Abstract
Virulent factors produced by pathogens play an important role in the infectious process, which is regulated by a cell-to-cell communication mechanism called quorum sensing (QS). Pseudomonas aeruginosa is an important opportunistic human pathogen, which causes infections in patients with compromised immune systems and cystic fibrosis. The QS systems of P. aeruginosa use N-acylated homoserine lactone (AHL) as signal molecules. Previously we have demonstrated that Panax ginseng treatment allowed the animals with P. aeruginosa pneumonia to effectively clear the bacterial infection. We postulated that the ability to impact the outcome of infections is partly due to ginseng having direct effect on the production of P. aeruginosa virulence factors. The study explores the effect of ginseng on alginate, protease and AHL production. The effect of ginseng extracts on growth and expression of quorum-sensing (QS)-controlled virulence factors on the prototypic P. aeruginosa PAO1 and its isogenic mucoid variant (PAOmucA22 or PDO300) was determined. Ginseng did not inhibit the growth of the bacteria, enhanced the extracellular protein production and stimulated the production of alginate. However, ginseng suppressed the production of LasA and LasB and down-regulated the synthesis of the AHL molecules. Ginseng has a negative effect on the QS system of P. aeruginosa, which might be part of the mechanisms that ginseng helped the bacterial clearance from the animal lungs in vivo in our previous animal study. It is possible that enhancing and repressing activities of ginseng are mutually exclusive as it is a complex mixture, as shown with the HPLC anaylsis of the hot water extract of ginseng that was performed in this study. Though ginseng is a promising natural synergetic remedy, it is important to isolate and evaluate the ginseng compounds associated with the anti-QS activity.
Keywords: Ginseng, Pseudomonas aeruginosa, anti-quorum sensing, LasA, LasB, Alginate
Introduction
The leading pathogen responsible for morbidity and mortality among patients with cystic fibrosis (CF) is Pseudomonas aeruginosa that grows in a biofilm-mode in the airways. The initial and intermittent colonization of CF lungs by nonmucoid P. aeruginosa can be eradicated by early aggressive antibiotic therapy but such treatment usually fails if the colony morphology of bacteria isolated from sputum samples is mucoid owing to overproduction of a capsule-like polysaccharide called alginate. Extensive efforts have been carried out to elucidate the molecular mechanisms responsible for overproduction of alginate in planktonic cells. However, despite our knowledge on the regulation of alginate genes in P. aeruginosa (Mathee et al., 2002), the successful control, if not complete elimination, still remains a challenge in the treatment of P. aeruginosa infection in CF patients.
The infection of CF patients with P. aeruginosa strains, especially after their conversion to alginate-producing mucoid form, is rarely eradicated by antibiotics (Høiby, 1974; Pedersen et al., 1992). In the hope of finding new and efficacious measures for the treatment of P. aeruginosa pneumonia in CF patients, alternative treatments have also been explored. Immunization with P. aeruginosa vaccines and adjuvants, or the treatment with IFN-γ or Chinese herbal medicine, Daphne giraldii Nitsche, could decrease the inflammatory response and enhance bacterial clearance in a rat model (Johansen et al., 1994; Johansen et al., 1995; Johansen et al., 1996; Song et al., 1996). The possibility of using the even more popular Chinese herbal supplement, ginseng has also been studied.
Ginseng is one of the best-known Chinese medicinal herbs and it has been widely used in China for thousands of years (Huang, 1993; O’Hara et al., 1998). The toxicity of ginseng is quite low, the LD50 for intravenous injection in mice is 16.5 mg/kg (Bensky, Gamble, 1993). The herb is reported to influence the cardiovascular, nervous, endocrine and immune systems; in addition, it affects metabolism, possesses some anti-cancer, anti-inflammation and anti-aging effects (Huang, 1993). As a modulator of the endocrine system and immune system, ginseng has been shown to have roles in modulating the production of specific antibodies and the functions of phagocytes and natural killer (NK) cells (Huang, 1993; Yang et al., 1983; Yang et al., 1987).
Several studies using animal models have shown that ginseng might play a role in enhancing immune response and bacterial clearance (Song et al., 1997a; Song et al., 1997b; Song et al., 1998; Song et al., 2002). Ginseng treatment appears to down-regulate the antibody response or shift the immune reaction from a Th2 type to a Th1 type and improve phagocytosis by PMNs, and perhaps downregulate inflammation (Song et al., 1997b). Furthermore, ginseng treatment significantly decreases the lung pathology and enhances bacterial clearance from the lung in the animal models of chronic P. aeruginosa lung infection (Song et al., 1997a; Song et al., 2002). It also down-regulates the serum IgG response against P. aeruginosa antigens, improves the blood PMN oxidative burst, and activates the phagocytosis of P. aeruginosa by blood PMNs and alveolar macrophages (Song et al., 1998). In addition, serum IgG1 decreases, IgG2a increases and mast cell numbers in the lung foci decrease. Furthermore, production of IFN-γ in the lung cell culture in the ginseng-treated group increased and IL-4 production decreased. These suggest that the therapeutic effect of ginseng is associated with its Th1-like modulating effect. The down-regulation of serum IgG leads to less formation of immune complexes, which in turn reduces the infiltration of PMNs and the damage of lung tissues (Song et al., 1997a; Song et al., 1997b).
All the studies described above have addressed the in vivo efficacy of ginseng against P. aeruginosa infection and the potential mechanisms involved using an animal model system. In this study we determined if ginseng directly affected the bacterial expression of virulence factors using a set of appropriate isogenic strains containing a well-characterized mutation. PAOmucA22 (PDO300) carries a mutation in the mucA gene that encodes the anti-sigma factor MucA, resulting in a constitutive mucoid phenotype (Mathee et al., 1999a; Mathee et al., 1999b) Since the efficacy of antibiotics and antibacterials against the mucoid and non-mucoid forms of P. aeruginosa differs significantly, we chose to determine the effect of ginseng extracts on the growth and expression of virulence factors under the control of quorum sensing (QS), in the wild type PAO1 (Holloway, Matsumoto, 1984) and the mucoid PDO300 (Mathee et al., 1999b) strains of P. aeruginosa. Our results suggest that although ginseng inhibited the expression of virulence factors such as LasA protease and LasB elastase in the non-mucoid strain, PAO1, addition of a lower concentration of ginseng (< 2.5 μg) stimulated growth and alginate production in the mucoid strain, PDO300. Therefore, for ginseng to be effectively used as a therapeutic agent, it becomes imperative to consider several factors including the concentration of ginseng extract, as well as the mucoid or non-mucoid state of the P. aeruginosa infection.
