Abstract
A straightforward method for the simultaneous preparation of (2S, 3R, 2′R)- and (2S, 3R, 2′S)-2′-hydroxy-ceramides (2′-OHCer) from (2S, 3R)-sphingosine acetonide precursors and racemic mixtures of 2-hydroxy fatty acids (2-OHFAs) is described. The obtained 2′-OH-C4-, -C6-, -C12-, -C16-Cer and 2′-OH-C6-dhCer pairs of diastereoisomers were characterized thoroughly by TLC, MS, NMR and optical rotation. Dynamic and multidimensional NMR studies provided evidence that polar interfaces of 2′-OHCers are extended and more rigid than observed for the corresponding non-hydroxylated analogs. Stereospecific profile on growth suppression of MCF7 cells was observed for (2′R)- and (2′S)-2′-OH-C6-Cers and their dihydro analogs. The (2′R)-isomers were more active than the (2′S)-isomers (IC50 ~ 3μM/8μM and IC50 ~ 8 μM/12 μM, respectively), surpassing activity of the ordinary C6-Cer (IC50 ~12μM) and C6-dhCer (IC50 ~ 38μM). Neither isomer of 2′-OH-C6-Cers and 2′-OH-C6-dhCers was metabolized to their cellular long chain 2′-OH-homologs. Surprisingly, the most active (2′R)-isomers did not influence the levels of the cellular Cers nor dhCers. Contrary to this, the (2′S)-isomers generated cellular Cers and dhCers efficiently. In comparison, the ordinary C6-Cer and C6-dhCer also significantly increased the levels of their cellular long chain homologs. These peculiar anabolic responses and SAR data suggest that (2′R)-2′-OHCers/dhCers may interact with some distinct cellular regulatory targets in a specific and more effective manner than their non-hydroxylated analogs. Thus, stereoisomers of 2′-OHCers can be potentially utilized as novel molecular tools to study lipid-protein interactions, cell signaling phenomena and to understand the role of hydroxylated sphingolipids in cancer biology, pathogenesis and therapy.
Keywords: ceramide, (2′R)-2′-hydroxy-ceramide, (2′S)-2′-hydroxy-ceramide, stereospecificity, synthesis, LC-MS/MS, NMR, cytotoxicity, cellular sphingolipids
1. Introduction
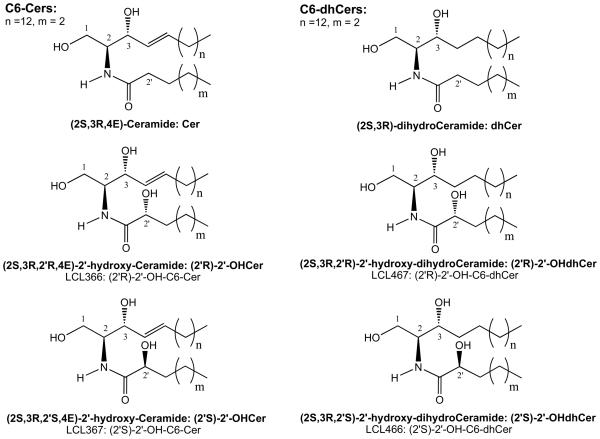
Ceramides (Cers, Scheme 1) have emerged as key modulators of cancer cell growth and apoptosis.1-4 They are a highly diversified structures consisting of different sphingoid base backbones and an amide linked varied chain fatty acids (FAs; mostly C14-C26 saturated, unsaturated and hydroxylated FAs).5
Scheme 1.
Chemical structures of D-erythro-Cer/dhCer and (2′R)- and (2′S)-isomers of D-erythro-2′-OHCer/dhCers
Naturally occurring 2′-hydroxy-Cers (2′-OHCers), namely (2S, 3R, 2′R, 4E)-2′-hydroxy-Cers, Scheme 1), contain an additional hydroxyl group located in the alpha position to the carbonyl group in their N-acyl moieties. The formation of an additional chiral center in these molecules gives rise to the possibility to create 2 stereoisomers: 2′R or 2′S. Natural 2′-OHCers represent (2′R)-isomer.6-14
A large body of recent research showed that sphingolipids (SPLs) containing 2′-OHCers as their building blocks are common components of mammalian and plant specific lipid membranes, cells, organs and tissues including brain, epidermis and epithelial tissue of the digestive tract.15-18 Despite their prevalence in the nervous system, epidermis and kidney as well as suggested and confirmed contributions to the pathogenesis of many diseases such as Farber's disease19,20, hereditary leukodystrophy21 and cancer22,23, their physiological functions remain mostly unknown.18
2′-OH-Sphingomyelin, glucosyl-2′-OH-Cer, lactosyl-2′-OH-Cer and their complex glyco-SPL analogs have been isolated from different natural sources.18,24-26 Galactosylceramide, commonly called “cerebroside”, containing cerebronic acid in the N-acyl part of Cer (2′-OH-C24-Cer) is a major glycosphingolipid in brain and nervous tissues and builds up its complex analogs: sulfatide, ganglioside GM4 (sialylgalactosyl-Cer) and Gal2Cer (galabiosyl-Cer).27 Interestingly, the ratio of hydroxy- to nonhydroxy-FA in cerebrosides increases with the complexity of the central nervous system.28 This cerebroside was also identified in intestines from the Japanese quail and Runner and Kidney beans.29,30
2′-OHCers are present as sub-components of the skin (Cer 4-7, 9 sub-classes).15,31-33 They are thought to have structural roles to facilitate tight packing of lipids and to influence the conformation of the head groups via the hydrogen bonds.33-35 This class of Cers was also identified in human erythrocytes, the small intestine liver and kidney of rats and the Harderian gland of guinea pigs.18,36 Some special 2′-OHCers (C3, C16-C26 and C29) were found to have a remarkable biological effects, including cytotoxicity and antiproliferative activities related to activation of caspase 3.37-40
2′-OHCers may be produced by the action of fatty acid 2-hydroxylase (FA2H), which has been recently identified molecularly.18,41,42 Mutation of this enzyme results in inherited human neurodegenerative disorders.21,43
Interestingly, deficiency in lysosomal CDase causes a lysosomal storage disease, resulting in the accumulation of 2′-OHCers and Cers in the kidneys, cerebrum, lungs, and stomach of Farber's disease patients.19,20,44,45 Studies performed in certain animal cells showed that 2′-OHCers were preferably converted to galactosylceramides, whereas Cers with normal fatty acids were used for glucosylceramides formation, but this was not a universal rule.46 Elevated cellular toxicity and plant growth regulatory activity of some natural 2′-OHCers, were also reported.11,14
To date we do not know what factors predominantly control the structure of 2′-OHCer or are responsible for its distinct biological functions. The following are lines of evidence confirming the roles of 2′-OH group of 2′-OHCers: (i) formation of the extensive network of hydrogen bonds between the galactose head group of complex 2′-OH-SPL and the polar head of Cer, (ii) promotion of Cers monolayer condensation to a close-packed arrangement, and (iii) increased effects on the phase transition temperatures and gel phases stabilization in comparison to non-hydroxylated analogs.35,47,48
Although ceramide has been extensively studied as a mediator of apoptotic responses, 2′-OHCers have never been considered as ubiquitous signaling molecules. However, our preliminary studies showed that 2′-OHCers were ubiquitously present at a very low level in numerous cancer cell types (e.g. ~5 % of total Cers in MCF7 cells, unpublished) suggesting that these Cers may play a role in Cer-mediated cell signaling.
Current knowledge on the potential involvement of 2′-OHCers in metabolism and cell signaling phenomena as well as access to advanced structure-biological activity correlation data is very limited due to the lack of well-characterized synthetic 2′-OHCers to be potentially used as molecular probes and analytical standards. To address the above issues and to have non-restricted access to varied-chain model 2′-OHCers, we developed a simple and efficient method for the simultaneous preparation of their pure isomers from easily available precursors and reagents.
In this publication we describe synthesis of (2′R)- and (2′S)-2′-OH-C4-, C6-, C12-, C16-Cer and (2′R)- and (2′S)-2′-OH-C6-dhCer isomers and present their full physiochemical and NMR characterization. Basic biological effects of the newly synthesized 2′-OH-C6-Cer and 2′-OH-C6-dhCer diastereoisomers were examined in breast carcinoma MCF7 cells and compared to the activity of C6-Cer and C6-dhCer. We also studied metabolism and effects of these compounds on cellular SPLs.
2. Results and Discussion
2. 1. Chemistry
2. 1. 1. Synthetic strategy and selection of model 2′-OHCers
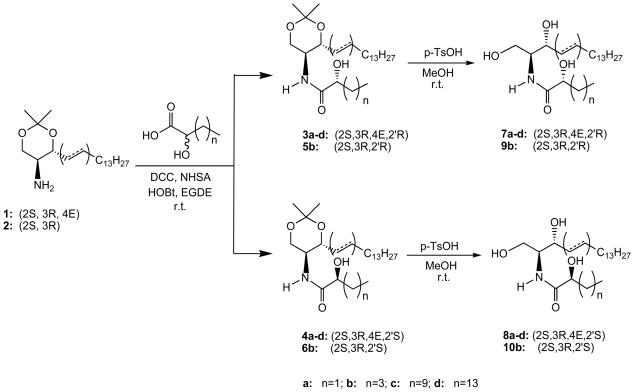
The most important challenge in the synthesis of naturally occurring 2′-OHCer is to construct the amide bond between the sphingoid base backbone and the appropriate 2-OHFA moiety, while preserving the stereochemistry of the used building blocks.6,7,9,11,39 In all reported cases, beside the necessity to make the appropriate sphingoid base precursor, the synthesis of the R-isomer of any long chain 2-OHFAs was also required, since these lipids are not yet commercially available. Generally, all these methods suffer from many drawbacks, namely tedious synthesis procedures and multiple purification steps, which make them impractical for a large-scale preparation of varied chains 2′-OHCers. Faced with this predicament, we undertook an attempt to simplify that strategy and develop a practical method of synthesis of 2′-OHCers capable of producing a chromatographically separable set of their two diastereoisomers simultaneously, when racemic mixtures of 2-OHFAs are used in the key synthetic step. The rationale for this approach was based on two encouraging observations. First, the introduction of the acetonide protective group into the sphingolipid system facilitated the amide bond formation reaction and secondly, this protecting group was used as a separation handle for polyol isomers and their analogs.9,39,49,50
We introduce C4-, C6-, C12- and C16 homologs of (2S, 3R, 4E, 2′R)-OHCer and their corresponding (2S, 3R, 4E, 2′S)-isomers as well as (2S, 3R, 2′R)-C6-OHdhCer and (2S, 3R, 2′S)-C6-OHdhCer. In order to validate the selected method and confirm the structures of 2′-OHCer and 2′-OHdhCer we compared their properties and spectra to the model (2′R)- and (2′S)-2′-OH-C4-Cer stereoisomers, which were prepared first from enantiomerically pure 2-hydroxybutyric acids as well as to the commercially available natural 2′-OH-C18-Cer, isolated from cerebroside.
2. 1. 2. Synthesis of 2′-OHCer stereoisomers
The synthetic routes of 2′-OHCers 7a-d, 8a-d, 9b and 10b are summarized in Scheme 2. To demonstrate the amenability of our strategy toward the synthesis of a separable set of varied chain diastereisomers of 2′-OHCers, we first investigated a condensation of commercially available R- and S-isomers of 2-hydroxybutyric acid with the known acetonide of sphingosine.9,39 Using a standard procedure for the amide bond formation, i.e., dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide (NHS) and 1-hydroxybenzotriazole (HOBt) in ethylene glycol dimethyl ether (EGDE) solution, ceramides 3a and 4a were obtained in 82% and 95% yields, respectively. Most importantly, these model compounds showed a quite different TLC Rf values (0.36 and 0.64, respectively) and opposite optical rotations ([α]22D = + 8.9 and −14.7, respectively). Rf values of 3a and 4a strongly suggested that their mixtures should be easily separated under normal column chromatography conditions. We then explored whether a condensation of the longer chain 2-OHFA reacemic mixtures with sphingosine acetonides would follow the same pattern.
Scheme 2.
Synthetic outline for the preparation of 2′-OHCers and 2′-OHdhCers.
Indeed, the coupling of the amino component 1 or 2 with the corresponding racemic 2-OHFAs delivered the expected mixtures of (R)-and (S)-isomers of 2′-OHCers (3b-d, 4b-d, 5b and 6b), which were easily separated by normal column chromatography (ΔTLC Rf = 0.18-0.2). Final treatment of the separated 2′-OHCer acetonide isomers with p-TsOH in methanol at room temperature provided the desired pure isomers of 7a-d, 8a-d, 9b and 10b in a satisfactory overall yield (26-33%). All synthesized (2′R)-OHCers gave positive values for their optical rotations, which is in full agreement with the reported data for these natural isomers.6-14
2. 2. NMR characterization of 2′-OHCers
To date, all reported NMR studies of 2′-OHCers and their analogs were focused on the structure determination or confirmation of the isolated and synthesized compounds.6-14,38,39,51 Strategically, most of these experiments focused on the identification of D2O exchangeable doublet at δ 7.20, indicative of the NH proton of the amide part located at the center of Cer structure. Using this proton as a starting point and applying COSY, TOCSY, ROESY and HSQC experiments, the putative structure of Cer having 2-OHFA moiety was easily confirmed.52,53 However, there are no reports dealing with the detailed analysis of 2′-OHCer diastereoisomers spectra and their comparison with the ordinary Cers.
In our studies NMR experiments were used to: (i) discriminate between the 2′R and 2′S-isomers of the synthesized short and long chain 2′-OHCers, (ii) compare their spectra to the natural long chain 2′-OHCers and model non-hydroxylated analogs and, (iii) analyze the influence of the additional hydroxy group in the Nacyl part on the structure of the extended polar head.
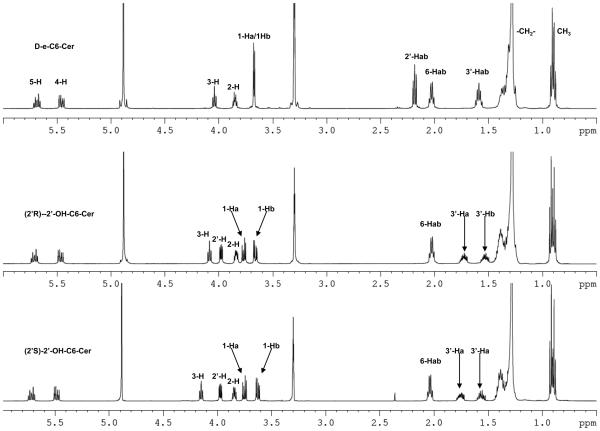
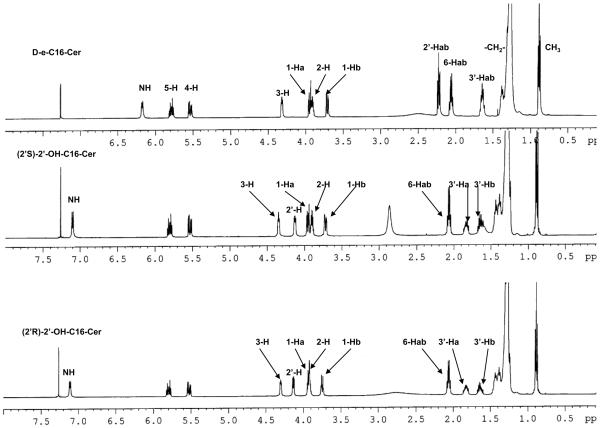
One and two-dimensional 1H- and 13C-NMR experiments clearly exhibited connectivity, chemical shift values and coupling features expected for 2′-OHCers (Fig. 1A-D and data listed in the Experimental). Also, they showed significant differences in the values of δH and δC for the polar head protons as well as carbons in comparison to the parent Cers.
