111/j.1469-1809.1996.tb01183.x. [DOI] [PubMed] [Google Scholar]
- 9.Piazza A, Mayr WR, Contu L, Amoroso A, Borelli I, Curtoni ES, Marcello C, Moroni A, Olivetti E, Richiardi P. Genetic and population structure of four Sardinian villages. Ann Hum Genet. 1985;49(Pt 1):47–63. doi: 10.1111/j.1469-1809.1985.tb01675.x. [DOI] [PubMed] [Google Scholar]
- 10.Songini M, Casu A. Epidemiology of childhood diabetes. Acta Biomed. 2005;76(Suppl 3):19–25. [PubMed] [Google Scholar]
- 11.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288(14):1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 12.Gabbay RA, Khan L, Peterson KL. Critical features for a successful implementation of a diabetes registry. Diabetes Technol Ther. 2005;7(6):958–967. doi: 10.1089/dia.2005.7.958. [DOI] [PubMed] [Google Scholar]
- 13.Locatelli M, Songini M, Bottazzo GF. [IDDM-Sardinia Project: a study model on the etiopathogenesis of insulin-dependent diabetes mellitus and other autoimmune pathologies. Gruppi di studio IDDM-Sardegna] Ann Ist Super Sanita. 1999;35(2):253–263. [PubMed] [Google Scholar]
- 14.Green A, Gale EA, Patterson CC. Incidence of childhood-onset insulin-dependent diabetes mellitus: the EURODIAB ACE Study. Lancet. 1992;339(8798):905–909. doi: 10.1016/0140-6736(92)90938-y. [DOI] [PubMed] [Google Scholar]
- 15.Bottazzo GF, Loviselli A, Velluzzi F, Mariotti S, Cossu E, Cirillo R, Balestrieri A, Delitala G, Sepe V, Songini M. The “Sardinia-IDDM study:” an attempt to unravel the cause of insulin-dependent diabetes mellitus in one of the countries with the highest incidence of the disease in the world. Ann Ist Super Sanita. 1997;33(3):417–424. [PubMed] [Google Scholar]
- 16.Cherubini V, Chiarelli F, Altobelli E, Verrotti A, Carle F. Regional variability in the epidemiology of childhood diabetes in Italy. J Pediatr Endocrinol Metab. 1997;10(5):471–478. doi: 10.1515/jpem.1997.10.5.471. [DOI] [PubMed] [Google Scholar]
- 17.Songini M, Bernardinelli L, Clayton D, Montomoli C, Pascutto C, Ghislandi M, Fadda D, Bottazzo GF. The Sardinian IDDM study: 1. Epidemiology and geographical distribution of IDDM in Sardinia during 1989 to 1994. Diabetologia. 1998;41(2):221–227. doi: 10.1007/s001250050893. [DOI] [PubMed] [Google Scholar]
- 18.Songini M, Casu A, Ashkenazi I, Laron Z. Seasonality of birth in children (0-14 years) and young adults (0-29 years) with type 1 diabetes mellitus in Sardinia differs from that in the general population. The Sardinian Collaborative Group for Epidemiology of IDDM. J Pediatr Endocrinol Metab. 2001;14(6):781–783. doi: 10.1515/jpem.2001.14.6.781. [DOI] [PubMed] [Google Scholar]
- 19.Songini M. Diabete di tipo I in Sardegna: il progetto “hot and cold spots.”. Aggiornamento Medico. 1997;21(1):27–33. [Google Scholar]
- 20.Songini M, Loche M, Muntoni S, Stabilini M, Coppola A, Dessi G, Green A, Bottazzo GF, Muntoni S. Increasing prevalence of juvenile onset type 1 (insulin-dependent) diabetes mellitus in Sardinia: the military service approach. Diabetologia. 1993;36(6):547–552. doi: 10.1007/BF02743272. Jun. [DOI] [PubMed] [Google Scholar]
- 21.Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32(4):457–467. doi: 10.1016/j.immuni.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Knip M, Veijola R, Virtanen SM, Hyöty H, Vaarala O, Akerblom HK. Environmental triggers and determinants of type 1 diabetes. Diabetes. 2005;54(Suppl 2):S125–136. doi: 10.2337/diabetes.54.suppl_2.s125. [DOI] [PubMed] [Google Scholar]
- 23.Muntoni S, Fonte MT, Stoduto S, Marietti G, Bizzarri C, Crinò A, Ciampalini P, Multari G, Suppa MA, Matteoli MC, Lucentini L, Sebastiani LM, Visalli N, Pozzilli P, Boscherini B, Muntoni S. Incidence of insulin-dependent diabetes mellitus among Sardinian- heritage children born in Lazio region, Italy. Lancet. 1997;349(9046):160–162. doi: 10.1016/s0140-6736(96)04241-9. [DOI] [PubMed] [Google Scholar]