Materials and methods
Bacterial strains
Non-mucoid wild-type P. aeruginosa PAO1 (Holloway, Matsumoto, 1984) and its isogenic mucoid variant Alg+ PAOmucA22 (PDO300) (Mathee et al., 1999a; Mathee et al., 1999b) were used. LB media containing various concentrations of ginseng were used for bacterial growth under rapid shaking at 37°C. For growth curve experiments, culture samples were removed every hour for measurement of OD600 for 20 hours.
Ginseng preparation
Six-year old ginseng roots from Jilin, People’s Republic of China were dried and ground into powder. Luria-Bertani (LB) broth extracts of different concentrations of ginseng were prepared as described previously (Song et al., 1997b). In brief, different amounts of ginseng powder were mixed with LB medium (1% tryptone, 0.5% yeast extract, and 0.5% sodium chloride), heated to 90°C for 30 minutes, filtered and then used for culturing bacteria.
Determination of acylhomoserine lactones (AHL)
AHLs were extracted from ABT culture supernatants with acidified ethyl acetate, dried under nitrogen, and quantified by electrospray mass spectroscopy (ESMS) after the methods of Makemson et al (Makemson et al., 2006). Peak intensities for BHL (m/z = 172) and OdDHL (m/z = 298), and their sodium adducts (m/z=194 and 230 respectively) were combined and converted to concentration using a standard curve generated from the pure compounds. Background readings from samples extracted with alkaline ethyl acetate were subtracted from those of the acid-extracted bacterial cultures before conversion. Acetic acid preserves the AHL while NaOH opens up the charged lactone ring, resulting in the loss of AHL during the extraction with organic solvent, ethyl acetate.
LasA staphylolytic assay
LasA protease activity was determined by measuring the ability of culture supernatants to lyse boiled S. aureus cells (Kong et al., 2005). Supernatants from 18-hour culture at 37°C under shaking condition were taken for protease activity evaluation. A 100-μl aliquot of P. aeruginosa LB culture supernatant with or without plant extracts was added to 900 μl of boiled S. aureus suspension. OD600 was determined after 0, 5, 10, 20, 30, 45, and 60 minutes. Activity was expressed as ΔOD600/hour per μg protein.
Elastase activity
LasB protease (elastase) activity was measured in a spectrophotometric assay using elastin Congo red (ECR; Sigma, St. Louis, MO) as a substrate as reported previously (Ohman et al., 1980). A 100-μl aliquot was added to 900 μl of ECR buffer (100 mM Tris, 1 mM CaCl2, pH 7.5) containing 20 mg ECR. This was then incubated with shaking for 3 hours at 37°C. Insoluble ECR was removed by centrifugation, and the absorption of the supernatant was measured at 495 nm. Cell-free AB medium, alone, and with ginseng were used as negative controls. Activity was expressed as ΔOD495 per μg protein.
Quantification of alginate
The content of uronic acid polymers (the component of alginate) in the supernatants of over-night culture was analyzed by the carbazole-borate assay with D-mannuronic acid lactone (Sigma) (Knutson, Jeanes, 1968).
Quantification of proteins
Bio-Rad dye-reagent concentrate was diluted to 25% with distilled water. 20 μl of the sample was mixed with 20 μl of Triton X-100 or NaOH solutions in varying concentrations at 20°C and incubated for 1 minute prior to the addition of 1.0 ml of the diluted dye reagent. The optical density was read at 595 nm in the interval of 5 to 60 minutes after addition of the dye reagent.
High performance liquid chromatography (HPLC) analysis
The hot water extract was analyzed using an HPLC Spectra system P4000 (Thermo Finnigan, Waltham, MA) with a spherical reverse phase column (5 μm, 4.6 mm × 150 mm) (Waters Spherisorb® ODS2, Milford, MA). In addition, six ginsenoside standards, Rb1, Rb2, Rc, Rd, Re, and Rg1, were purchased (ChromaDex™, Irvine, CA), that were used as controls to confirm the presence of these ginsenosides in the extract. A mixture containing all six ginsenosides at a concentration of 30 mg/ml dissolved in water was made and analyzed on HPLC. To spike the water extract with the standards, 20 μl of the ginsenoside standards mixture (30 mg/ml) was added to 300 μl of the water extract. Samples were introduced by autoinjector, with a 10 μl or 20 μl injection volume. Using a slightly modified protocol that was previously described (Li et al., 1996), mobile phases were (A) water and (B) acetonitrile (HPLC grade; Fisher Chemical, Pittsburgh, PA), with a flow rate of 1 ml/min and the following gradient for B: 0–22 min, 20–22%; 22–40 min, 22–50%; 40–50 min, 50–55%; 52–54 min, 65–20%; and 54–70 min, 20%. The peaks were detected by UV spectrophotometry at 205 nm.