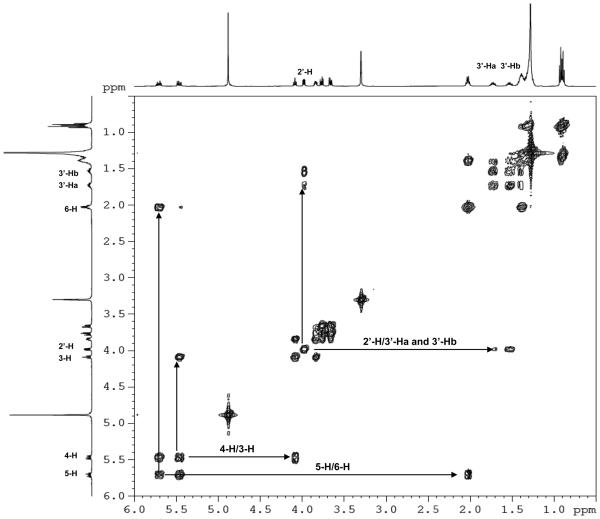
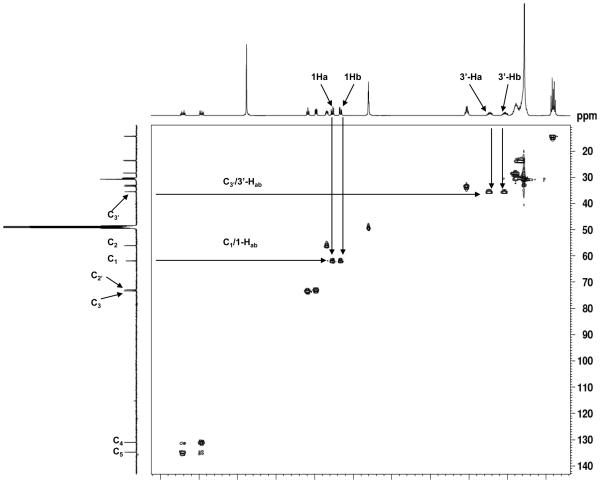
Figure 1.
500 MHz NMR spectra of 2′-OHCers and Cers. (A) Comparison of the NMR spectra of 2′-OH-C6-Cer diastereoisomers with C6-Cer in CD3OD solution. Signature shift of the 3-H proton and anisochronism of the polar interface 1Hab/3′-Hab protons. (B) Comparison of (2′R)-, (2′S)-2′-OH-C16-Cers and C16-Cer spectra in CDCl3 solution. (C) 1H-1H-COSY spectrum of (2′R)-2′-OH-C6-Cer in CD3OD solution. (D) 1H-13C-HSQC spectrum of (2′R)-2′-OH-C6-Cer in CD3OD solution.
Summarizing key findings of the study: (i) strong deshielding effects for the 2′-H protons (Δδ2′H = 1.72 -1.78 ppm, Fig.1A and 1B) and the chiral C2′ carbons (ΔδC2′ = 35-36 ppm, Fig.1D) in both isomers were observed, (ii) the NH protons were significantly shifted to the lower field (ΔδNH = 0.9 ppm, Fig.1B) in comparison to the corresponding protons in non-hydroxylated Cers, and (iii) well-separated resonance signals for each methylene 1-Hab and 3′-Hab protons in both 2′-OHCer isomers, in CDCl3 and CD3OD solutions, were present (Fig.1A and B). This is quite a different behavior than we usually observe in the spectra of ordinary Cers, where the 1-Ha, 1-Hb and the N-acyl chain methylene proton signals always appeared as one resonance signal (narrow doublet or multiplet) in CD3OD solution.54,55 All of the above spectral features most likely resulted from the extensive intramolecular H-bonding interactions between the hydroxyl groups and the amide group of the polar interface of 2′-OHCer. As a consequence, elevated rigidity of the extended polar heads in 2′-OHCers is expected.54
Generally, all NMR spectra of varied chain 2′-OHCer isomers are similar however, the (2′S)-isomers showed higher deshielding effects for their 3-H protons than (2′R)-isomers (Δδ3-H = 0.06-0.17 ppm). The observed difference can be the consequence of a proximal position of the 2′-hydroxy amide unit to the 3-H proton in (2′S)-isomer. The juxtaposition of the amide group may potentially cause a deshielding effect of the C=O group on 3-H proton (spatial magnetic anisotropy).56 Also, the 3-OH group of 2′-OHCer have increased opportunity to form more H-bonds between the remaining proton-donor and acceptor centers than in non-hydroxylated Cer. Nevertheless, to achieve the full capability of interactions, the polar head of Cer must have its all H-donor/acceptor groups in the gauche spatial disposition (-sc staggered conformer).54 In order to fulfill this spatial arrangement and launch effective H-bonding interactions inside the polar interface, the (2′S)- and (2′R)-isomers may need to twist their N-acyl parts out of the line with the sphingosine backbone. The degree of this distortion may have impact on the overall structure of 2′-OHCers. At this point of the study we do not know exactly which particular isomer, if not both, may have their backbones in a different spatial arrangements (i.e., syn or anti) in comparison to the ordinary Cers. In summary, these structural features can be responsible for the observed distinct deshielding effect for the 3-H protons of 2′-OHCers. Moreover, their NMR resonance signals (δ3H= ~4.30 ppm/(2′S)-isomers vs ~4.13 ppm/(2′R)-isomers) can be directly used as diagnostics to monitor and confirm the level of optical purity of the prepared 2′-OHCer diastereoisomers.
Based on this preliminary exploration, 2′-OHCer can be described as a distinct lipophilic multidentate ligand possessing a set of two rigid three-carbon subunits (C1/C2/C3 and C (O) C′2/C′3) in the vicinity of its polar interface. This kind of ligand has the capability to control its own shape by utilizing electronic and steric factors: H-bonding interactions to impose conformational restriction on polar head and fix the spatial arrangement of both chains. Also, when its environment will change, the multidentate and rigid polar head of 2′-OHCer may interact efficiently with its target protein or other lipids. The proper assessment of 2′-OHCer diastereoisomers' conformational preferences in solution will need more advanced NMR and molecular modeling studies.
2. 3. Inhibitory effects of 2′-OHCers/dhCers on MCF7 breast carcinoma cells growth
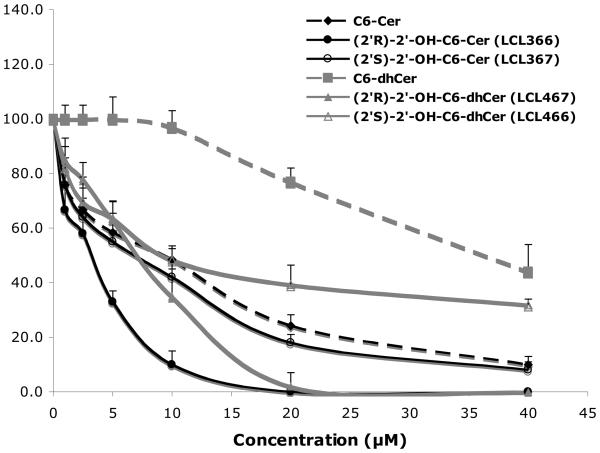
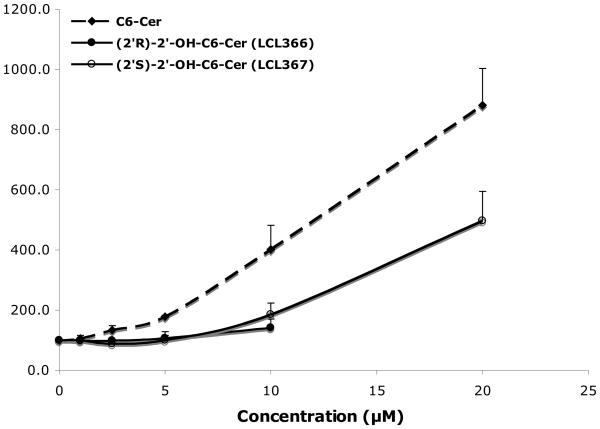
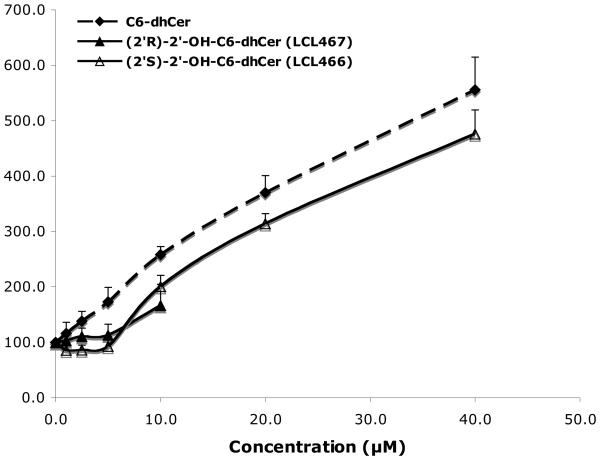
The effects of the newly obtained compounds were examined in MCF7 cells, using 2′-OH-C6-Cer and 2′-OH-C6-dhCer stereoisomers, representing cell permeable short chain homologs of 2′-OHCers/dhCers. Their activity was compared to the effects of D-e-C6-Cer and D-e-C6-dhCer, basic tools commonly used in the sphingolipid research.55,57,58 When added to culture media, these analogs showed high antiproliferative activities on cell growth (Fig. 2, 24 h treatments). IC50 values for (2′R)- and (2′S)-isomer of 2′-OH-C6-Cers and 2′-OHC6-dhCers were established as 3 μM/8μM and 8μM/12μM, respectively. Under identical conditions, the IC50 values for C6-Cer and C6-dhCer were 12μM and 38μM, respectively. Interestingly, all synthesized 2′-OH-Cers/dhCer were more potent than the parent compounds and the naturally occurring (2′R)-isomers had significantly stronger antiproliferative effects as compared to the (2′S)-isomers. We tested also cytotoxic effect of 2′-OH-C4-Cer isomers. Both isomers showed a very similar inhibitory effects on cell growth with IC50 values corresponding to 7 μM and 9μM for the (2′R)- and the (2′S)- isomer, respectively. Interestingly, as was previously reported, the (2′S)-2′-OH-C3-Cer was much more potent then its (2′R)- isomer in HL60 leukemia cells. 39
Figure 2.
Inhibitory effects of 2′-OH-C6-Cer and 2′-OHC6-dhCer stereoisomers and corresponding C6-Cer and C6-dhCer on MCF7 cell growth. Concentration-dependent effect at 24h.
The potent antiproliferative effect of the (2′R)-2′-OH-C6-Cer was also observed with human cervical cancer HeLa cells and human lung adenocarcinoma A549 cells, indicating that the effect is not limited to breast cancer cells only (data not shown). A similar trend, higher cytotoxic activity of 2′-OHCers isolated from different natural sources in comparison to their nonhydroxylated analogs was previously reported in HL60, K562, A-549, HepG2, Hep3B and BGC-823 cells lines.37,38,40
In summary, our data show that the introduction of the hydroxyl group at position 2′ in the N-acyl part of Cer not only maintains but also enhances the activity of 2′-OH-C6-dhCer over its normal and desaturated analogs. These findings are also of interest when considering other studies, showing higher cytotoxicity of phytoCers than the corresponding dhCer and Cer analogs in SKN-BE(2)C cells.59 All these observations extend the existing “Cer cytotoxicity paradigm”, which states that the trans double bond between C4 and C5 atoms in the Cer backbone is essential for its elevated activity and pro-apoptotic responses.1,57,60,61 Thus, the cytotoxic activity of Cer seems to be rendered by the presence of the double bond in the vicinity of its polar head and/or presence of an additional hydroxyl group at position 2′ or 4. Our and other NMR studies pinpoint these electronic features of the Cer structure as responsible for its polar head enhanced rigidity.62 This may facilitate the molecule's molecular recognition and enhance its binding to the target regulatory proteins.4,63 The formation of that lipid-protein complex can be responsible for the observed elevated cytotoxicity of 2′-OHCers and 2′-OHdhCe (vide infra part 5.1.2.1).
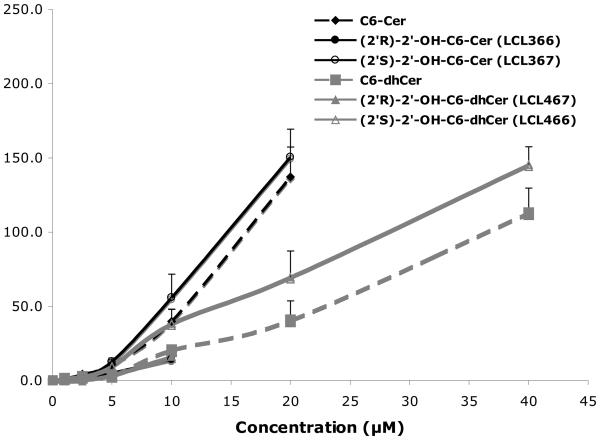
2. 4. Cellular levels of 2′-OHCers/dhCers
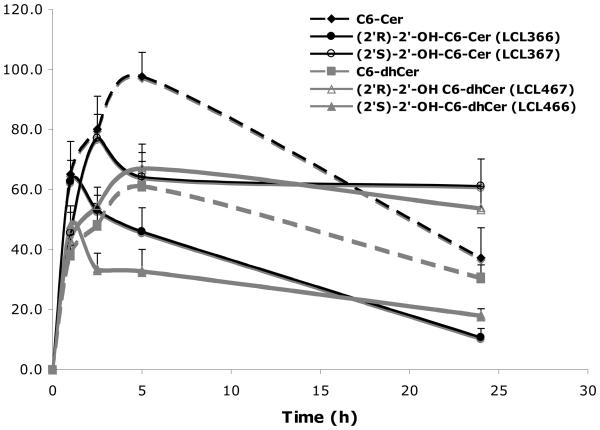
Cellular levels of the representative C6-analogs in MCF7 cells were established by MS analysis. Experimental data from cell treatments at 24h showed a dose dependent increase for all tested analogs, however at different rates (Fig. 3A). (2′S)-Isomer (LCL367) showed the highest level of the C6-Cer series whereas the (2′R)-isomer (LCL366) showed the lowest level and C6-Cer was in the middle. A similar pattern was observed for the C6-dhCer series.
Figure 3.
Cellular level of selected analogs in MCF7 cells. (A) Concentration dependent levels at 24h. (B) Time dependent levels for 10 μM treatments.
Cellular uptakes for 10μM treatments were fast and at comparable levels up to ~1h, with the highest levels for LCL366 and C6-Cer (Fig. 3B). Following a time course, LCL366 was progressively decreased to the lowest level among all tested analogs at 24h. LCL367 was elevated up to 2.5 h followed by a small decrease at 5h and plateau to 24 h. C6-Cer was progressively increased up to 5 h followed by decrease at 24h. C6-dhCer followed the curve of C6-Cer, however at a lower rate. The 2′S-isomer of 2′-OH-C6-dhCer, LCL466, was elevated up to 5h reaching the level of C6-dhCer and remaining constant up to 24h. The (2′R)-isomer of 2′-OHC6-dhCer, LCL467, showed the lowest level, remaining in a plateau between 2.5-24h.
In summary, we noticed differences in the cellular levels between (2′R)- and (2′S)-isomers for 2′-OHCers and 2′-OHdhCers, with (2′R)-isomers being utilized over time similarly to the parent compounds, whereas (2′S)-isomers being stable.
These results may suggest different metabolism of these compounds and/or their binding to the specific protein targets.