- 24.Sebastiani L, Visalli N, Adorisio E, Suppa MA, Buzzetti R, De Cicco AL, Giovannini C, Comerci MD, Negri M, Pozzilli P. A 5-year (1989-1993) prospective study of the incidence of IDDM in Rome and the Lazio region in the age-group 0-14 years. Diabetes Care. 1996;19(1):70–73. doi: 10.2337/diacare.19.1.70. [DOI] [PubMed] [Google Scholar]
- 25.Calori G, Gallus G, Bognetti E, Chiumello G. Insulin-dependent diabetes mellitus in Sardinian-heritage children living in Lombardy. Lancet. 1998;351(9098):263–264. doi: 10.1016/S0140-6736(98)24004-9. [DOI] [PubMed] [Google Scholar]
- 26.Tenconi MT, Devoti G, Nasetti G, Piazza M, Songini M, Bottazzo GF. Diabete mellito di tipo 1 nei sardi emigrati in provincia di Pavia. Il Diabete. 1999;11(suppl 2):102–103. [Google Scholar]
- 27.Tenconi MT, Devoti G, Rizzo M, Roncarolo F, Bernasconi A, Lanati N, Calcaterra V, Songini M, Locatelli M, Bottazzo GF. Type 1 diabetes risk and autoantibody positivity in Sardinian migrants in the province of Pavia. North Am J Med Sci. 2009;1:48–53. [PMC free article] [PubMed] [Google Scholar]
- 28.Guaita G, Shattock M, Cossu E, Sepe V, Songini M, Cirillo R, Bottazzo GF, SNI study group The Sardinian newborn-IDDM (SNI) study. North to south gradient for ICA in 2933 cord blood samples from Sardinian newly born children. Diabetologia. 1995;38(Suppl 1):A86. [Google Scholar]
- 29.Bruno G, Pagano G, Faggiano F, De Salvia A, Merletti F. Effect of Sardinian heritage on risk and age at onset of type 1 diabetes: a demographic case-control study of Sardinian migrants. Int J Epidemiol. 2000;29(3):532–535. [PubMed] [Google Scholar]
- 30.Muntoni S. Genetic versus environmental factors in insulin-dependent diabetes mellitus. Lancet. 1997;349(9065):1626. doi: 10.1016/S0140-6736(05)61666-2. [DOI] [PubMed] [Google Scholar]
- 31.Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222(4630):1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- 32.Payton MA, Hawkes CJ, Christie MR. Relationship of the 37,000- and 40,000-M(r) tryptic fragments of islet antigens in insulin-dependent diabetes to the protein tyrosine phosphatase-like molecule IA-2 (ICA512) J Clin Invest. 1995;96(3):1506–1511. doi: 10.1172/JCI118188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De Camilli P. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 34.Bonifacio E, Lampasona V, Genovese S, Ferrari M, Bosi E. Identification of protein tyrosine phosphatase-like IA2 (islet cell antigen 512) as the insulin-dependent diabetes-related 37/40K autoantigen and a target of islet-cell antibodies. J Immunol. 1995;155(11):5419–5426. [PubMed] [Google Scholar]
- 35.Seissler J, Hatziagelaki E, Scherbaum WA. Modern concepts for the prediction of type 1 diabetes. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S304–316. doi: 10.1055/s-2001-18590. [DOI] [PubMed] [Google Scholar]
- 36.Strebelow M, Schlosser M, Ziegler B, Rjasanowski I, Ziegler M. Karlsburg type 1 diabetes risk study of a general population: frequencies and interactions of the four major type 1 diabetes-associated autoantibodies studied in 9419 schoolchildren. Diabetologia. 1999;4:661–670. doi: 10.1007/s001250051213. [DOI] [PubMed] [Google Scholar]
- 37.LaGasse JM, Brantley MS, Leech NJ, Rowe RE, Monks S, Palmer JP, Nepom GT, McCulloch DK, Hagopian WA, Washington State Diabetes Prediction Study Successful prospective prediction of type 1 diabetes in schoolchildren through multiple defined autoantibodies: an 8-year follow-up of the Washington State Diabetes Prediction Study. Diabetes Care. 2002;25(3):505–511. doi: 10.2337/diacare.25.3.505. [DOI] [PubMed] [Google Scholar]