Results
Effect of ginseng on bacterial growth
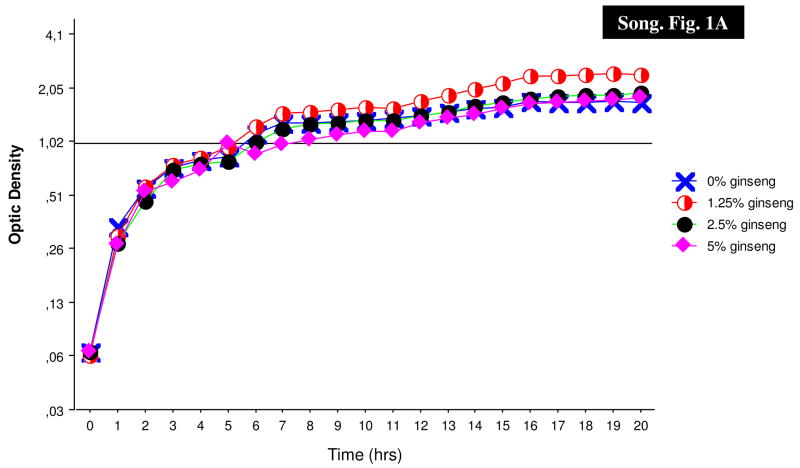
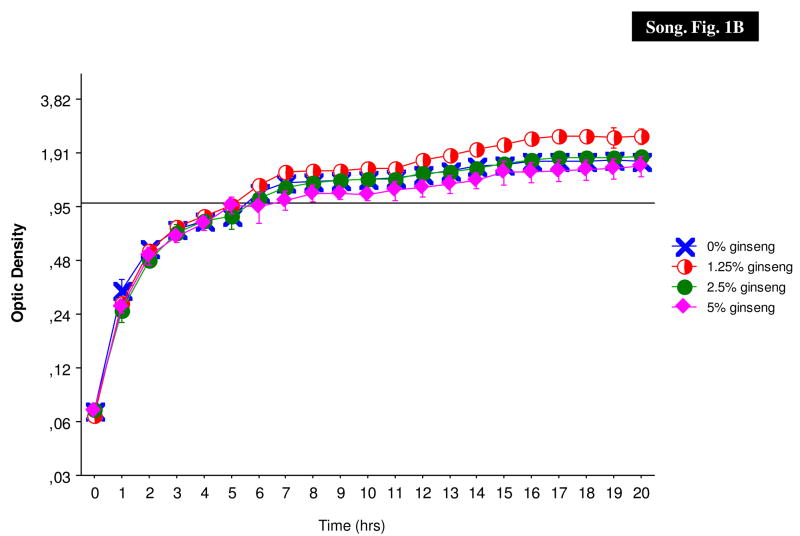
To determine if ginseng has any effect on P. aeruginosa growth, PAO1 and PAOmucA22 (PDO300) were grown in the presence of 1.25, 2.5 and 5% ginseng (Fig. 1). In general, the growth of these two strains was not significantly inhibited by the three different concentrations of ginseng in LB medium. In fact, 1.25% of ginseng seemed enhanced growth of the bacteria; the growth of the bacteria was exactly the same in 2.5% of ginseng as in the LB control, whereas the growth of P. aeruginosa in 5% of ginseng showed a relatively slower growth from hour 6 to 12 in PAO1 and hour 6 to 15 in PAOmucA22.
Fig. 1.
Growth curves of P. aeruginosa. The prototypic PAO1 (A) and PDO300 (B) were grown in the absence (X) and presence of 1.25% (open circle), 2.5% (filled circle) and 5% (diamonds) of ginseng.
Effects of ginseng on the synthesis of alginate
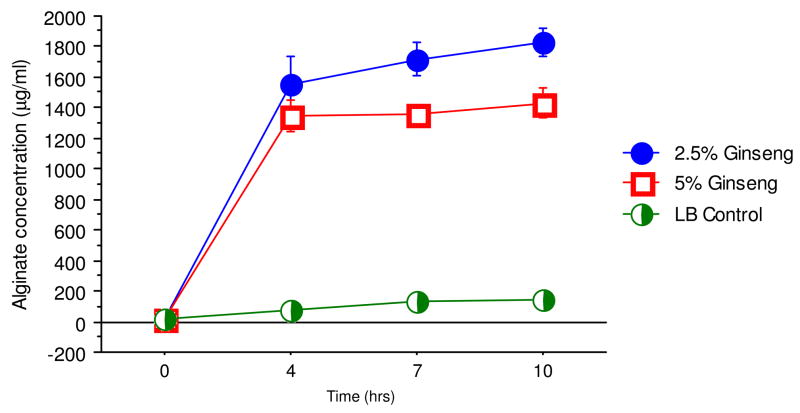
The prototypic PAO1 does not produce alginate unlike its isogenic variant PAOmuc22. The effect of ginseng on alginate production was compared between the strains with 2.5% and 5% ginseng (Fig. 2). Both concentrations did not stimulate any alginate production in PAO1. However, both 2.5% and 5% ginseng stimulated the production of alginate from the mucoid strain in a non-dosage-dependent way after over-night culture in LB medium.
Fig. 2.
Alginate production in P. aeruginosa. The amount of alginate produced in the absence (X) and presence of 2.5% (filled circle) and 5% (diamonds) of ginseng were quantified.