2. 5. Effects of 2′-OHCers/dhCers on bioactive SPLs in MCF7 human breast carcinoma cells
Cellular effects of 2′-OHCers on endogenous SPLs and their comparison to the actions of ordinary Cers have not yet been investigated. Using the LC-MS/MS method and monitoring changes in Cer species-Sph-S1P balance we generated a set of preliminary data pinpointing metabolic junctures and differences between the actions of model 2′-OH-C6-Cer diastereoisomers and their parent analogs.
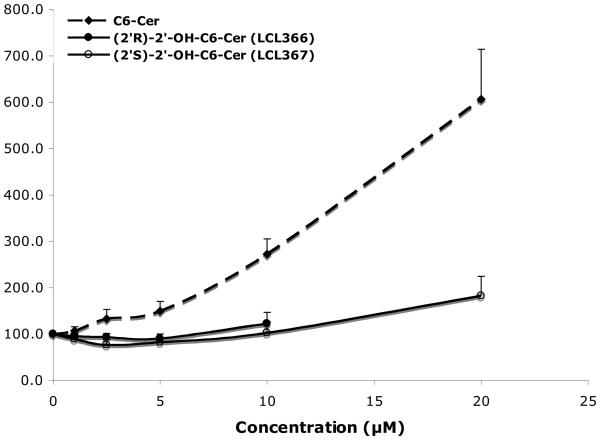
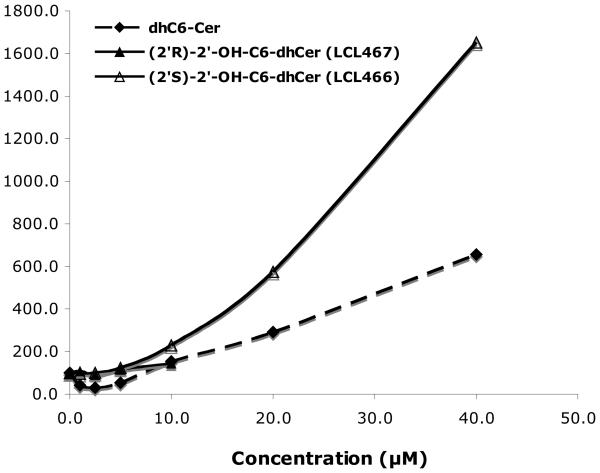
Studies assessing the roles of Cer in signal transduction phenomena and in regulating tumor cell responses to chemotherapy have shown that the active agonists, stress factors or cytotoxic drugs, usually cause an increase of the endogenous Cers, especially C14-, C16- and C18-Cers, followed later by cell death.2,4,64 This response was always observed after cell treatment with exogenous C2- and C6-Cers at the appropriate concentration.1,60-65 C2- and C6-dhCers are commonly used as a negative control for ceramide action.60,66 However, it is limited only to C2-dhCer as was previously reported by our and other group.60,67 As shown in Fig. 2, C6-dhCer caused an inhibitory effect on cell growth at concentrations higher than 10μM and was metabolized to C6-Cer and cellular dhCers followed by their desaturation to the cellular Cers (Figs. 6-8).
Figure 6.
Direct conversion of 2′-OHC6-dhCer stereoisomers and C6-dhCer to the respective unsaturated analogs 2′-OHC6-Cer and C6-Cer. Concentration dependent effects for 24h treatments.
Figure 8.
Time dependent effects of 10μM 2′-OH-C6-dhCer diastereoisomers and C6-dhCer on cellular SPLs. (A) Effect on cellular dhCers. (B) Effect on cellular Cers.
MCF7 control cells showed the following Cer composition: ~87% Cers, 8% dhCers and ~ 5% 2′-OHCers. Among the Cers species C24-, C24:1 and C16-Cers are the major components (~31%, 44% and 20%, respectively). The remaining C14-, C18-, C18-1 and C20- Cers represent almost equal values. Among the dhCer species C16-dhCer is the major component (~ 60-%) followed by C24:1-dhCer (~ 12 %). The remaining C14-, C20-, and C24-dhCers represent almost equal values. Among the 2′-OHCer species only C24-, C26-, C16-, C24:1- and C26:1 (~24 %, 22%, 19%, 18 %, and 17%, respectively) represent components of this class. Similar delinquent presence and elevated levels of long chain 2′-OHCer species were found in some mammalian tissues and organs.18
2. 5. 1. Stereospecific effects of 2′-OH-C6-Cers on cellular 2′-OHCers, Cers, Sph and S1P
2. 5. 1. 1. Concentration dependent effects
Surprisingly, as observed for 24h treatment, both tested isomers of 2′-OH-C6-Cers (2.5-20μM) showed no effect on endogenous 2′-OHCer levels (data not shown). For the maximal concentration used, 10μM LCL366 and 20μM LCL367, only a small increase was observed (up to 130%; C24- and C24:1-Cers for LCL366 ; C16-, C24- and C24:1-Cers for LCL367). The results suggest that 2′-OH-C6-Cers may not serve as good competitive substrates/inhibitors for enzymes of Cer metabolism or they are not efficiently delivered to the cellular compartment where their active metabolic target can be present. However, it was previously shown that natural 2′-OHCer served as a good substrate for neutral ceramidase in vitro.68 It was also shown that acidic ceramidase controlled intracellular 2′-OHCer level in vivo.44 Interestingly, C6-Cer itself was not converted to the cellular 2′-OHCer analogs (data not shown) but an increase in Sph level was clearly noticed in this case (vide infra, Fig 4B). It may suggest that the level of 2-OHFA-CoA or FA2H enzyme expression in cancer cells are low. All these observations are in conflict with results showing formation of 2′-OH-C18-Cer from 2-OH-C18-FA-CoA (racemic mixure) and dhSph in HEK293T cells.69
Figure 4.
Concentration dependent effect of 2′-OHC6-Cer isomers on endogenous bioactive SPLs for 24 h treatment. (A) Effect on total Cer. (B) Effect on Sph.
Isomers of 2′-OHC6-Cers did not elevate cellular Cer as effectively as C6-Cer (Fig. 4A). Also, they did not elevate cellular Sph as was observed for C6-Cer (Fig. 4B). Both, C6-Cer and LCL367 (2′S-isomer), elevated cellular S1P (800% and 600% for 20μM treatments, respectively), whereas LCL366 (2′R-isomer), had no effect on S1P (data not shown).
C6-Cer caused a significant dose dependent increase of endogenous Cer and Sph (Fig. 3A, up to 900% and 600% for 20μM, respectively). LCL367 followed the pattern of C6-Cer but with a much lower effect (up to 500%/Cer and 150%/Sph for 20 μM). LCL366 only slightly increased endogenous Cer (up to 130%) and had no effect on Sph as noticed for 10μM treatment (the highest possible concentration used).
Next, we examined effects of these compounds on endogenous Cer species (data not shown). The results showed that C6-Cer elevated all Cer species starting from 5μM. The highest extent was observed for C18-Cer, C14-and C16-Cers (~80-, 40- and 20- fold, respectively, for 20μM treatment). Increase in the other Cers was to a much lower extent (C24:1-Cer, 5-fold and C24-Cer only ~0.2 fold). LCL367, the 2′S-isomer, followed the pattern of C6-Cer with a specific increase in C18-Cer (30-fold), C14-Cer (20 fold), C16-Cer (7 fold), C24:1-Cer (4 fold) and C24-Cer (2 fold). LCL366, the 2′R-isomer, up to 5μM had no effect on Cer species, however at 10μM a very moderate elevation of C18-Cer (~5-fold), C14-Cer (3 fold), C16-Cer (1.7 fold) and decrease of C24-Cer and C24:1-Cer (to 70 % and 50%, respectively) were observed. Again, the (2′R)- isomer behaved differently from (2′S)- isomer and C6-Cer.
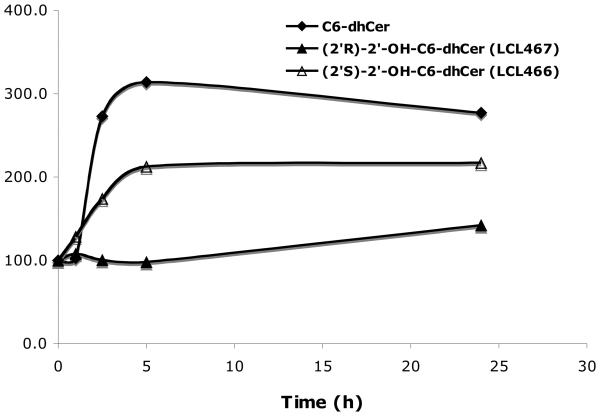
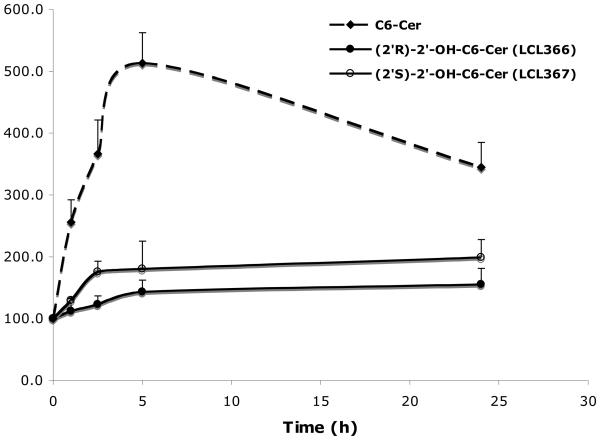
2. 5. 1. 2. Time dependent effects
Time dependent effects on cellular Cers were determined for 10μM treatments (Fig. 5). Both, LCL366 (2′R) and LCL367 (2′S) were not effective in formation of cellular Cer as compared to C6-Cer. Some elevations (~140%/LCL366 and ~180%/LCL367) were observed up to 5h with a plateau up to 24h. These effects were different from the action of C6-Cer, where cellular Cers increased rapidly (500%) up to 5h and decreased (350%) at 24 h. Again, a small increase in cellular Cer by LCL366 did not correlate with its strong antiproliferative effect (IC50/24h= 3μM, Fig. 2A).
Figure 5.
Time dependent effects of 10μM 2′-OH-C6-Cer stereoisomers and C6-Cer on cellular Cers
In summary, isomers of 2′-OHC6-Cers influenced differently bio-transformations of the cellular SPLs as compared to C6-Cer. (2′S)-isomer showed some similarities to the action of C6-Cer if used at higher concentration and during longer treatment. The observed lack of influence of the (2′R)-isomer on basic cellular SPLs in MCF7 cells and its high cytotoxicity can not be related to its anabolic transformations, which usually favor hydrolysis and generation of Sph and long chain Cers.57,58,60,61 Results suggest that this isomer or its unidentified yet metabolites may directly target some key regulatory enzymes (e.g. phosphatases, kinases or caspases).3,4,70 Our preliminary data support this hypothesis, since some (2′R)-OHCers had a higher potency than nonhydroxylated-Cer for the PPA2 enzyme activation (P. Roddy, D. Perry and Y. A. Hannun, unpublished data).
In conclusion, an uncommon profile of biological action for the (2′R)-isomer, representing natural 2′-OHCers, was discovered by comparison to the effects of C6-Cer and its unnatural (2′S)-isomer.
2. 5. 2. Stereospecific effects of 2′-OH-C6-dhCers on cellular SPLs
Since a metabolic conversion of C6-dhCer to C6-Cer was observed for 24 h treatment (Fig. 6), we also investigated conversions of 2′-OH-C6-dhCer diastereoisomers, LCL466 (2′S-isomer) and LCL467 (2′R-isomer) to their desaturated counterparts as well as effects on endogenous dhCers, Cers and both sphingoid bases.
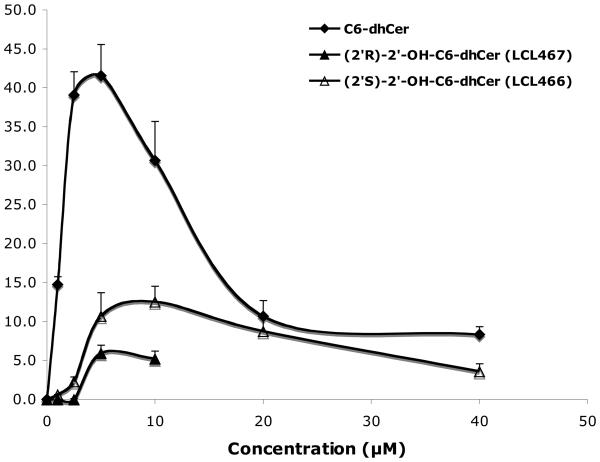
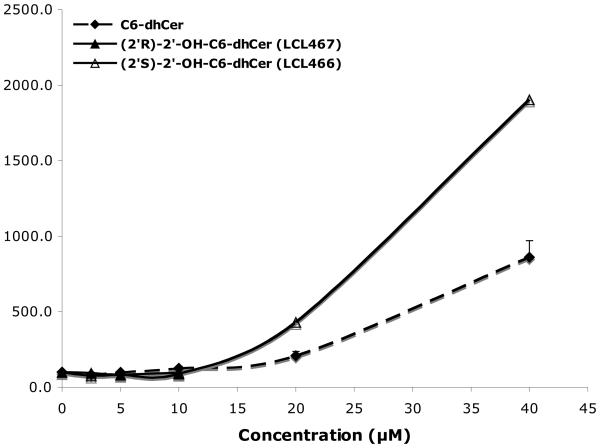
In general, 2′-OH-C6-dhCers did not serve as a good substrate for dhCer desaturase since only a small percent of conversion (up to 12%) of LCL466 to the corresponding LCL367 and LCL467 to the corresponding LCL366 (up to 5%) was observed as compared to 42% conversion for C6-dhCer.
2. 5. 2. 1. Concentration dependent effects of 2′OH-dhCers on endogenous SPLs
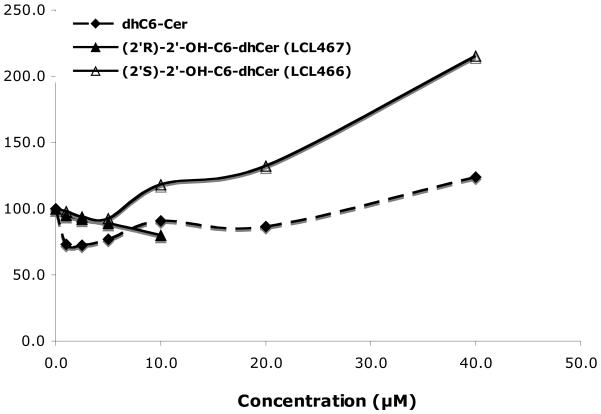
Effect on endogenous dhCers (Fig. 7A)
Figure 7.
Effect of 2′-OH-C6-dhCer stereoisomers and C6-dhCer on cellular SPLs at 24h. (A). Concentration dependent effects on cellular dhCers. (B) Concentration dependent effects on cellular Cer. (C) Concentration dependent effects on cellular dhSph. (D) Concentration dependent effects on cellular Sph
Up to 10μM of C6-dhCer and both isomers of 2′-OHC6-dh-Cer did not induce elevation of cellular dhCers. This concentration represented the highest concentration tested for (2′R)-isomer, since 20μM treatment caused total cell death as shown in Fig. 2. LCL466, (2′S)-isomer, elevated cellular dhCer to the highest extent (~19 fold/40μM treatment). In comparison, C6-dhCer increased cellular dhCer only ~7 fold/40μM treatment. Regarding dhCer species (results not shown), both LCL466 and C6-dhCer elevated mostly dhC16-Cer, the major dhCer for MCF7 control cells (up to 25 and 13 folds respectively). LCL467 did not elevate C16-dhCer. Interestingly, it slightly decreased the other dhCers, especially C14-dhCer (to 25% control).