- 38.Devendra D, Liu E, Eisenbarth GS. Type 1 diabetes: recent developments. BMJ. 2004;328(7442):750–754. doi: 10.1136/bmj.328.7442.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambert AP, Gillespie KM, Thomson G, Cordell HJ, Todd JA, Gale EA, Bingley PJ. Absolute risk of childhood-onset type 1 diabetes defined by human leukocyte antigen class II genotype: a population-based study in the United Kingdom. J Clin Endocrinol Metab. 2004;89(8):4037–4043. doi: 10.1210/jc.2003-032084. [DOI] [PubMed] [Google Scholar]
- 40.Lammi N, Karvonen M, Tuomilehto J. Do microbes have a causal role in type 1 diabetes? Med Sci Monit. 2005;11(3):RA63–69. [PubMed] [Google Scholar]
- 41.Robles DT, Eisenbarth GS. Type 1A diabetes induced by infection and immunization. J Autoimmun. 2001;16(3):355–362. doi: 10.1006/jaut.2000.0483. [DOI] [PubMed] [Google Scholar]
- 42.Akerblom HK, Vaarala O, Hyöty H, Ilonen J, Knip M. Environmental factors in the etiology of type 1 diabetes. Am J Med Genet. 2002;115(1):18–29. doi: 10.1002/ajmg.10340. [DOI] [PubMed] [Google Scholar]
- 43.Helgason T, Jonasson MR. Evidence for a food additive as a cause of ketosis-prone diabetes. Lancet. 1981;2(8249):716–720. doi: 10.1016/s0140-6736(81)91048-5. [DOI] [PubMed] [Google Scholar]
- 44.Verge CF, Stenger D, Bonifacio E, Colman PG, Pulcher C, Bingley PJ, Eisenbarth GS. Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: combinatorial islet autoantibody workshop. Diabetes. 1998;47(12):1857–1866. doi: 10.2337/diabetes.47.12.1857. Dec. [DOI] [PubMed] [Google Scholar]
- 45.Bottazzo GF, Cossu E, Cirillo R, Loviselli A, Velluzzi F, Mariotti S, Balestrieri A, Delitala G, Sepe V, Songini M, Sardinia-IDDM Study Groups Sardinia: a battlefield approach to type I diabetes epidemiology. Horm Res. 1997;48(Suppl 4):64–66. doi: 10.1159/000191317. [DOI] [PubMed] [Google Scholar]
- 46.Loviselli A, Velluzzi F, Mossa P, Cambosu MA, Secci G, Atzeni F, Taberlet A, Balestrieri A, Martino E, Grasso L, Songini M, Bottazzo GF, Mariotti S, Sardinian Schoolchildren Study Group The Sardinian Autoimmunity Study: 3. Studies on circulating antithyroid antibodies in Sardinian schoolchildren: relationship to goiter prevalence and thyroid function. Thyroid. 2001;11(9):849–857. doi: 10.1089/105072501316973109. [DOI] [PubMed] [Google Scholar]
- 47.Karvonen M, Jäntti V, Muntoni S, Stabilini M, Stabilini L, Muntoni S, Tuomilehto J. Comparison of the seasonal pattern in the clinical onset of IDDM in Finland and Sardinia. Diabetes Care. 1998;21(7):1101–1109. doi: 10.2337/diacare.21.7.1101. [DOI] [PubMed] [Google Scholar]
- 48.Meloni T, Marinaro AM, Mannazzu MC, Ogana A, La Vecchia C, Negri E, Colombo C, Meloni T, Marinaro AM, Mannazzu MC, Ogana A, La Vecchia C, Negri E, Colombo C. Diabetes Care. 1997;20(3):340–342. doi: 10.2337/diacare.20.3.340. [DOI] [PubMed] [Google Scholar]
- 49.Casu A, Carlini M, Contu A, Bottazzo GF, Songini M. Type 1 diabetes in sardinia is not linked to nitrate levels in drinking water. Diabetes Care. 2000;23(7):1043–1044. doi: 10.2337/diacare.23.7.1043. [DOI] [PubMed] [Google Scholar]
- 50.Fava D, Leslie RD, Pozzilli P. Relationship between dairy product consumption and incidence of IDDM in childhood in Italy. Diabetes Care. 1994;17(12):1488–1490. doi: 10.2337/diacare.17.12.1488. [DOI] [PubMed] [Google Scholar]
- 51.Muntoni S, Loddo S, Stabilini M, Stabilini L, Muntoni S. Cow's milk consumption and IDDM incidence in Sardinia. Diabetes Care. 1994;17(4):346–347. doi: 10.2337/diacare.17.4.346. [DOI] [PubMed] [Google Scholar]