Effect of ginseng on P. aeruginosa protease activities
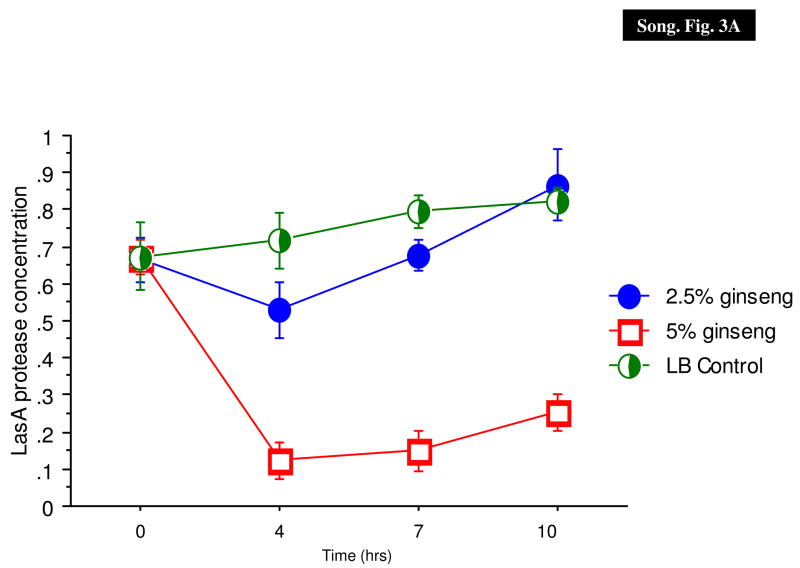
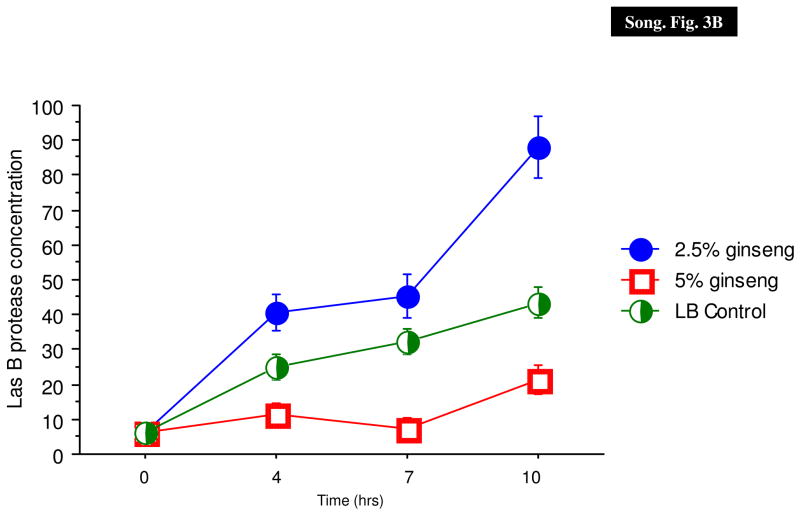
In a previous paper, we have shown that P. aeruginosa PAO1 and PAOmucA22 expressed similar levels of protease activities (Mathee et al., 1999a; Mathee et al., 1999b). The effect of ginseng on the production of LasA and LasB proteases was determined in P. aeruginosa PAO1 (Fig. 3).
Fig. 3.
Effect of ginseng on P. aeruginosa PAO1 protease activities. The staphylolytic LasA (A) and elastolytic LasB (B) activities were monitored in the absence (X) and presence of 2.5% (filled circle) and 5% (diamonds) of ginseng.
LasA staphylolytic protease is a 20-kDa zinc metalloendopeptidase belonging to the β-lytic endopeptidase family of proteases (Kessler, 1995). LasA production was inhibited only slightly by 2.5% ginseng, and completely with 5% of ginseng (Fig. 3A).
LasB elastase is a zinc metalloprotease capable of destroying or inactivating a wide range of biological tissues and immunological agents (Bever, Iglewski, 1988). There was a significant suppression of elastase activity with 5% ginseng, whereas 2.5% of ginseng slightly enhanced the production (Fig. 3B).
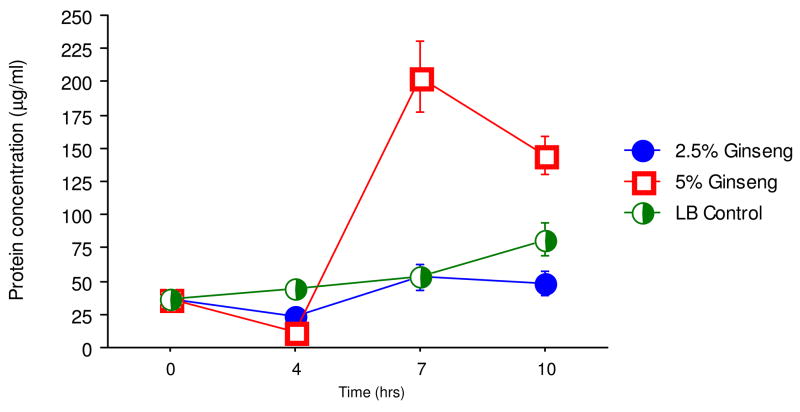
Effect of ginseng on total extracellular protein concentrations in culture supernatants
Total extracellular protein concentration was measured to determine if suppression of the protease activity is correlated with decrease in protein production. The synthesis of extracellular proteins from P. aeruginosa PAO1 was slightly downregulated in the two media containing different concentrations of ginseng on hour 4 of the culture. However, 5% ginseng greatly upregulated the synthesis of extracellular protein from the bacteria on hours 7 and 10, whereas 2.5% ginseng did not affect the synthesis of extracellular protein (Fig. 4).
Fig. 4.
Extracellular protein levels in P. aeruginosa PAO1. The protein levels in the supernatant were quantified in the absence (X) and presence of 2.5% (filled circle) and 5% (diamonds) of ginseng.
Effect of ginseng on the production of AHLs
To determine if ginseng interfered with the production of AHL molecules, the amount of BHL and OdDHL produced in the supernatant was quantitated using mass spectroscopy in the absence and presence of ginseng. Ginseng inhibited the production of both BHL and OdDHL in the supernatants of over-night culture of P. aeruginosa PAO1 in a concentration-dependent manner (Fig. 5).
Fig. 5.