Effect on endogenous Cers (Fig. 7B)
Effect of these analogs on endogenous Cer was different from their effects on dhCers (Fig. 7A). The highest elevation was observed for C6-dhCer for the full range of the concentration used, reaching 550% for 40 μM. LCL 466, the (2′S)-isomer, starting from 5μM followed the effect of C6-dhCer, however to a lower extent (~ 450% for 40 μM treatment). LCL467 as similarly shown for cellular dhCer, did not effect cellular Cer up to 5 μM, however a 10μM treatment increased Cer to ~ 150%. Regarding Cer species, all compounds elevated mostly C18- and C14-Cers, followed by C24:1-Cer, however at different rates (results not shown). C16-Cer was elevated up to ~50 fold by 40μM LCL466 and C6-dhCer, whereas 1μM to 5μM LCL467, caused a rather similar decrease (up to 60%) of this Cer.
Effect on sphingoid bases
MCF7 control cells (1×10*6) contain ~ 20 pmols Sph and only ~1 pmol of dhSph. Dose dependent increase of cellular dhSph was caused by LCL466, (2′S)-isomer, increasing ~ 16 fold for 40μM treatments, whereas LCL467 did not effect cellular dhSph. C6-dhCer had small effect on dhSph elevation (~ 5 folds/40μM) (Fig. 7C). LCL466 also caused elevation of cellular Sph (Fig. 7D), however to much lower extends in comparison to dhSph. LCL467 showed the same small decrease of Sph (to ~80%), whereas C6-dhCer decreased Sph at a low concentration (to ~ 70%), showing some recovery at a higher concentration.
2. 5. 2. 2. Time dependent effects
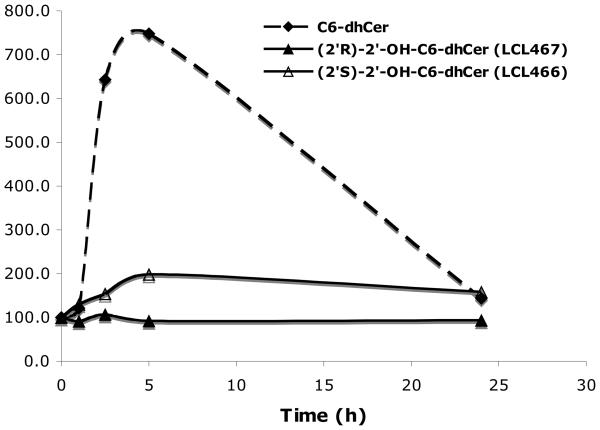
Time dependent elevation of cellular dhCer (Fig. 8A) and Cer (Fig. 8B) were observed for 10 μM LCL466, up to 5h, reaching 200% for both lipids. 10μM C6-dhCer elevated cellular dhCers to 750% and Cers to 310%. LCL467, the (2′R)-isomer, did not affect cellular dhCers nor Cers for this period of time; however, at 24h Cer was slightly elevated (~ 140%) whereas dhCer remained unchanged. At 24h, dhCer generated by C6-dhCer decreased to almost control level whereas cellular Cer was only slightly decreased (from 310% to 280%). Regarding effect of LCL466 at 24 h, both cellular dhCer and Cer were the same as for 5h treatment. Among the dhCer species, LCL466 and C6-dhCer elevated all dhCer species, whereas LCL467 decreased long chain dhCers (~ 10-25%) and increased very long chain dhCers (~20-30%), data not shown. LCL466 and C6-dhCer elevated all Cer species, particularly C18-, C14- and C16-Cers however to very different extent (data not shown). Cellular dhSph followed the curves of dhCer whereas Sph was decreased by both 2′-OH-dhCers and slightly increased by C6-dhCer (data not shown).
In conclusion, 2′-OH-C6-dhCer diastereoisomers showed different effects on cellular dhCers, Cers, dhSph and Sph. The (2′R)-isomer was not effective, whereas (2′S)-isomer acted as a very potent inducer of these lipids being three time more effective than C6-dhCer. These results suggest that LCL466 may undergo anabolic transformations and/or may regulate the action of dhCer metabolizing enzymes. DhCer/Cer synthases or dhCer desaturase can be considered as its targets. Most likely, LCL466 may act as an inhibitor of dhCer synthase or activator of dhCer ceramidase, since elevation of dhSph was observed. Moreover, LCL466 may additionally act as an inhibitor of dhCer desaturase, since cellular dhCers generated by this compound were not so efficiently transformed to their oxidized analogs as those generated by the ordinary C6-dhCer (50/25 and 50/13 fold ratio for C16-Cer/C16-dhCer, respectively).
Summary and Conclusion
This work reveals a set of varied chain (2′R)- and (2′S)-isomers of 2′-OHCers and 2′-OHdhCers as novel molecular tools to study Cer and dhCer biology. Applying these tools and pursuing SAR and metabolomic studies in MCF7 cells we contribute new insights into the “Cer cytotoxicity paradigm”.
In summary, we have developed a simple and convenient method for the simultaneous preparation of (2′R)- and (2′S)-isomers of 2′-OHCers and 2′-OHdhCers starting from sphingoid base precursors and a racemic mixture of 2-OHFA. The developed protocol may provide an unrestricted access to pure (2′R)- and (2′S)-isomers of varied chain 2′-OHCers and 2′-OHdhCers with enormous possibilities to synthesize sub-classes of skin Cers and other complex 2′-OHSPLs for structural and biological studies. The NMR studies showed that additional presence of the hydroxyl group in the N-acyl part of Cer increased the hydrogen bonding capacity of Cer/dhCer polar head and restricted its conformational flexibility. Thus, the presence or absence of these electronic and steric features can be responsible for a different molecular recognition and binding affinities of 2′-OHCers/dhCers and their non-hydroxylated analogs by metabolic and regulatory enzymes.
MCF7 cells, and other cell lines, responded differently to the action of these two diastereoisomers in comparison to their non-hydroxylated analogs. In general, both isomers of 2′-OH-C6-Cers and 2′-OH-C6-dhCers were more potent in cell killing than corresponding C6-Cer and C6-dhCer. Moreover, they were not metabolized to their long chain 2′-OHCers and showed divergent stereospecific effect on cellular Cer. Interestingly, 2′-OH-C6-dhCers did not serve as a good substrate for dhCer desaturase as compared to C6-dhCer. Data suggest that the observed high cytotoxic effects of (2′R)-isomers are not the result of their anabolic inter-conversions to sphingoid bases and long chain Cers or direct regulatory actions on the Cer metabolizing enzymes.
In conclusion, the observed similarities in the early cellular uptakes of (2′R)- and (2′S)-isomers, differentiated for the longer treatments, may suggest a higher metabolic rate for (2′R)-isomers or their efficient binding to some important and specific proteins. However, taking under consideration the following facts, that the (2′R)-isomers of 2′-OH-C6- and 2′-OH-dhC6-Cers were not effective in the generation of cellular Cers, sphingoid bases nor their GluCer and GalCer analogs (our preliminary observation), we can rather suggest that their high antiproliferative effects are the result of their direct actions on the regulatory targets. Finally, the observed divergences between (2′S)-OHCer/dhCer isomers and Cer/dhCer actions on anabolic transformation of the cellular SPLs pinpoint regulation of CDase, dhCerS and dhCer desaturase enzymes as the cause of their elevated cytotoxicity.
The observations presented here, correlating steric and electronic influences on Cer conformation with its cytotoxicity and metabolic interconversions, should prove their usefulness as a guide in defying key factors which control SPL-protein interactions.
Further studies on effects of 2′-OH-C6-Cer/dhCer diastereoisomers on the complex SPLs, search for their direct cellular targets and mechanism of cell death are pending.
4. Experimental
4. 1. Chemistry
All solvents, general reagents and short chain (2R)- and (2S)-4-hydroxybutyric acids were purchased from Aldrich-Sigma & Fluka. D-e-Sph and D-e-dhSph were purchased from Avanti. Long chain 2-hydroxy-FAs and a model sample of naturally occurring (2′R)-2′-hydroxy-C18-ceramide were purchased from Matreya. The homogeneity and optical purity of these lipids were checked by NMR, MS spectrometry and optical rotation.
Natural (2′R)-2′-hydroxy-C18-ceramide (from Matreya, cat. #1323): TLC Rf = 0.40-42 (CHCl3-CH3OH, 45 : 5, v/v); 1H NMR (500MHz, CDCl3, δ in ppm) : 7.08 (d, 1H, J = 8.2, NH), 5.79 (dtd, 1H, J = 15.1, 6.8, 1.0, 5-H), 5.52 (ddt, 1H, J = 15.1, 6.8, 1.1, 4-H), 5.34(m, ~0.5H, C′H=C′H), 4.30 (t, 1H, J = 6.0, 3-H), 4.13 (m, 1H, 2′-HR), 3.92 (m, 2H, 1-Ha and 2-H), 3.74 (m, 1H, 1-Hb), 2.0 (m, ~3H, C(6)H2 and C′H2), 1.82 (m, 1H, 3′-Ha), 1.64 (m, 1H, 3′Hb), 1.26 (m, ~48H, CH2), 0.88 (t, 6H, J = 7.1, CH3); 13C NMR (CDCl3) δ 174.4 (C=O), 134.3 (C5), 130.0 (C′H=C′H), 128.4 (C4), 74.3 (C3), 72.48 (C′2), 62.2 (C1), 54.5 (C2), 34.8 (C′3), 32.3 (C6), 31.9, 29.7, 29.6, 29.5, 29.4, 29.3, 29.2, 29.1, 27.2, 25.0 and 22.7 (C7-C17 and C′4-C′15), 14.0 (C′H3 and CH3). ESI-MS (CH3OH, relative intensity, %) m/z 664.6 ([M1H]+, 32), 628.6 ([M1H-H2O]+, 4), (582.6 ([M2H]+, 40), 564.2 ([M2H-H2O]+, 8), 546.7 (([M2H-2H2O]+, 4). Calcd. for C42H82NO4 m/z 663.62 [M1] and C36H71NO4 m/z 581.54 [M2].
1,3-Isopropylidene-(2S,3R,4E)-sphingosine (1) and 1,3-isopropylidene-(2S,3R)-dihydrosphingosine (2) were prepared from the corresponding sphingoid bases by a slightly modified procedure described previously.9 Purity of the synthesized compounds was confirmed by NMR spectroscopy and the measurement of their optical rotation values. Reaction progress was monitored by the analytical normal and reverse phase thin layer chromatography (NP TLC or RP TLC) using aluminum sheets with 0.25 mm silica gel 60-F254 (Merck) and 0.150 mm C18-silica gel (Sorbent Technologies). Detection was done by the PMA reagent (ammonium heptamolybdate tetrahydrate cerium sulfate, 5 : 2, g/g) in 125 mL of 10% H2SO4) and the Dragendorff reagent (Fluka) following heating of the TLC plates at 170°C or by the UV (254 nm). Flash chromatography was performed using EM Silica Gel 60 (230-400 mesh) with the indicated eluent systems. Melting points were determined in open capillaries on Electrothermal IA 9200 melting point apparatus and are reported uncorrected. Optical rotation data were acquired using a Jasco P-1010 polarimeter. 1H- and 13C-NMR spectra were recorded on Bruker AVANCE 500MHz spectrometer equipped with Oxford Narrow Bore Magnet. Chemical shifts are reported in ppm on the d scale from the internal standard of residual chloroform (7.26 ppm). Mass spectral data were recorded in a positive ion electrospray ionization (ESI) mode on Thermo Finnigan TSQ 7000 triple quadrupole mass spectrometer. Samples were infused in methanol solution with an ESI voltage of 4.5 kV and capilary temperature of 200°C. Specific structural features were established by fragmentation pattern upon electrospray (ESI/MS/MS) conditions, specific for each of the compounds studied.71,72
4. 1. 2. Preparation of 1, 3-isopropylidene-sphingoid bases
4. 1. 2. 1. 1, 3-Isopropylidene-(2S,3R,4E)-sphingosine (1)
A mixture of (2S,3R, 4E)-sphingosine (300 mg, 1.0 mmol), pyridinium p-tolulenesulfonate (98%, 252 mg, 1.0 mmol) and 2,2-dimethoxypropane (6 mL) in dry toluene (10 mL) was stirred at reflux for 4 hours. The reaction mixture was then cooled to room temperature and diluted with ethyl acetate (10 mL), washed with saturated sodium bicarbonate solution and water. The collected organic layer was dried over anhydrous magnesium sulfate and filtered. The solvent was evaporated under reduced pressure and the obtained residue was purified by flash column chromatography (CHCl3 : CH3OH : NH4OH conc, 15 : 1 : 0.025 v/v/v) to give pure 1 (312 mg, 92%) as a pale yellow oil. This material formed a waxy solid upon storage in refrigerator (+4°C) overnight. TLC Rf = 0.43 (CHCl3 : CH3OH : NH4OH Conc, 15 : 1 : 0.025, v/v/v); [α]25D = +11.0 (c = 1, CHCl3 ); [α]25365 = +38.7 (c = 1, CHCl3) ; 1H NMR (500 MHz, CDCl3) δ 5.82 (dtd, 1H, J = 15.2, 7.4 and 1.0, 5-H), 5.37 (ddt, 1H, J = 15.1, 7.6 and 1.4, 4-H), 3.85 (m, 2H, 1-Ha and 3-H), 3.54 (dd, 1H, J = 10.8 and 11.2, 1-Hb), 2.70 (m, 1H, 2-H), 2.01 (m, 2H, C(6)H2), 1.49 (s, 3H, CH3), 1.42 (s, 3H, CH3), 1.35 (m, 2H, CH2), 1.24 (m, 22H, CH2), 0.88 (t, 3H, J = 7.4, CH3), 0.87 (t, 3H, J = 7.1, CH3). 13C NMR (500 MHz, CDCl3) δ 137.3, 127.8, 127.7, 100.4, 98.7, 78.4, 65.6, 49.3, 32.6, 32.1, 29.9,29.8, 29.7, 29.6, 29.5, 29.2, 22.9, 19.8, 19.4, 14.9, 14.4. MS (CH3OH) m/z 340.8 ([MH]+, 100), 282.3 ([MH]+- C3H6O, 15); Calcd for C21H41NO2 m/z 339.3 [M].
4. 1. 2. 2. 1,3-Isopropylidene-(2S,3R)-dihydrosphingosine (2)
The same procedure, as described for the synthesis of 1, was performed using (2S, 3R)-dihydrosphingosine as a starting material. The crude product was purified by flash chromatography (CHCl3 : CH3OH, 25 : 2 v/v) to give pure 2 in 99%yield as a pale yellow oil. This material solidified upon storage in refrigerator (+4°C) overnight. TLC Rf = 0.58 (CHCl3 : CH3OH, 25 : 2 v/v); [α]23D = + 31.8 (c = 1, CHCl3 ); [α]23365 = +97.0 (c = 0.5, CHCl3). Remaining spectral and analytical data are identical to the literature references.9
4.1. 3. General procedure for the preparation of 1,3-isopropylidene-2′-OH-Cers (3a-d, 4a-d) and 1,3-isopropylidene-2′-OH-C6-dihydroCers (5b, 6b)
A well-stirred mixture of 1,3-ispropylidene-(2S,3R,4E)-sphingosine (1, 105 mg, 0.31 mmol) or 1,3-ispropylidene-(2S,3R)-dihydrosphingosine (2, 106 mg, 0.31 mmol), 2-hydroxy fatty acid (0.36 mmol), DCC (99%, 75 mg, 0.36 mmol), NHS (97%, 42 mg, 0.36 mmol) and HOBt (~5% water, 49 mg, 0.36 mmol) in dry EGDE (5 mL) was stirred at room temperature for 4 hours. The reaction mixture was evaporated to dryness under reduced pressure and the obtained residue was purified by flash column chromatography using the suitable solvent system.