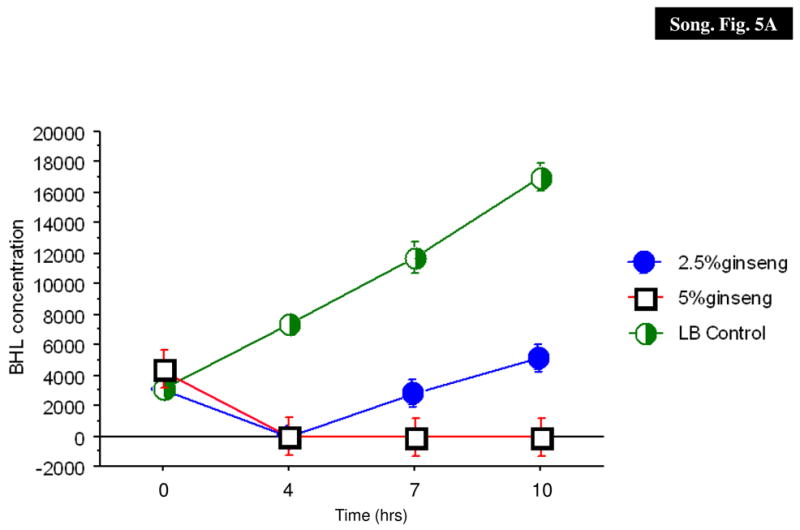
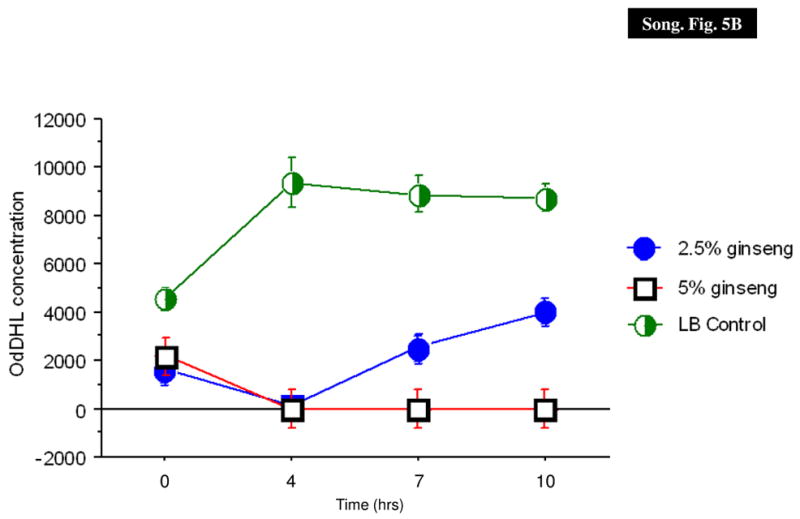
AHL production in P. aeruginosa PAO1. The BHL (A) and OdDHL (B) levels in the supernatant were quantified in the absence (X) and presence of 2.5% (filled circle) and 5% (diamonds) of ginseng.
HPLC analysis of the ginseng extract
In order to provide a general compositional view of the extract as well as confirmation of the presence of ginsenosides, HPLC of the aqueous extract was done and compared using the elution profile of ginsenoside standards (Figs. 6A and 6B). Initially, retention times were determined for each ginsenoside standard individually. Subsequently, the six ginsenoside standards were combined and the HPLC retention times were determined. The ginsenoside standards produced six peaks with retention times of 26.8 min, 28.0 min, 37.0 min, 37.6 min, 38.3 min, and 39.6 min corresponding to Rg1, Re, Rb1, Rc, Rb2, and Rd, respectively (Fig. 6A). The water extract of ginseng contained six peaks with similar retention times, indicating the presence of Rb1, Rb2, Rc, Rd, Re, and Rg1 (Fig. 6B). In addition, the hot water extract was spiked with standards to provide further confirmation of the presence of the ginsenosides (Fig. 6C). The peaks corresponding to the ginsenoside standards retention time were increased in size, thus verifying the presence of the six ginsenosides (Fig. 6C).
Fig. 6.
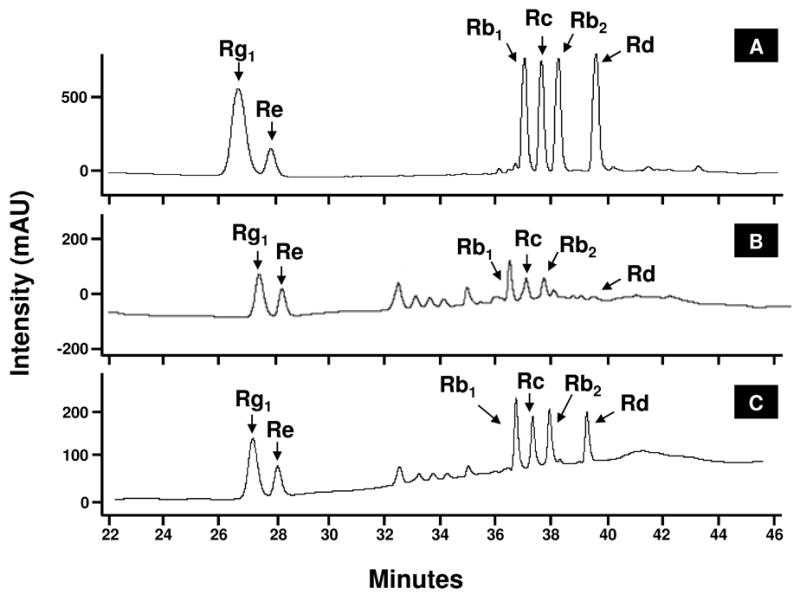
HPLC - UV analysis of ginseng extract. The chromatogram show combined ginsenoside standards (A) and aqueous ginseng extracts (B). Peaks are a function of intensity measured in milliabsorption units over time in minutes. Peaks corresponding to the six ginsensosides used as standards are labeled.
Discussion
P. aeruginosa is the major pathogen responsible for the secondary infections in patients with burn trauma, diffused pan-bronchitis, chronic obstructive pulmonary disease, CF and with immune defects (Driscoll et al., 2007). P. aeruginosa remains one of the major causes of nosocomial infections (Rosenthal et al., 2008). The pathogenesis of P. aeruginosa is associated closely with the production of a myriad of extracellular virulence factors and the formation of biofilm, both of which are regulated by QS systems in the bacterium (Davies et al., 1998; Kharazmi, 1989; Meyer et al., 1996; Morihara, Homma, 1985). There is increasing evidence indicating that QS might be a novel target for developing new antimicrobial agents (Kaufmann et al., 2008).
Previously, we found that ginseng treatment helped to speed up the clearance of P. aeruginosa from animal lungs with P. aeruginosa pneumonia, to decrease the severity of lung pathology by changing the type of immune response from a TH2 to a TH1 response and to stimulate the phagocytosis by neutrophils and monocytes (Song et al., 1997a; Song et al., 1997b; Song et al., 1998; Song et al., 2002; Song et al., 1999). It has also been shown that P. aeruginosa virulence can be reduced using anti-QS inhibitors (Hentzer et al., 2003). Thus, we postulated that protective effect of ginseng could be partially due to its ability to reduce the efficacy of virulence factor production that is often under QS-control.
Ginseng exhibits no toxicity against P. aeruginosa
The toxicity of ginseng in mice is quite low, the LD50 for intravenous injection is 16.5 mg/kg (Bensky, Gamble, 1993). In the present study, ginseng did not inhibit the growth of neither mucoid nor non-mucoid P. aeruginosa (Fig. 1). Instead, lower concentrations of ginseng seemed to stimulate growth. Thus, it is likely that the effect of ginseng seen in in vivo studies is not due to bacterial killing. One should also be mindful not to prescribe a lower dose of ginseng that might enhance its growth properties and consequently have a negative outcome in patients.
Ginseng stimulates alginate production
Recovering alginate-producing P. aeruginosa patients spells poor prognosis for CF patients (Høiby, 1974). Early aggressive antibiotic therapy is able to eradicate initial and intermittent colonization of the CF lungs by P. aeruginosa (Frederiksen et al., 1997). However, when the colony morphology of bacteria isolated from sputum samples is observed to convert to the Alg+ form, the organisms can no longer be eliminated from the lungs despite aggressive antibiotic therapy (Frederiksen et al., 1997). The selection pressure for mucoid conversion common to P. aeruginosa strains that thrive in the complex CF respiratory environment is not well understood. However, it has been established that repeated exposure of a P. aeruginosa biofilm in vitro to activated polymorphonuclear leukocytes (PMNs), or to low-levels of hydrogen peroxide, can give rise to mucoid variants with defects in mucA gene, mimicking that seen in vivo (Mathee et al., 1999b). Thus, any antibacterial or anti-QS agents used have to suppress alginate production for effective eradication of P. aeruginosa infection. In this study, ginseng stimulated alginate production (Fig. 2). The animal studies to date point to ginseng as a promising natural medicine for stimulation of the immune system in CF patients with P. aeruginosa lung infections. However, these studies were done largely with nonmucoid strains. The results from this study suggest that ginseng might not be appropriate for patients with chronic infections of mucoid P. aeruginosa. Moreover, similar results were obtained in mice infected with PAO1 and PAOmucA22 (Song et al, unpublished observations). However, the caveat is that the effect of ginseng on alginate production in vivo is not known. We believe that it can certainly be used for early infection where the immune response has to be stimulated as seen in an activation of innate immunity after ginseng treatment (Song et al., 1997a). Alternatively the ginseng components with anti-QS activity should be separated from the alginate-enhancing components.
Ginseng exhibits anti-QS activity
It has been demonstrated that the success of P. aeruginosa as an opportunistic pathogen is due to the battery of QS-regulated arsenals that it produces to combat host-associated aggression (antibiotics and immune response) (Davies et al., 1998; Kharazmi et al., 1989; Meyer et al., 1996; Morihara, Homma, 1985). As such, targeting bacterial QS as potential anti-infective therapy has been of interest (Kaufmann et al., 2008; Fast, 2003). Synthesizing chemical analogs has yielded compounds with anti-QS activity, but they are yet to make their way to pharmaceutical use (Rice et al., 2005; Janssens et al., 2007). However, the promise is in the naturally occurring compounds that can be potentially non- or mildly toxic. Anti-QS activity has been demonstrated in algae and terrestrial plants (Manefield et al., 1999; Adonizio et al., 2006; Adonizio et al., 2008a; Adonizio et al., 2008b; Singh et al., 2009). This study clearly demonstrates that 5 % ginseng has the ability to counter the QS system. Ginseng was able to suppress the protease activities (Fig. 3). This suppression is not due to a decrease in cellular growth or protein synthesis, as 5% ginseng stimulated the synthesis of extracellular proteins in the supernatants, but not the virulence factors (Fig. 4). It appears that the reduction of protease activity may be due to the decrease in the production of BHL and OdDHL (Fig. 3), critical components that stimulate the production of virulence factors. It is of concern that 2.5% ginseng stimulated the synthesis of elastase from P. aeruginosa. Thus, it is important to determine the appropriate dose for ginseng to be administered as an effective natural remedy to combat P. aeruginosa infection.
Ginseng has been used widely as dietary supplement in the orient. In recent years, efforts have been made to validate its use. This study suggests that ginseng is a potentially promising remedy to treat P. aeruginosa infections in future. Ginseng extract is a complex mixture containing ginsenosides saponins and other root components such as polysaccharides, sterols, organic acids, phenolic acids, flavonoids, essential oils, vitamins and trace elements. The aqueous extract used in this study contains ginsenosides as well as other unidentified compounds (Fig. 6B). It is known that traditional Chinese medicine is characterized by multiple compounds working synergistically and isolating these activities are difficult (Lee, 2000). A number of these compounds could be responsible for the anti-QS activity. An acidic polysaccharide isolated from P. ginseng displayed anti-adhesive activity against pathogenic bacteria (Lee et al., 2006). The activity of other ginseng-derived compounds has not been researched as extensively as ginsenosides. While ginsenosides have been shown to have a beneficial effect on a number of mammalian systems, the effects of ginsenosides on bacteria have not been widely examined (Liu, 1992). It is very likely the components that enhance growth, alginate production are different from those that exhibit anti-QS activity. It is important that these components are separated and tested before ginseng can be advocated for use against P. aeruginosa infections.