4. 1. 3. 1. (2S, 3R, 2′R, 4E)-1,3-isopropylidene-2′-hydroxy-C4-ceramide (3a)
Prepared from 1 and (2R)-2-hydroxybutanoic acid in 82% yield after purification by column chromatography (CHCl3-CH3OH, 25:2, v/v). Analytical sample of 3a was prepared by crystallization from ethyl acetate-n-hexane (3 : 1, v/v) as a white microcrystalline powder, mp 75-77°C. TLC Rf = 0.36 (CHCl3-CH3OH, 25 : 2, v/v); [α]22D = + 8.9 (c = 1, CHCl3); [α]22365 = + 19.2 (c = 1, CHCl3); 1H NMR (500 MHz, CDCl3) δ 6.31 (d, 1H, J = 8.6, NH), 5.74 (dtd, 1H, J = 15.4, 7.6 and 1.0, 5-H), 5.42 (ddt, 1H, J = 15.4, 7.6 and 1.4, 4-H), 4.07 (m, 2H, 2′-HR and 3-H), 3.97 (dd, 1H, J = 5.3 and 11.2, 1-Ha), 3.86 (m, 1H, 2-H), 3.64 (dd, 1H, J = 9.5 and 11.2, 1-Hb), 2.01 (m, 2H, C(6)H2), 1.82 (m, 3′-Ha, 1H), 1.65 (m, 3′-Hb, 1H), 1.49 (s, 3H, CH3), 1.42 (s, 3H, CH3), 1.24 (m, 22H, CH2), 0.93 (t, 3H, J = 7.4, CH3), 0.87 (t, 3H, J = 7.1, CH3). MS (CH3OH) m/z 873.0 ([2M+Na]+, 23), 449.0 ([M+Na]+, 100), 368.0 ([MH-(CH3)2CO]+, 5). Calcd for C25H47NO4 m/z 425.35 [M].
4. 1. 3. 2. (2S, 3R, 2′S, 4E)-1,3-isopropylidene-2′-hydroxy-C4-ceramide (4a)
Prepared from 1 and (2S)-2-hydroxybutanoic acid in 95% yield after purification by column chromatography (CHCl3-CH3OH, 25:2, v/v). Analytical sample of 4a was prepared by crystallization from ethyl acetate-n-hexane (3:1, v/v) as a white microcrystalline powder, mp 62-63°C. TLC Rf = 0.64 (CHCl3-CH3OH, 25:2, v/v); [α]22D = −14.7 (c = 1, CHCl3 ); [α]22365 = −62.3 (c = 1, CHCl3); 1H NMR (500 MHz, CDCl3) δ 6.25 (d, 1H, J = 8.2, NH), 5.79 (dtd, 1H, J = 15.4, 7.6 and 1.0, 5-H), 5.44 (ddt, 1H, J = 15.4, 7.6 and 1.4, 4-H), 4.16 (dd, 1H, J = 7.8 and 9.4, 3-H), 4.06 (dd, 1H, J = 4.8 and 7.3, 2′-HS ), 4.02 (dd, 1H, J = 5.2 and 11.2, 1-Ha), 3.84 (m, 1H, 2-H), 3.70 (dd, 1H, J = 9.6 and 11.2, 1-Hb), 2.05 (m, 2H, C(6)H2), 1.87 (m, 3′-Ha, 1H), 1.68 (m, 3′-Hb, 1H), 1.53 (s, 3H, CH3), 1.46 (s, 3H, CH3), 1.24 (m, 22H, CH2), 0.98 (t, 3H, J = 7.4, CH3), 0.90 (t, 3H, J = 7.1, CH3); MS (CH3OH) m/z 873.0 ([2M+Na]+, 60), 870.0 ([2M-2H+Na]+, 100), 449.0 ([M+Na]+, 5), 368.0 ([MH-(CH3)2CO]+, 23). Calcd. for C25H47NO4 m/z 425.35 [M].
4. 1. 3. 3. (2S, 3R, 2′R, 4E)-1,3-isopropylidene-2′-hydroxy-C6-ceramide (3b)
Prepared from 1 and DL-2-hydroxyhexanoic acid in 41% yield after purification by column chromatography (ethyl acetate-n-hexane, 2:1, v/v). Analytical sample of 3b was prepared by crystallization from ethyl acetate-n-hexane (1 : 3, v/v) as a white microcrystalline powder, mp 77-79°C. TLC Rf = 0.22 (ethyl acetate-nhexane, 1:1, v/v); [α]22D = +5.3 (c =0.5, CHCl3 ); [α]22365 = +16.8 (c =0.5, CHCl3); 1H NMR (500 MHz, CDCl3) δ 6.36 (d, 1H, J = 8.8, NH), 5.77 (dtd, 1H, J = 15.1, 7.4, 1.0, 5-H), 5.42 (ddt, 1H, J = 15.1, 7.5, 1.4, 4-H), 4.09 (m, 2H, 2′-HR and 3-H), 3.97 (dd, 1H, J = 5.6 and 11.2, 1-Ha), 3.88 (m, 1H, 2-H), 3.66 (dd, 1H, J = 9.6 and 11.2, 1-Hb), 2.38 (d, 1H, J = 4.8, 2′-OH), 2.04 (m, 2H, C(6)Ha), 1.87 (m, 2H, CH2), 1.81 (m, 1H, 3′-Ha), 1.70 (m, 2H, CH2), 1.60 (m, 1H, 3′-Hb), 1.50 (s, 3H, CH3), 1.43 (s, 3H, CH3), 1.24 (m, 32H, CH2), 1.15 (m, 2H, CH2), 0.88 ( m, 6H, 2×CH3). MS (CH3OH) m/z 929.7 ([2M+Na]+, 2), 449.5 (100), 414.4 (([MH-(CH3)2C], 6) 396. 2([MH(CH3)2CO]+, 48). Calcd. for C27H51NO4 m/z 453.38 [M].
4.1. 3. 4. (2S, 3R, 2′S, 4E)-1,3-isopropylidene-2′-hydroxy-C6-ceramide (4b)
Prepared from 1 and DL-2-hydroxyhexanoic acid in 40% yield after purification by column chromatography (ethyl acetate-n-hexane, 2 : 1, v/v). Analytical sample of 4b was prepared by crystallization from ethyl acetate-n-hexane (1 : 3, v/v) as a white microcrystalline powder, mp 68-69°C. TLC Rf = 0.41 (ethyl acetate-n-hexane, 1: 1, v/v); [α]22D = −11.4 (c = 0.5, CHCl3 ); [α]22365 = −43.4 (c = 0.5, CHCl3 ); 1H NMR (400 MHz, CDCl3) δ 6.24 (d, 1H, J = 8.4, NH), 5.76 (dtd, 1H, J = 15.1, 7.4, 1.0, 5-H), 5.42 (ddt, 1H, J = 15.1, 7.5, 1.4, 4-H), 4.15 (dd, 1H, J = 8 and 9.6, 3-H), 4.06 (q, 1H, J = 4.0, 2′-HS), 4.0 (dd, 1H, J = 5.6 and 11.2, 1-Ha), 3.81 (m, 1H, 2-H), 3.66 (dd, 1H, J = 9.6 and 11.2, 1-Hb), 2.26 (d, 1H, J = 4.8, 2′-OH), 2.02 (m, 2H, C(6)Ha), 1.80 (m, 1H, 3′-Ha), 1.58 (m, 1H, 3′-Hb), 1.52 (s, 3H, CH3), 1.43 (s, 3H, CH3), 1.24 (m, 32H, CH2), 1.15 (m, 2H, CH2), 0.85 ( m, 6H, 2×CH3). MS (CH3OH) m/z 929.7 ([2M+Na]+, 2), 449.5 (100), 414.4 ([MH-(CH3)2C], 6) 396.2 ([MH-(CH3)2CO]+, 48). Calcd. for C27H51NO4 m/z 453.38 [M].
4. 1. 3. 5. (2S, 3R, 2′R, 4E)-1,3-isopropylidene-2′-hydroxy-C12-ceramide (3c)
Prepared from 1 and DL-2-hydroxydodecanoic acid in 33% yield after purification by column chromatography (ethyl acetate-n-hexane, 2:3, v/v). Analytical sample of 3c was prepared by crystallization from ethyl acetate-nhexane (3 : 1, v/v) as a white microcrystalline powder, mp 85-87°C. TLC Rf = 0.22 (ethyl acetate-n-hexane, 2:3, v/v); [α]23D = +5.60 (c = 0.5, CHCl3 ); [α]23365 = +11.0 (c = 0.5, CHCl3); 1H NMR (500 MHz, CDCl3) δ 6.32 (d, 1H, J = 8.8, NH), 5.74 (dtd, 1H, J = 15.1, 7.4, 1.0, 5-H), 5.40 (ddt, 1H, J = 15.1, 7.5, 1.4, 4-H), 4.04 (m, 2H, 2′-HR and 3-H), 3.96 (dd, 1H, J = 5.2 and 11.2, 1-Ha), 3.86 (m, 1H, 2-H), 3.64 (dd, 1H, J = 9.6 and 11.2, 1-Hb), 2.24 (d, 1H, J = 4.4, 2′-OH), 2.01 (m, 2H, C(6)Ha), 1.92 (m, 2H, CH2), 1.78 (m, 1H, 3′-Ha), 1.64 (m, 2H, CH2), 1.59 (m, 1H, 3′-Hb), 1.48 (s, 3H, CH3), 1.41 (s, 3H, CH3), 1.24 (m, 32H, CH2), 1.05 (m, 2H, CH2), 0.88 ( t, 6H, J = 7.1, CH3). MS (CH3OH) m/z 538.6 ([MH]+, 2), 498.5.6 ([MH-2H2O]+, 10), 488.4 ([MH-(CH3)2CO]+, 100), 449.5 (10). Calcd. for C33H63NO4 m/z 537.48 [M].
4. 1. 3. 6. (2S, 3R, 2′S, 4E)-1,3-isopropylidene-2′-hydroxy-C12-ceramide (4c)
Prepared from 1 and DL-2-hydroxydodecanoic acid in 37% yield after purification by column chromatography (ethyl acetate-n-hexane, 2:3, v/v). Analytical sample of 4c was prepared by crystallization from ethyl acetate-n-hexane (3 : 1, v/v) as a white microcrystalline powder, mp 80-82°C. TLC Rf = 0.40 (ethyl acetate-n-hexane, 2:3, v/v); [α]23D = −6.84 (c = 0.5, CHCl3 ); [α]23365 = −34.2 (c = 0.5, CHCl3 ); 1H NMR (400 MHz, CDCl3) δ 6.21 (d, 1H, J = 8.4, NH), 5.74 (dtd, 1H, J = 15.0, 7.5, 1.0, 5-H), 5.40 (ddt, 1H, J = 15.1, 7.5, 1.4, 4-H), 4.12, (dd, 1H, J= 7.6 and 9.2, 3-H), 4.04 (q, 1H, J = 4.2, 2′-HS), 3.97 (dd, 1H, J = 5.2 and 11.2, 1-Ha), 3.79 (m, 1H, 2-H), 3.64 (dd, 1H, J = 9.6 and 11.2, 1-Hb), 2.24 (d, 1H, J = 4.4, 2′-OH), 2.0 (m, 2H, C(6)Ha), 1.92 (m, 0.5H, CH2), 1.78 (m, 1H, 3′-Ha), 1.67 (m, 0.5H, CH2), 1.58 (m, 1H, 3′-Hb), 1.48 (s, 3H, CH3), 1.42 (s, 3H, CH3), 1.24 (m, 36.5H, CH2), 1.05 (m, 0.5H, CH2), 0.88 ( t, 6H, J = 7.1, CH3). MS (CH3OH) m/z 538.6 ([MH]+, 2), 498.5.6 ([MH-2H2O]+, 10), 488.4 ([MH-(CH3)2CO]+, 100), 449.5 (10). Calcd. for C33H63NO4 m/z 537.48 [M].
4.1. 3. 7. (2S, 3R, 2′R, 4E)-1,3-isopropylidene-2′-hydroxy-C16-ceramide (3d)
Prepared from 1 and DL-2-hydroxyhexadecanoic acid in 40% yield after purification by column chromatography (ethyl acetate-n-hexane, 2 : 3, v/v). Analytical sample of 3d was prepared by crystallization from ethyl acetate-nhexane (3 : 1, v/v) as a white microcrystalline powder, mp 100-101°C. TLC Rf = 0.25 (ethyl acetate-n-hexane, 2:3, v/v); [α]22D = +8.6 (c = 0.8, CHCl3 ); [α]22365 = +17.5 (c = 0.8, CHCl3); 1H NMR (500 MHz, CDCl3) δ 6.29 (d, 1H, J = 8.6, NH), 5.74 (dtd, 1H, J = 15.4, 7.6, 1.0, 5-H), 5.42 (ddt, 1H, J = 15.4, 7.7, 1.4, 4-H), 4.07 (m, 2H, 2′-HR and 3-H), 3.97 (dd, 1H, J = 4.4 and 11.4, 1-Ha), 3.86 (m, 1H, 2-H), 3.64 (dd, 1H, J = 9.6 and 11.4, 1-Hb), 2.01 (m, 2H, C(6)Ha), 1.92 (m, 0.5H, CH2), 1.78 (m, 1H, 3′-Ha), 1.68 (m, 0.5H, CH2), 1.57 (m, 1H, 3′-Hb), 1.49 (s, 3H, CH3), 1.42 (s, 3H, CH3), 1.24 (m, 44.5H, CH2), 1.1 (m, 0.5H, CH2), 0.88 ( t, 6H, J = 7.1, CH3). MS (CH3OH) m/z 616.6 ([M+Na]+, 4), 536.5 ([MH-(CH3)2CO]+, 100), 449.5 (85). Calcd. for C73713NO4 m/z 593.54 [M].
4.1. 3. 8. (2S, 3R, 2′S, 4E)-1,3-isopropylidene-2′-hydroxy-C16-Ceramide (4d)
Prepared from 1 and DL-2-hydroxyhexadecanoic acid in 38% yield after purification by column chromatography (ethyl acetate-n-hexane, 2:3, v/v). Analytical sample of 4d was prepared by crystallization from ethyl acetate-n-hexane (3 : 1, v/v) as a white microcrystalline powder, mp 81- 83°C. TLC Rf = 0.44 (ethyl acetate-n-hexane, 2:3, v/v); [α]22D = −7.6 (c = 0.8, CHCl3); [α]22365 = −36.8 (c = 0.8, CHCl3); 1H NMR (500 MHz, CDCl3) δ 6.20 (d, 1H, J = 8.2, NH), 5.75 (dtd, 1H, J = 15.3, 6.7, 1.0, 5-H), 5.40 (ddt, 1H, J = 15.3, 6.6, 1.2, 4-H), 4.13 (dd, 1H, J = 7.7 and 9.6, 3-H), 4.03 (q, 1H, J = 4.1, 2′-HS ), 3.98 (dd, 1H, J = 5.2 and 11.2, 1-Ha), 3.80 (m, 1H, 2-H), 3.68 (dd, 1H, J = 9.6 and 11.2, 1-Hb), 2.22 (d, 1H, J = 4.9, 2′-OH), 2.02 (m, 2H, C(6)H2), 1.78 (m, 1H, 3′-Ha), 1.58 (m, 1H, 3′-Hb), 1.49 (s, 3H, CH3), 1.42 (s, 3H, CH3), 1.24 (m, 46H, CH2), 0.87 (t, 6H, J = 7.1, CH3). MS (CH3OH) m/z 616.6 ([M+Na]+, 4), 536.5 ([MH-(CH3)2CO]+, 100), 449.5 (85). Calcd. for C73713NO4 m/z 593.54 [M].
4. 1. 3. 9. (2S, 3R, 2′R)-1,3-isopropylidene-2′-hydroxy-C6-dihydroceramide (5b)
Prepared from 2 and DL-2-hydroxyhexanoic acid in 32% yield after purification by column chromatography (ethyl acetate-n-hexane, 1 : 1, v/v). Analytical sample of 5b was prepared by crystallization from ethyl acetate-n-hexane (3 : 1, v/v) as a white microcrystalline powder, mp 61-62°C. TLC Rf = 0.26 (ethyl acetate-n-hexane, 1:1, v/v); [α]23D = +29.4 (c = 0.5, CH3OH); [α]23365 = +93.4 (c = 0.5, CH3OH); 1H NMR (500 MHz, CDCl3) δ 6.43 (d, 1H, J = 8.4, NH), 4.14 (q, 1H, J= 4.2, 2′-HR), 3.86 (m, 2H, 1-Ha and 3-H), 3.53 (m, 2H, 2-H and 1-Hb), 1.82 (m, 3′-Ha, 1H), 1.63 (m, 3′-Hb, 1H), 1.23-1.52 (m, 36H, CH2 and 2xCH3), 0.85 (m, 6H, 2xCH3); MS (CH3OH) m/z 456.4 ([MH]+, 100), 398.4 ([MH-(CH3)2CO]+, 30). Calcd. for C27H31NO4 m/z 455.71 [M].
4. 1. 3. 10. (2S, 3R, 2′S)-1,3-isopropylidene-2′-hydroxy-C6-dihydroceramide (6b)
Prepared from 2 and DL-2-hydroxyhexanoic acid in 35% yield after purification by column chromatography (ethyl acetate-n-hexane, 1 : 1, v/v). Analytical sample of 6b was prepared by crystallization from ethyl acetate-nhexane (3 : 1, v/v) as a white microcrystalline powder, mp 83-84°C. TLC Rf = 0.46 (ethyl acetate-n-hexane, 1:1, v/v); [α]23D = +11.0 (c = 0.5, CH3OH); [α]23365 = +23.4 (c = 0.5, CH3OH) ; 1H NMR (500 MHz, CDCl3) δ 6.35 (d, 1H, J = 8.5, NH), 4.12 (q, 1H, J= 4.1, 2′-HS), 3.90 (m, 2H, 1-Ha and 3-H), 3.55 (m, 2H, 2-H and 1-Hb), 1.82 (m, 3′-Ha, 1H), 1.60 (m, 3′-Hb, 1H), 1.20-1.52 (m, 36H, CH2 and 2xCH3), 0.90 (m, 6H, 2xCH3). MS (CH3OH) m/z 456.4 ([MH]+, 100), 398.4 ([MH(CH3)2CO]+, 30). Calcd. for C27H31NO4 m/z 455.71 [M].
4.1. 4. General procedure for the preparation of 2′-OHCers (7b-d, 8b-d) and 2′-OH-C6-dhCers (9b, 10b)
A mixture of 1,3-isopropylidene-2′-hydroxy-ceramide (0.07 mmol) and ptoluenesulfonic acid monohydrate (19 mg, 0.10 mmol) in dry methanol (2 mL) was stirred at room temperature for 4 hrs. The reaction mixture was evaporated under reduced pressure to dryness. The obtained residue was purified by flash column chromatography using the suitable solvent system.
4. 1. 4. 1. (2S, 3R, 2′R, 4E)-2′-hydroxy-C4-ceramide (7a)
Prepared from 3a in 80% yield after purification by column chromatography (CHCl3 : CH3OH, 45 : 5, v/v v/v). Analytical sample of 7a was prepared by crystallization from acetone as a white microcrystalline powder, mp 94-96°C. TLC Rf = 0.32 (CHCl3 : CH3OH, 45:5, v/v); [α]22D = +18.4 (c = 0.25, CHCl3); [α]22365 = +43.1 (c = 0.25, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.12 (d, 1H, J = 7.7, NH), 5.78 (dtd, 1H, J = 15.1, 6.8, 1.0, 5-H), 5.52 (ddt, 1H, J = 15.1, 6.8, 1.1, 4-H), 4.31 (m, 1H, 3-H), 4.12 (dd, 1H, J= 4.1 and 7.2, 2′-HR), 3.93 (m, 2H, 1-Ha and 2-H), 3.74 (dd, 1H, J = 3.1 and 11.0, 1-Hb), 2.04 (q, 2H, J = 7.0, C(6)H2), 1.87 (m, 1H, 3′-Ha), 1.70 (m, 1H, 3′-Hb), 1.39 (m, 2H, C(7)H2 ), 1.23 (m, 20H, CH2), 0.99 (t, 3H, J = 7.1, C′H3), 0.87 (t, 3H, J = 7.1, CH3); 13C NMR (CDCl3) δ 174.7 (C=Ο), 134.6 (C5), 128.3 (C4), 74.3 (C3), 73.2 (C′2), 62.1 (C1), 54.3 (C2), 32.3 (C′3), 31.9 (C6), 29.6, 29.4, 29.3, 29.2, 29.0, 27.6 and 22.6 (C7-C17), 14.1 (C′H3), 9.15 (CH3). (CD3OD) δ 176.80 (C=Ο), 134.78 (C5), 130.80 (C4), 73.83 (C3), 73.21 (C′2), 61.79 (C1), 55.97 (C2), 33.23 (C′3), 32.94 (C6), 30.66, 30.58, 30.52, 30.34, 30.29, 30.11, 28.59 and 23.61 (C7-C17), 14.35 (C′H3), 9.68 (CH3) MS (CH3OH) m/z 368.1 ([MH-H2O]+, 14), 386.0 ([MH]+, 10), 793.1 ([2M + Na]+, 100). Calcd. for C22H49NO4 m/z 385.32 [M].
4. 1. 4. 2. (2S, 3R, 2′S, 4E)-2′-hydroxy-C4-ceramide (8a)
Prepared from 4a in 81% yield after purification by column chromatography (CHCl3 : CH3OH, 45:5, v/v). Analytical sample of 8a was prepared by crystallization from acetone as a white microcrystalline powder, mp 80-82°C. TLC Rf = 0.34 (CHCl3 : CH3OH, 45:5, v/v); [α]22D = −25.1(c = 0.25, CHCl3), [α]22365 = −95.8 (c = 0.25, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.13 (d, 1H, J = 7.4, NH), 5.82 (dtd, 1H, J = 15.1, 7.0, 1.0, 5-H), 5.54 (ddt, 1H, J = 15.1, 7.0, 1.1, 4-H), 4.38 (t, 1H, J = 5.4, 3-H), 4.15 (dd, 1H, J = 4.1 and 7.1, 2′-Hs), 4.01 (dd, 1H, J = 4.0 and 11.4, 1-Ha), 3.84 (m, 1H, 2-H), 3.75 (dd, 1H, J = 4.1 and 11.4, 1-Hb), 2.08 (q, 2H, J = 7.0, C(6)H2), 1.91 (m, 1H, 3′-Ha), 1.74 (m, 1H, 3′-Hb), 1.41 (m, 2H, C(7)H2 ), 1.23 (m, 20H, CH2), 1.03 (t, 3H, J = 7.1, C′H3), 0.92 (t, 3H, J = 7.1, CH3); (CD3OD) δ 5.70 (dtd, 1H, J = 15.4, 7.0, 1.0, 5-H), 5.49 (ddt, 1H, J = 15.4, 7.0, 1.0, 4-H), 4.15 (t, 1H, J = 7.0, 3-H), 3.94 (dd, 1H, J = 5.6 and 7.0, 2′-HS ), 3.84 (m, 1H, 2-H), 3.75 (dd, J = 4.9 and 11.2, 1-Ha), 3.63 (dd, 1H, J = 4.2 and 11.2, 1-Hb), 2.04 (q, 2H, J = 7.0, C(6)H2), 1.77 (m, 1H, 3′-Ha), 1.62 (m, 1H, 3′Hb), 1.37 (m, 2H, CH2) 1.28 (m, 42H, CH2), 0.94 (t, 6H, J = 7.0, CH3), 0.89 (t, 6H, J = 7.0, CH3); (CD3OD) δ 177.01 (C=Ο), 134.86 (C5), 130.92 (C4), 74.03 (C3), 73.36 (C′2), 62.09 (C1), 56.44 (C2), 33.55 (C′3), 33.23 (C6), 30.97, 30.96, 30.95, 30.92, 30.81, 30.49, 30.47, 28.80 and 23.89 (C7-C17), 14.58 (C′H3), 9.77 (CH3). MS (CH3OH) m/z 385.9 ([MH]+, 28), 368.1 ([MH-H2O]+, 25), 793.1 ([2M + Na]+, 100). Calcd. for C22H49NO4 m/z 385.32 [M].
4. 1. 4. 3. (2S, 3R, 2′R, 4E)-2′-hydroxy-C6-ceramide (7b, LCL366)
Prepared from 3b in 78% yield after purification by column chromatography (CHCl3 : CH3OH, 45 : 5, v/v). Analytical sample of 7b was prepared by crystallization from acetone as a white microcrystalline powder, mp 85-86°C. TLC Rf = 0.38 (CHCl3 : CH3OH, 45:5, v/v); [α]22D = +7.7 (c = 0.25, CHCl3); [α]22365 = +28.6 (c = 0.25, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.21 (d, 1H, J = 7.0, NH), 5.77 (dtd, 1H, J = 15.1, 6.8, 1.0, 5-H), 5.52 (ddt, 1H, J = 15.1, 6.8, 1.1, 4-H), 4.29 (t, 1H, J = 5.0, 3-H), 4.13 (dd, 1H, J = 3.7 and 8.2, 2′-HR ), 3.92 (m, 2H, 1-Ha and 2-H), 3.75 (dd, 1H, J = 5.7 and 12.0, 1-Hb), 2.05 (q, 2H, J = 7.1, C(6)H2), 1.82 (m, 1H, 3′-Ha), 1.62 (m, 1H, 3-′Hb), 1.37 (m, 4H, CH2), 1.26 (m, 22H, CH2), 0.91 (t, 3H, C′H3), 0.88 (t, 3H, J = 7.1, CH3); (CD3OD) δ 5.73 (dtd, 1H, J = 15.1, 7.0, 1.0, 5-H), 5.47 (ddt, 1H, J = 15.1, 7.0, 1.1, 4-H), 4.09 (t, 1H, J = 7.0, 3-H), 3.97 (dd, 1H, J = 3.8 and 8.1, 2′-HR ), 3.83 (m, 1H, 2-H), 3.77 (dd, 1H, J = 5.4 and 11.2, 1-Ha), 3.66 (dd, 1H, J = 4.1 and 11.2, 1-Hb), 2.03 (q, 2H, J = 7.1, C(6)H2), 1.74 (m, 1H, 3′-Ha), 1.53 (m, 1H, 3-′Hb), 1.4 (m, 4H, CH2), 1.28 (m, 22H, CH2), 0.91 (t, 3H, J = 7.1, C′H3), 0.89 (t, 3H, J = 7.1, CH3); 13C NMR (CDCl3) δ 175.5 (C=Ο), 134.6 (C5), 128.3 (C4), 74.0 (C3), 72.4 (C′2), 62.0 (C1), 54.4 (C2), 34.2 (C′3), 32.3 (C6), 32.0, 29.7, 29.5, 29.3, 29.2, 29.1, 27.2, 22.7 and 22.5 (C7-C17, C′4 and C′5), 14.1 (C′H3), 13.9 (CH3); (CD3OD) δ 177.1 (C=Ο), 134.8 (C5), 131.0 (C4), 73.3 (C3), 72.9 (C′2), 61.8 (C1), 56.0 (C2), 35.5 (C′3), 33.4 (C6), 33.0, 30.7, 30.6, 30.4, 30.3, 30.2, 28.4, 23.7 and 23.6 (C7-C17, C′4 and C′5), 14.4 (C′H3), 14.3 (CH3); MS (CH3OH) m/z 436.4 ([M+Na]+, 20), 414.4 ([MH]+, 100), 396.4 ([MH-H2O]+, 10). Calcd. for C24H47NO4 m/z 413.35[M].
4. 1. 4. 4. (2S, 3R, 2′S, 4E)-2′-hydroxy-C6-ceramide (8b, LCL367)
Prepared from 4b in 88% yield after purification by column chromatography (CHCl3 : CH3OH, 45 : 5, v/v). Analytical sample of 8b was prepared by crystallization from acetone as a white microcrystalline powder, mp 80-82°C. TLC Rf = 0.40 (CHCl3 : CH3OH, 45:5, v/v); [α]22D = −35.4 (c = 0.25, CHCl3); [α]22365 = −124.8 (c = 0.25, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.13 (d, 1H, J = 7.7, NH), 5.80 (dtd, 1H, J = 15.1, 6.8, 1.0, 5-H), 5.52 (ddt, 1H, J = 15.1, 6.8, 1.1, 4-H), 4.35 (t, 1H, J = 5.2, 3-H), 4.14 (dd, 1H, J = 3.9 and 8.0, 2′-HR ), 3.97 (dd, 1H, J = 4.0 and 11.4, 1-Ha), 3.90 (m, 1H, 2-H), 3.72 (dd, 1H, J = 3.5 and 11.4, 1-Hb), 2.05 (q, 2H, J = 7.1, C(6)H2), 1.83 (m, 1H, 3′-Ha), 1.65 (m, 1H, 3-′Hb), 1.37 (m, 4H, CH2), 1.26 (m, 22H, CH2), 0.92 (t, 3H, J = 7.1, C′H3), 0.88 (t, 3H, J = 7.1, CH3); (CD3OD) δ 7.56 (d, 0.5H, J = 9.0, NH), 5.70 (dtd, 1H, J = 15.1, 7.0, 1.0, 5-H), 5.47 (ddt, 1H, J = 15.1, 7.0, 1.1, 4-H), 4.15 (t, 1H, J = 6.7, 3-H), 3.97 (dd, 1H, J = 3.8 and 8.1, 2′-HS ), 3.84 (m, 1H, 2-H), 3.75 (dd, 1H, J = 5.4 and 11.1, 1-Ha), 3.63 (dd, 1H, J = 4.4 and 11.1, 1-Hb), 2.03 (q, 2H, J = 7.1, C(6)H2), 1.74 (m, 1H, 3′-Ha), 1.57 (m, 1H, 3-′Hb), 1.4 (m, 4H, CH2), 1.28 (m, 22H, CH2), 0.91 (t, 3H, C′H3), 0.89 (t, 3H, J = 7.1, CH3); 13C NMR (CDCl3) δ 175.2 (C=O), 134.5 (C5), 127.9 (C4), 73.7 (C3), 72.3 (C′2), 62.2 (C1), 54.5 (C2), 34.3 (C′3), 32.3 (C6), 31.9, 29.6, 29.4, 29.3, 29.2, 29.1, 27.2, 22.6 and 22.4 (C7-C17, C′4 and C′5), 14.1 (C′H3), 13.9 (CH3); (CD3OD) δ 177.0 (C=O), 134.6 (C5), 131.7 (C4), 73.1 (C3), 72.8 (C′2), 61.9 (C1), 56.2 (C2), 35.4 (C′3), 33.3 (C6), 33.0, 30.8, 30.7, 30.6, 30.4, 30.3, 28.3, 23.7 and 23.6 (C7-C17, C′4 and C′5), 14.4 (C′H3), 14.3 (CH3); MS (MeOH) m/z 436.4 ([M+Na]+, 20), 414.4 ([MH]+, 100), 396.4 ([MH-H2O]+, 10). Calcd. for C24H47NO4 m/z 413.35[M].