Acknowledgments
We gratefully acknowledge the support of National Institute of Health, National Center for Alternative and Complementary Medicine (1-R15-AT002626, KM), Cystic Fibrosis Foundation (MATHEE0110, KM), Florida International University Teaching Assistantship (KFK). We would also like to thank Mathee Lab Crew and Professor Wagner for their critical reading of this manuscript and their invaluable suggestions and comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adonizio A, Kong KF, Mathee K. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob Agents Chemother. 2008a;52:198–203. doi: 10.1128/AAC.00612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adonizio A, Leal SM, Jr, Ausubel FM, Mathee K. Attenuation of Pseudomonas aeruginosa virulence by medicinal plants in a Caenorhabditis elegans model system. J Med Microbiol. 2008b;57:809–813. doi: 10.1099/jmm.0.47802-0. [DOI] [PubMed] [Google Scholar]
- Adonizio AL, Downum K, Bennett BC, Mathee K. Anti-quorum sensing activity of medicinal plants in southern Florida. J Ethnopharmacol. 2006;105:427–435. doi: 10.1016/j.jep.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Bensky D, Gamble A. Chinese herbal medicine: Materia Medica, Revised edition. Washington: Eastland Press, Inc; 1993. [Google Scholar]
- Bever RA, Iglewski BH. Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene. J Bacteriol. 1988;170:4309–4314. doi: 10.1128/jb.170.9.4309-4314.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D, Parsek M, Pearson J, Iglewski B, Costerton J, Greenberg E. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs. 2007;67:351–368. doi: 10.2165/00003495-200767030-00003. [DOI] [PubMed] [Google Scholar]
- Fast W. Molecular radio jamming: autoinducer analogs. Chem Biol. 2003;10:1–2. doi: 10.1016/s1074-5521(03)00005-x. [DOI] [PubMed] [Google Scholar]
- Frederiksen B, Koch C, Høiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr Pulmonol. 1997;23:330–335. doi: 10.1002/(sici)1099-0496(199705)23:5<330::aid-ppul4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P, Manefield M, Costerton JW, Molin S, Eberl L, Steinberg P, Kjelleberg S, Høiby N, Givskov M. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway BW, Matsumoto M. Pseudomonas aeruginosa PAO. In: O’Brien SJ, editor. Genetic Maps. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1984. pp. 194–197. [Google Scholar]
- Høiby N. Pseudomonas aeruginosa infection in cystic fibrosis: relationship between mucoid strains of Pseudomonas aeruginosa and the humoral immune response. Acta Pathol Microbiol Scand. 1974;82:551–558. [PubMed] [Google Scholar]
- Huang KC. The pharmacology of Chinese herbs. Boca Raton, Florida: CRC Press, Inc; 1993. [Google Scholar]
- Janssens JC, Metzger K, Daniels R, Ptacek D, Verhoeven T, Habel LW, Vanderleyden J, De Vos DE, De Keersmaecker SC. Synthesis of N-acyl homoserine lactone analogues reveals strong activators of SdiA, the Salmonella enterica serovar Typhimurium LuxR homologue. Appl Environ Microbiol. 2007;73:535–544. doi: 10.1128/AEM.01451-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen H, Espersen F, Cryz SJ, Hougen H, Fomsgaard A, Rygaard J, Høiby N. Immunization with Pseudomonas aeruginosa vaccines and adjuvant can modulate the type of inflammatory response subsequent to infection. Infect Immun. 1994;62:3146–3155. doi: 10.1128/iai.62.8.3146-3155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen HK, Hougen HP, Cryz SJ, Jr, Rygaard J, Høiby N. Vaccination promotes TH1-like inflammation and survival in chronic Pseudomonas aeruginosa pneumonia in rats. Am J Respir Crit Care Med. 1995;152:1337–1346. doi: 10.1164/ajrccm.152.4.7551392. [DOI] [PubMed] [Google Scholar]
- Johansen HK, Hougen HP, Rygaard J, Høiby N. Interferon-gamma (IFN-γ) treatment decreases the inflammatory response in chronic Pseudomonas aeruginosa pneumonia in rats. Clin Exp Immunol. 1996;103:212–218. doi: 10.1046/j.1365-2249.1996.d01-618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann GF, Park J, Janda KD. Bacterial quorum sensing: a new target for anti-infective immunotherapy. Expert Opin Biol Ther. 2008;8:719–724. doi: 10.1517/14712598.8.6.719. [DOI] [PubMed] [Google Scholar]
- Kessler E. β-lytic endopeptidases. Methods Enzymol. 1995;248:740–756. doi: 10.1016/0076-6879(95)48050-1. [DOI] [PubMed] [Google Scholar]
- Kharazmi A. Interactions of Pseudomonas aeruginosa proteases with the cells of the immune system. In: Høiby N, Pedersen SS, Shand GH, Döring G, Holder IA, editors. Basic and Clinical aspects of Pseudomonas aeruginosa. Basel: Antibiotics and Chemotherapy. S. Karger; 1989. pp. 42–49. [DOI] [PubMed] [Google Scholar]
- Kharazmi A, Bibi Z, Nielson H, Høiby N, Döring G. Effect of Pseudomonas aeruginosa rhamnolipid on human neutophil and monocyte function. Acta Pathol Microbiol Immunol Scand. 1989;97:1068–1072. [PubMed] [Google Scholar]
- Knutson C, Jeanes A. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal Biochem. 1968;24:470–481. doi: 10.1016/0003-2697(68)90154-1. [DOI] [PubMed] [Google Scholar]
- Kong KF, Jayawardena SR, Indulkar SD, Del Puerto A, Koh CL, Høiby N, Mathee K. Pseudomonas aeruginosa AmpR is a global transcriptional factor that regulates expression of AmpC and PoxB β-lactamases, proteases, quorum sensing, and other virulence factors. Antimicrob Agents Chemother. 2005;49:4567–4575. doi: 10.1128/AAC.49.11.4567-4575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Shim JS, Lee JS, Kim MK, Chung MS, Kim KH. Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydr Res. 2006;341:1154–1163. doi: 10.1016/j.carres.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Lee KH. Research and future trends in the pharmaceutical development of medicinal herbs from Chinese medicine. Public Health Nutr. 2000;3:515–522. doi: 10.1017/s1368980000000604. [DOI] [PubMed] [Google Scholar]