4. 1. 4. 5. (2S, 3R, 2′R, 4E)-2′-hydroxy-C12-ceramide (7c)
Prepared from 4c in 75% yield after column chromatography purification (silica gel 60; CHCl3-MeOH, 45: 5, v/v). Analytical sample of 7c was prepared by crystallization from acetone as a white microcrystalline powder. TLC Rf = 0.37 (CHCl3-MeOH, 45 : 5, v/v); [α]23D = +8.4° (c = 0.25, CHCl3 ); [α]23365 = +19.0° (c = 0.25, CHCl3); 1H NMR (500 MHz, CD3OD-CDCl3, 3 : 1, v/v)/ δ 5.63 (dtd, 1H, J = 15.1, 6.8, 1.0, 5-H), 5.35 (ddt, 1H, J = 15.1, 6.8, 1.1, 4-H), 4.0 (t, 1H, J = 5.6, 3-H), 3.95 (m, H2O and 2′-HR ), 3.65 (m, 2H, 1-Ha and 2-H), 3.57 (dd, 1H, J = 3.6 and 11.2, 1-Hb), 1.95 (q, 2H, J = 7.1, C(6)H2), 1.65 (m, 1H, 3′-Ha), 1.44 (m, 1H, 3′-Hb), 1.15 (m, 38H, CH2), 0.76 (t, 6H, J = 7.1, CH3); ESI-MS (CH3OH, relative intensity, %) m/z. 498.5 ([MH]+, 100), 480.4 ([MH-H2O]+, 15). Calcd for C30H59NO4 m/z 497.44 [M].
4. 1. 4. 6. (2S, 3R, 2′S, 4E)-2′-hydroxy-C12-ceramides (8c)
Prepared from 4c in 70% yield after column chromatography purification (silica gel 60; CHCl3-CH3OH, 45: 5, v/v). Analytical sample of 8c was prepared by crystallization from acetone as a white microcrystalline powder. TLC Rf = 0.46 (CHCl3-CH3OH, 45 : 5, v/v); [α]23D = −26.9° (c = 0.25, CHCl3,); [α]23365 = −99.0° (c = 0.25, CHCl3 ); 1H NMR (500 MHz, CDCl3) δ 7.30 (d, 1H, J = 8.4, NH), 5.70 (dtd, 1H, J = 15.1, 6.8, 1.0, 5-H), 5.40 (ddt, 1H, J = 15.1, 6.8, 1.1, 4-H), 4.17 (m, 1H, 3-H), 3.93 (dd, 1H, J = 4.0 and 8.4, 2′-HS ), 3.72 (m, 2H, 1-Ha and 2-H), 3.53 (dd, 1H, J = 5.6 and 13.2, 1-Hb), 1.96 (q, 2H, J = 7.0, C(6)H2), 1.72 (m, 1H, 3′-Ha), 1.48 (m, 1H, 3′-Hb), 1.20 (m, 38H, CH2), 0.79 (t, 6H, J = 7.1, CH3). ESI-MS (CH3OH, relative intensity, %) m/z. 498.5 ([MH]+, 100), 480.4 ([MH-H2O]+, 15). Calcd for C30H59NO4 m/z 497.44 [M].
4. 1. 4. 7. (2S, 3R, 2′R, 4E)-2′-hydroxy-C16-ceramide (7d)
Prepared from 3d in 88% yield after column chromatography purification as a white microcrystalline powder (silica gel 60; CHCl3-CH3OH, 45 : 5, v/v). Analytical sample of 7d was prepared by crystallization from acetone as a white microcrystalline powder, mp 101-103°C. TLC Rf = 0.38 (CHCl3-CH3OH, 45 : 5, v/v); [α]23D = +5.2° (c = 0.25, MeOH-CHCl3, 2 : 8, v/v); [α]23365 = +32.2° (c = 0.25, CH3OH-CHCl3, 2 : 8, v/v); 1H NMR (500 MHz, CD3OD-CDCl3, 1 : 3, v/v) δ 5.76 (dtd, 1H, J = 15.1, 6.8, 1.0, 5-H), 5.47 (ddt, 1H, J = 15.1, 6.8, 1.1, 4-H), 4.12 (t, 1H, J = 6.0, 3-H), 4.01 (dd, 1H, J = 3.7 and 8.2, 2′-HR ), 3.85 (m, 1H, 2-H), 3.80 (dd, J = 8.6 and 11.3, 1-Ha), 3.68 (dd, 1H, J = 3.7 and 11.3, 1-Hb), 2.05 (q, 2H, J = 7.1, C(6)H2), 1.78 (m, 1H, 3′-Ha), 1.58 (m, 1H, 3′Hb), 1.26 (m, 46H, CH2), 0.88 (t, 6H, J = 7.1, CH3); (CDCl3) δ 7.11 (d, 1H, J = 8.2, NH) 5.78 (dtd, 1H, J = 15.1, 6.8, 1.0, 5-H), 5.52 (ddt, 1H, J = 15.1, 6.8, 1.1, 4-H), 4.30 (t, 1H, J = 6.0, 3-H), 4.13 (dd, 1H, J = 3.5 and 8.0, 2′-HR ), 3.93 (m, 2H, 1-Ha and 2-H), 3.74 (dd, 1H, J = 5.7 and 13.0, 1-Hb), 2.05 (q, 2H, J = 7.1, C(6)H2), 1.82 (m, 1H, 3′-Ha), 1.64 (m, 1H, 3′-Hb), 1.26 (m, 46H, CH2), 0.88 (t, 6H, J = 7.1, CH3); (CD3OD, 30°C, 700MHz) δ 5.72 (dtd, 1H, J = 15.3, 7.0, 0.9, 5-H), 5.47 (ddt, 1H, J = 15.3, 7.3, 0.9, 4-H), 4.08 (t, 1H, J = 7.4, 3-H), 4.12 (dd, 1H, J = 3.8 and 7.9, 2′-HR ), 3.84 (m, 1H, 2-H), 3.76 (dd, J = 4.9 and 11.2, 1-Ha), 3.64 (dd, 1H, J = 5.0 and 11.2, 1-Hb), 2.05 (q, 2H, J = 7.0, C(6)H2), 1.76 (m, 1H, 3′-Ha), 1.57 (m, 1H, 3′Hb), 1.40 (m, 4H, CH2) 1.29 (m, 42H, CH2), 0.90 (t, 6H, J = 7.1, CH3); 13C NMR (CDCl3) δ 174.7 (C=O), 134.5 (C5), 128.7 (C4), 74.4 (C3), 72.46 (C′2), 62.3 (C1), 54.6 (C2), 34.9 (C′3), 32.3 (C6), 31.9, 29.7, 29.5, 29.4, 29.3, 29.2, 29.1, 25.0 and 22.7 (C7-C17 and C′4-C′15), 14.0 (C′H3 and CH3); (CD3OD) δ 177.3 (C=O), 135.0 (C5), 131.2 (C4), 73.4 (C3), 73.2 (C′2), 62.1 (C1), 56.1 (C2), 36.0 (C′3), 33.6 (C6), 33.2, 30.98, 30.96, 30.94, 30.92, 30.9, 30.88, 30.82, 30.7, 30.62, 30.61, 30.49), 26.3 and 23.8 (C7-C17 and C′4 -C′15), 14.5 (C′H3 and CH3). ESI-MS (CH3OH, relative intensity, %) m/z 1130.5 and 1129.4 ([2M + Na]+and ([2M-H + Na]+ 70 and 100), 1107.0 ([2M+H]+, 35), 554.1 (MH+, 16), 536.3 ([MH - H2O]+, 27). Calcd for C34H67NO4 m/z 553.51 [M].
4. 1. 4. 8. (2S, 3R, 2′S, 4E)-2′-hydroxy-C16-ceramides (8d)
Prepared from 4d in 81% yield after column chromatography purification as a white microcrystalline powder (silica gel 60; CHCl3-MeOH, 45 : 5, v/v). Analytical sample of 8d was prepared by crystallization from acetone as a white microcrystalline powder, mp 100-102°C. TLC Rf = 0.39 (CHCl3-CH3OH, 45 : 5, v/v); [α]23D = −16.1° (c = 0.25, MeOH-CHCl3, 2 : 8, v/v); [α]23365 = −50.7° (c = 0.25, CH3OH-CHCl3, 2 : 8, v/v); 1H NMR (500 MHz, CD3OD-CDCl3, 1 : 3, v/v) δ 7.42 (d, ~0.5H, J = 8.2, NH), 5.76 (dtd, 1H, J = 15.1, 6.8, 1.0, 5-H), 5.47 (ddt, 1H, J = 15.1, 6.8, 1.1, 4-H), 4.22 (t, 1H, J = 6.3, 3-H), 4.01 (dd, 1H, J = 3.8 and 8.3, 2′-HS ), 3.85 (m, 1H, 2-H), 3.79 (dd, 1H, J = 8.2 and 11.2, 1-Ha), 3.62 (dd, 1H, J = 5.6 and 11.2, 1-Hb), 2.04 (q, 2H, J = 7.0, C(6)H2), 1.78 (m, 1H, 3′-Ha), 1.58 (m, 1H, 3′-Hb), 1.27 (m, 46H, CH2), 0.88 (t, 6H, J = 7.1, CH3); (CDCl3) δ 7.10 (d, 1H, J = 8.2, NH) 5.80 (dtd, 1H, J = 15.1, 6.8, 1.0, 5-H), 5.53 (ddt, 1H, J = 15.1, 6.8, 1.1, 4-H), 4.34 (t, 1H, J = 5.0, 3-H), 4.12 (dd, 1H, J = 4.0 and 8.0, 2′-HR ), 3.95 (dd, J = 4.0 and 11.5, 1-Ha), 3.89 (m, 1H, 2-H), 3.72 (dd, 1H, J = 3.5 and 11.5, 1-Hb), 2.06 (q, 2H, J = 7.0, C(6)H2), 1.82 (m, 1H, 3′-Ha), 1.64 (m, 1H, 3′Hb), 1.26 (m, 46H, CH2), 0.88 (t, 6H, J = 7.1, CH3); (CD3OD, 30°C, 700MHz) δ 5.73 (dtd, 1H, J = 15.3, 7.0, 1.0, 5-H), 5.49 (ddt, 1H, J = 15.3, 7.0, 1.0, 4-H), 4.16 (t, 1H, J = 6.3, 3-H), 4.12 (q, 1H, J = 5.6, 2′-HS ), 3.84 (m, 1H, 2-H), 3.79 (dd, J = 5.1 and 11.2, 1-Ha), 3.64 (dd, 1H, J = 4.0 and 11.2, 1-Hb), 2.04 (q, 2H, J = 7.0, C(6)H2), 1.71 (m, 1H, 3′-Ha), 1.55 (m, 1H, 3′Hb), 1.37 (m, 4H, CH2) 1.29 (m, 42H, CH2), 0.90 (t, 6H, J = 7.1, CH3); 13C NMR (CDCl3) δ 174.6 (C=O), 134.5 (C5), 128.5 (C4), 74.2 (C3), 72.38 (C′2), 62.4 (C1), 54.7 (C2), 35.0 (C′3), 32.9 (C6), 31.9, 29.7, 29.6, 29.54 29.5, 29.4, 29.3, 29.2, 29.0, 25.0 and 22.6 (C7-C17 and C′4 -C′15), 14.0 (C′H3 and CH3); (CD3OD) δ 177.2 (C=O), 134.9 (C5), 130.9 (C4), 73.3 (C3), 73.03 (C′2), 62.1 (C1), 56.5 (C2), 30.98, 30.96, 30.94, 30.92, 30.9, 30.88, 30.82, 30.7, 30.62, 30.61, 30.49, 26.2 and 23.9 (C7-C17 and C′4 -C′15), 14.5 (C′H3 and CH3).
4.1. 4. 9. (2S, 3R, 2′R)-2′-hydroxy-C6-dihydroceramide (9b, LCL447)
Prepared from 5b in 93 % yield after column chromatography purification as a white microcrystalline powder (silica gel 60; CHCl3-CH3OH, 10 : 1, v/v). Analytical sample of 9b was prepared by crystallization from acetone as a white microcrystalline powder, mp 112-114.°C. TLC Rf = 0.24 (CHCl3-CH3OH, 45 : 5, v/v); [α]23D = +38.20° (c = 0.125, MeOH); [α]23365 = +124.91° (c = 0.125, CH3OH); 1H NMR (500 MHz, CDCl3) δ 7.28 (d, 1H, J = 8.4, NH), 4.12 (q, 1H, J= 3.6, 2′-HS), 3.95 (dd, 1H, J = 3.6 and 11.2, 1-Ha), 3.82 (m, 2H, 2-H and 3-H), 3.78 (m, 1H, J = 3.1 and 11.2, 1Hb), 1.82 (m, 1H, 3′-Ha), 1.61 (m, 1H, 3′-Hb ), 1.51 (m, 2H, C(4)H2), 1.25 (m, 36H, CH2), 0.90 (t, 3H, J = 7.1, C′H3), 0.87 (t, 3H, J = 7.1, CH3). MS (CH3OH) m/z 416.4 ([MH]+, 100), 498.3 ([MH-H2O]+, 4). Calcd. for C24H49NO4 m/z 415.37[M].
4. 1. 4. 10. (2S, 3R, 2′S)-2′-hydroxy-C6-dihydroceramide (10b, LCL446)
Prepared from 6b in 95 % yield after column chromatography purification as a white microcrystalline powder (silica gel 60; CHCl3-CH3OH, 10 : 1, v/v). Analytical sample of 10b was prepared by crystallization from acetone as a white microcrystalline powder, mp 90-91°C. TLC Rf = 0.33 (CHCl3-CH3OH, 45 : 5, v/v); [α]23D = −17.20° (c = 0.125, CH3OH); [α]23365 = −62.03° (c = 0.125, MeOH); 1H NMR (500 MHz, CDCl3) δ 7.28 (d, 1H, J = 8.4, NH), 4.14 (q, 1H, J= 3.8, 2′-HS), 4.02 (dd, 1H, J = 3.5 and 11.4, 1-Ha), 3.82 (m, 2H, 2-H and 3-H), 3.78 (m, 1H, J = 3.1 and 11.4, 1Hb), 1.86 (m, 3′-Ha, 1H), 1.68 (m, 3′-Hb, 1H), 1.54 (m, 2H, C(4)H2), 1.25 (m, 36H, CH2), 0.90 (t, 3H, J = 7.1, C′H3), 0.86 (t, 3H, J = 7.1, CH3). MS (CH3OH) m/z 416.4 ([MH]+, 100), 498.3 ([MH-H2O]+, 4). Calcd. for C24H49NO4 m/z 415.37[M].
4.2. Biology
4.2.1. Cell Culture
MCF7 cells (breast adenocarcinoma, pleural effusion) were purchased from American type Culture Collection (ATCC, Rockville, MD, USA) and grown in RPMI 1640 media (Life Technologies, Inc) supplemented with 10% fetal calf serum (FCS, Summit Biotechnology, CO, USA) and maintained under standard incubator conditions (humidified atmosphere 95% air, 5% CO2, 37 °C). A parallel set of cells was used to determine cell proliferation and to prepare lipid extracts for MS analysis.
4.2.2 Cell proliferation
MCF7 cells were seeded at a density of ~50 % (corresponding to 1×106 cells) in 10 ml of 10 % FCS and, after an overnight incubation, were treated with the compounds at (0-50 μM) in ethanol (<0.1%) and the changes in cell numbers after 24 h were determined and expressed as a percentage of the untreated controls. Briefly, media were removed, cells were washed twice with PBS, detached using 1% Trypsin and centrifuged at 800 rpm. Cell pellets were resuspended in PBS and Trypan blue (Sigma Chemicals, St. Louis, MO, USA) was added (1:1 dilution). Under a light microscope, the percentage of unstained and stained cells was assessed. The IC50 represents the drug concentration resulting in 50% growth inhibition compared to control cells.
4.2.3. LC-MS/MS analysis of endogenous SPLs and cellular levels of exogenously added compounds
Advanced analyses were performed on a Thermo Finnigan TSQ 7000, triple-stage quadrupole mass spectrometer operating in a Multiple Reaction Monitoring (MRM) positive ionization mode as described.71,72 Quantitative analysis of the cellular level of 2′-OH-C6-Cers/dhCers and C6-Cer/dhCer was based on the calibration curves generated by spiking an artificial matrix with known amounts of target standards and an equal amount of the internal standard (IS). The target analyte to IS peak area ratios from the samples were similarly normalized to their respective IS and compared to the calibration curves using a linear regression model. Final results were expressed as the level of the particular SPLs/phospholipids (Pi) determined from the Bligh & Dyer lipid extract and expressed as SPLs/Pi (pmol/nmol).
Acknowledgements
This work was supported by NIH Grant P20 RR017677 and NCI grant P01 CA097132-01A. Special acknowledgement is for NCRR—CO6RR018823 providing laboratory space for Lipidomics Shared Resource in CRI building.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Science. 1993;259:1769. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 2.Mimeault M. FEBS Lett. 2002;530:9. doi: 10.1016/s0014-5793(02)03432-4. [DOI] [PubMed] [Google Scholar]
- 3.Carpinteiro A, Dumitru C, Schenck M, Gulbins E. Cancer Lett. 2008;264:1. doi: 10.1016/j.canlet.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Kitatani K, Idkowiak-Baldys J, Hannun YH. Cell. Signal. 2008;20:1010. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. http://www.lipidmaps.org/
- 6.Inagaki M, Isobe R, Kawano Y, Maiyamato T, Komori T, Higuchi R. Eur. J. Chem. 1998:129. [Google Scholar]
- 7.Asai N, Fusetani N, Matsunaga S. J. Nat. Prod. 2001;64:1210. doi: 10.1021/np010177m. [DOI] [PubMed] [Google Scholar]
- 8.Masuda Y, Yoshida M, Mori K. Biosci. Biotechnol. Biochem. 2002;66:1531. doi: 10.1271/bbb.66.1531. [DOI] [PubMed] [Google Scholar]
- 9.Azuma H, Takao R, Niiro H, Shikata K, Tamagaki S, Tachibana T, Ogino K. J. Org. Chem. 2003;68:2790. doi: 10.1021/jo0206824. [DOI] [PubMed] [Google Scholar]
- 10.Tien NQ, Ngoc P-H, Minh PH, Kiem PV, Minh CV, Kim YH. Arch. Pharm. Res. 2004;27:1020. doi: 10.1007/BF02975424. [DOI] [PubMed] [Google Scholar]
- 11.Radhika P, Rao VL, Laatsch H. Chem. Pharm. Bull. 2004;52:1345. doi: 10.1248/cpb.52.1345. [DOI] [PubMed] [Google Scholar]
- 12.Masuda Y, Mori K. Eur. J. Org. Chem. 2005:4789. [Google Scholar]
- 13.Leon F, Brouard I, Rivera A, Torres F, Rubio S, Quintana J, Estevez F, Bermejo J. J. Med. Chem. 2006;49:5830. doi: 10.1021/jm0605334. [DOI] [PubMed] [Google Scholar]
- 14.Ishii T, Okino T, Mino Y. J. Nat. Prod. 2006;69:1080. doi: 10.1021/np050530e. [DOI] [PubMed] [Google Scholar]
- 15.Holleran WM, Takagi T, Uchida Y. FEBS Lett. 2006;580:545. doi: 10.1016/j.febslet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 16.Futerman AH, van Meer G. Nat. Rev. Mol. Cell Biol. 2004;5:554. doi: 10.1038/nrm1423. [DOI] [PubMed] [Google Scholar]
- 17.Iwamori M. Human Cell. 2005;18:117. doi: 10.1111/j.1749-0774.2005.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 18.Hama H. Biochim. Biophys. Acta. 2010;1801:405. doi: 10.1016/j.bbalip.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugita M, Iwamori M, Evans J, McCluer RH, Dulanej JT, Moser HW. J. Lipid Res. 1974;15:223. [PubMed] [Google Scholar]
- 20.Iwamori M, Moser WH. Clin. Chem. 1975;21:725. [PubMed] [Google Scholar]
- 21.Edwardson S, Hama H, Shaag A, Gomori JM, Berger I, Soffer D, Korman SH, Taustein I, Saada A, Elpeleg O. Am. J. Hum. Genet. 2008;83:643. doi: 10.1016/j.ajhg.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiguchi K, Iwamori Y, Suzuki N, Kobayashi Y, Ishizuka B, Ishiwata I, Kita T, Kikuchi Y, Iwamori M. Cancer Sci. 2006;97:1321. doi: 10.1111/j.1349-7006.2006.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richtie SA, Ahiahonu PWK, Jayasinghe D, Heath D, Liu J, Lu Y, Jin W, Kavianpour A, Yamazaki Y, Khan AM, Hossain M, SuMyat KK, Wood PL, Krenitsky K, Takemasa I, Miyake M, Sekimoto M, Monden M, Matsubara H, Nomura F, Goodenowe DB. BMC Medicine. 2010;8:13. doi: 10.1186/1741-7015-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson K, Nilsson K, Samuelsson BE, Steen GO. Biochim. Biophys. Acta. 1969;176:660. doi: 10.1016/0005-2760(69)90239-2. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson O, Svenerholm L. J. Lipid Res. 1982;23:327. [PubMed] [Google Scholar]
- 26.Riboni L, Acquotti D, Casellato R, Ghidoni R, Montagnolo G, Benevento A, Zecca L, Rubino F, Sonnino S. Eur. J. Biochem. 1992;201:107. doi: 10.1111/j.1432-1033.1992.tb19834.x. [DOI] [PubMed] [Google Scholar]
- 27.Dahiya R, Brasitus TA. Biochim. Biophys. Acta. 1986;875:220. doi: 10.1016/0005-2760(86)90171-2. [DOI] [PubMed] [Google Scholar]
- 28.Kishimoto Y, Akanuma H, Singh I. Mol. Cell Biochem. 1979;254:1050. doi: 10.1007/BF00223361. [DOI] [PubMed] [Google Scholar]
- 29.Hirabayashi Y, Hamaoka A, Matsumoto M, Nishimura K. Lipids. 1986;21:710. doi: 10.1007/BF02537245. [DOI] [PubMed] [Google Scholar]
- 30.Kojima M, Ohnishi M, Ito S. J. Agric. Food. Chem. 1991;39:1709. [Google Scholar]
- 31.Wertz PW, van der Bergh B. Chem. Phys. Lipids. 1998;91:85. doi: 10.1016/s0009-3084(97)00108-4. [DOI] [PubMed] [Google Scholar]
- 32.Ponec M, Weerheim A, Lankhorst P, Wertz P. J. Invest. Dermatol. 2003;120:581. doi: 10.1046/j.1523-1747.2003.12103.x. [DOI] [PubMed] [Google Scholar]
- 33.Bouwstra JA, Gooris SG, Dubbelaar FER, Ponec M. J. Lipid Res. 2001;42:1759. [PubMed] [Google Scholar]
- 34.Pascher I. Biochem. Biophys. Acta. 1976;455:433. doi: 10.1016/0005-2736(76)90316-3. [DOI] [PubMed] [Google Scholar]
- 35.Lofgren H, Pasher I. Chem. Phys. Lipids. 1977;20:273. doi: 10.1016/0009-3084(77)90068-8. [DOI] [PubMed] [Google Scholar]
- 36.Yasugi E, Kasama T, Seyama Y. J. Biochem. 1991;110:202. doi: 10.1093/oxfordjournals.jbchem.a123557. [DOI] [PubMed] [Google Scholar]
- 37.Minamino M, Sakaguch I, Naka T, Ikeda N, Kato Y, Tomiyasu I, Yano I, Kobayashi K. Microbiology. 2003;149:2071. doi: 10.1099/mic.0.25922-0. [DOI] [PubMed] [Google Scholar]
- 38.Kyogasima M, Tadano-Aritomi K, Aoyama T, Yusa A, Goto Y, Tomiya-Kozumi K, Ito H, Murate T, Kannagi R, Hara A. J. Biochem. 2008;144:95. doi: 10.1093/jb/mvn050. [DOI] [PubMed] [Google Scholar]
- 39.Azuma H, Takao R, Shikata K, Niiro H, Tachibana T, Ogino K. J. Med. Chem. 2003;46:3445. doi: 10.1021/jm030125p. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Sun D-D, Chen J-W, He L-W, Zhang H-Q, Xu H-Q. Fitoterapia. 2007;78:490. doi: 10.1016/j.fitote.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Alderson NL, Rembiesa BM, Walla MD, Bielawska A, Bielawski J, Hama H. J. Biol. Chem. 2004;279:48562. doi: 10.1074/jbc.M406649200. [DOI] [PubMed] [Google Scholar]
- 42.Uchida Y, Hama H, Alderson NL, Douangpanya S, Wang Y, Crumrine DA, Elias PM, Holleran WM. J. Biol. Chem. 2007;282:13211. doi: 10.1074/jbc.M611562200. [DOI] [PubMed] [Google Scholar]
- 43.Dick KJ, Eckhardt M, Paisán-Ruiz C, Alshehhi AA, Proukakis C, Sibtain NA, Maier H, Sharifi R, Patton MA, Bashir W, Koul R, Raeburn S, Gieselmann V, Houlden H, Crosby AH. Hum. Mutat. 2010;31:E1251. doi: 10.1002/humu.21205. [DOI] [PubMed] [Google Scholar]
- 44.Sun Y, Witte DP, Zamzow M, Ran M, Quinn B, Matsuda J, Grabowski GA. Hum. Mol. Genet. 2007;16:957. doi: 10.1093/hmg/ddm040. [DOI] [PubMed] [Google Scholar]
- 45.Park JH, Schuchman EH. Biochem. Biophys. Acta. 2006;1758:2133. doi: 10.1016/j.bbamem.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 46.Van der Bijl P, Strous GJ, Lopes-Cardozo M, Thomas-Oates J, van Meer G. Biochem. J. 1996;317:589. doi: 10.1042/bj3170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pascher I, Sundell S. Chem. Phys. Lipids. 1977;20:175. doi: 10.1016/0009-3084(85)90012-x. [DOI] [PubMed] [Google Scholar]
- 48.Boggs JM, Koshy KM, Rangaraj G. Biochim. Biophys Acta. 1988;938:361. doi: 10.1016/0005-2736(88)90134-4. [DOI] [PubMed] [Google Scholar]
- 49.Odinkov VN, Guliyautinov IV, Nedopekin DV, Veskina NA, Khalilov LM. Rus. J. Org. Chem. 2003;39:952. [Google Scholar]
- 50.Bode SE, Muller M, Wolberg M. Org. Lett. 2002;4:619. doi: 10.1021/ol017223u. [DOI] [PubMed] [Google Scholar]
- 51.Zhu JH, Yu R-M, Yang L, Li W-M. Fitoterapia. 2010;81:339. doi: 10.1016/j.fitote.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Costantino V, D'Esposito M, Fattorusso E, Mangoni A, Basilico N, Parapini S, Taramelli D. J. Med. Chem. 2005;48:7411. doi: 10.1021/jm050506y. [DOI] [PubMed] [Google Scholar]
- 53.Jung JH, Lee Ch.-O., Kim Y. Ch., Kang SS. J. Nat. Prod. 1996;59:319. doi: 10.1021/np960201+. [DOI] [PubMed] [Google Scholar]
- 54.Li L, Tang X, Taylor KG, DuPre DB, Yappert MC. Biophys. J. 2002;82:2067. doi: 10.1016/S0006-3495(02)75554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szulc ZM, Bielawski J, Gracz H, Gustilo M, Mayroo N, Hannun Y, Obeid LM, Bielawska A. Bioorg. Med. Chem. 2006;14:7083. doi: 10.1016/j.bmc.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 56.Abraham RJ, Mobil M, Smith RJ. Magn. Res. Chem. 2003;41:26. [Google Scholar]
- 57.Sultan I, Senkal CE, Ponnusamy S, Bielawski J, Szulc ZM, Bielawska A, Hannun YA, Ogretmen B. Biochem. J. 2006;393:513. doi: 10.1042/BJ20051083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chapman JV, Gouaze-Andersson V, Messner MC, Flowers M, karimi R, Kester M, Barth BM, Liu X, Giulano AE, Cabot MC. Biochem. Pharmacol. 2010;80:308. doi: 10.1016/j.bcp.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hwang O, Kim G, Jang YO, Kim SW, Choi G, Choi HJ, Jeon SY, Lee DG, Lee JD. Molec. Pharmacol. 2001;59:1249. doi: 10.1124/mol.59.5.1249. [DOI] [PubMed] [Google Scholar]
- 60.Bielawska A, Crane HM, Liotta D, Obeid LM, Hannun YA. J. Biol. Chem. 1993;268:26226. [PubMed] [Google Scholar]
- 61.Ogretmen B, Pettus BJ, Rossi MJ, Wood R, Usta J, Szulc Z, Bielawska A, Obeid LM, Hannun YA. J. Biol. Chem. 2002;277:12960. doi: 10.1074/jbc.M110699200. [DOI] [PubMed] [Google Scholar]
- 62.Ogretmen B, Pettus BJ, Rossi MJ, Wood R, Usta J, Szulc Z, Bielawska A, Obeid LM, Hannun YA. J. Biol. Chem. 2002;277:12960. doi: 10.1074/jbc.M110699200. [DOI] [PubMed] [Google Scholar]
- 63.Bieberich E. Future Lipidol. 2008;3:273. doi: 10.2217/17460875.3.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogretmen B, Hannun YA. Nat. Rev. Cancer. 2004;4:604. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 65.Park I-N, Cho I, Kim SG. Drug Metab. Dispos. 2004;9:893. [PubMed] [Google Scholar]
- 66.Chik CL, Li B, Karpinski E, Ho AK. Mol. Cell. Endicronol. 2004;218:175. doi: 10.1016/j.mce.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 67.Ridgeway ND, Merriam DJ. Biochim. Biophys. Acta. 1995;1256:57. doi: 10.1016/0005-2760(95)00010-a. [DOI] [PubMed] [Google Scholar]
- 68.El-Bawab S, Usta J, Roddy P, Szulc ZM, Bielawska A, Hannun YA. J. Lipid Res. 2002;43:141. [PubMed] [Google Scholar]
- 69.Mizutani Y, Kihara A, Chiba H, Tojo H, Igarashi Y. J. Lipid Res. 2008;49:2356. doi: 10.1194/jlr.M800158-JLR200. [DOI] [PubMed] [Google Scholar]
- 70.Mukhopadhyay A, Saddoughi SA, Song P, Sultan I, Ponnusamy S, Senkal CE, Snook CF, Arnoki HK, Sears RC, Hannun YA, Ogretmen B. FASEB J. 2009;23:751. doi: 10.1096/fj.08-120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Methods. 2006;39:82. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 72.Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, Bielawska A. Methods Mol. Biol. 2009;579:443. doi: 10.1007/978-1-60761-322-0_22. [DOI] [PubMed] [Google Scholar]