- Li TSC, Mazza G, Cottrell AC, Gao L. Ginsenosides in roots and leaves of American ginseng. J Agric Food Chem. 1996;44:717–720. [Google Scholar]
- Liu CX, Xiao PG. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;36:27–38. doi: 10.1016/0378-8741(92)90057-x. [DOI] [PubMed] [Google Scholar]
- Makemson J, Eberhard A, Mathee K. Simple electrospray mass spectrometry detection of acylhomoserine lactones. Luminescence. 2006;21:1–6. doi: 10.1002/bio.873. [DOI] [PubMed] [Google Scholar]
- Manefield M, de Nys R, Kumar N, Read R, Givskov M, Steinberg P, Kjelleberg S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology. 1999;145:283–291. doi: 10.1099/13500872-145-2-283. [DOI] [PubMed] [Google Scholar]
- Mathee K, Ciofu O, Givskov M, Ohman DE, Molin S, Høiby N, Kharazmi A. Induction of Pseudomonas aeruginosa alginate production in vivo mediated by inflammatory response in lungs of cystic fibrosis patients. Clin Microbiol. 1999a;5:S8–9. [Google Scholar]
- Mathee K, Ciofu O, Sternberg C, Lindum PW, Campbell JI, Jensen P, Johnsen AH, Givskov M, Ohman DE, Molin S, Høiby N, Kharazmi A. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology. 1999b;145:1349–1357. doi: 10.1099/13500872-145-6-1349. [DOI] [PubMed] [Google Scholar]
- Mathee K, Kharazmi A, Høiby N. Role of Exopolysaccharide in Biofilm Matrix Formation: The Alginate Paradigm. In: McLean RJC, Decho AW, editors. Molecular Ecology of Biofilms. UK: Horizon Press; 2002. [Google Scholar]
- Meyer JM, Neely A, Stintzi A, Georges C, Holder IA. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara K, Homma JY. Pseudomonas proteases. In: Holder IA, editor. Bacterial enzymes and virulence. Boca Raton, Fla: CRC Press, Inc; 1985. pp. 41–79. [Google Scholar]
- O’Hara M, Kiefer D, Farrell K, Kemper K. A review of 12 commonly used medicinal herbs. Arch Fam Med. 1998;7:523–536. doi: 10.1001/archfami.7.6.523. [DOI] [PubMed] [Google Scholar]
- Ohman DE, Cryz SJ, Iglewski BH. Isolation and characterization of a Pseudomonas aeruginosa PAO mutant that produces altered elastase. J Bacteriol. 1980;142:836–842. doi: 10.1128/jb.142.3.836-842.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SS, Moller H, Espersen F, Sorensen CH, Jensen T, Høiby N. Mucosal immunity to Pseudomonas aeruginosa alginate in cystic fibrosis. Acta Pathol Microbiol Scand Suppl. 1992;100:326–334. [PubMed] [Google Scholar]
- Rice SA, McDougald D, Kumar N, Kjelleberg S. The use of quorum-sensing blockers as therapeutic agents for the control of biofilm-associated infections. Curr Opin Investig Drugs. 2005;6:178–184. [PubMed] [Google Scholar]
- Rosenthal VD, Maki DG, Mehta A, Alvarez-Moreno C, Leblebicioglu H, Higuera F, Cuellar LE, Madani N, Mitrev Z, Duenas L, Navoa-Ng JA, Garcell HG, Raka L, Hidalgo RF, Medeiros EA, Kanj SS, Abubakar S, Nercelles P, Pratesi RD. International nosocomial infection control consortium report, data summary for 2002–2007, issued January 2008. Am J Infect Control. 2008;36:627–637. doi: 10.1016/j.ajic.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Singh BN, Singh BR, Singh RL, Prakash D, Sarma BK, Singh HB. Antioxidant and anti-quorum sensing activities of green pod of Acacia nilotica L. Food. Chem Toxicol. 2009;47:778–786. doi: 10.1016/j.fct.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Song Z, Johansen HK, Moser C, Høiby N. Effects of Chinese medicinal herbs on a rat model of chronic Pseudomonas aeruginosa lung infection. Acta Pathol Microbiol Scand Suppl. 1996;104:350–354. [PubMed] [Google Scholar]
- Song Z, Johansen HK, Faber V, Høiby N. Ginseng treatment enhances bacterial clearance and decreases lung pathology in athymic rats with chronic P. aeruginosa pneumonia. Acta Pathol Microbiol Scand. 1997a;105:438–444. [PubMed] [Google Scholar]
- Song Z, Johansen HK, Faber V, Moser C, Kharazmi A, Rygaard J, Høiby N. Ginseng treatment reduces bacterial load and lung pathology in chronic Pseudomonas aeruginosa pneumonia in rats. Antimicrob Agents Chemother. 1997b;41:961–964. doi: 10.1128/aac.41.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Kharazmi A, Wu H, Faber V, Moser C, Krogh H, Rygaard J, Høiby N. Effects of ginseng treatment on neutrophil chemiluminescence and immunoglobulin G subclasses in a rat model of chronic Pseudomonas aeruginosa pneumonia. Clin Diagn Lab Immunol. 1998;5:882–887. doi: 10.1128/cdli.5.6.882-887.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Kharazmi A, Wu H, Johansen HK, Faber V, Høiby N. Immunomodulatory properties of ginseng – effects on Pseudomonas aeruginosa lung infection. Clin Microbiol Infect. 1999;5:S37–38. [Google Scholar]
- Song Z, Wu H, Mathee K, Høiby N, Kharazmi A. Gerimax ginseng regulates both humoral and cellular immunity during chronic Pseudomonas aeruginosa lung infection. J Altern Complement Med. 2002;8:459–466. doi: 10.1089/107555302760253658. [DOI] [PubMed] [Google Scholar]
- Yang GZ, Bao T, Fu N, Geng PL. Preliminary study of the modulating effects of ginseng on immunity in vivo and in vitro. Acta Beth Med Univ (Chin) 1983;9:1–4. [Google Scholar]
- Yang GZ, Bao T, Yu YL. Immune modulating effects of ginseng in vivo and in vitro. Comm Med Res (Chin) 1987;16:220–221. [Google Scholar]